Evaluation of Maize Growth Following Early Season Foliar P Supply of Various Fertilizer Formulations and in Relation to Nutritional Status
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment 1
2.2. Experiment 2
2.3. Plant Sampling and Analysis
2.4. Statistical Analysis
3. Results
3.1. Development of Biomass, P Concentration, and P Content Over Time
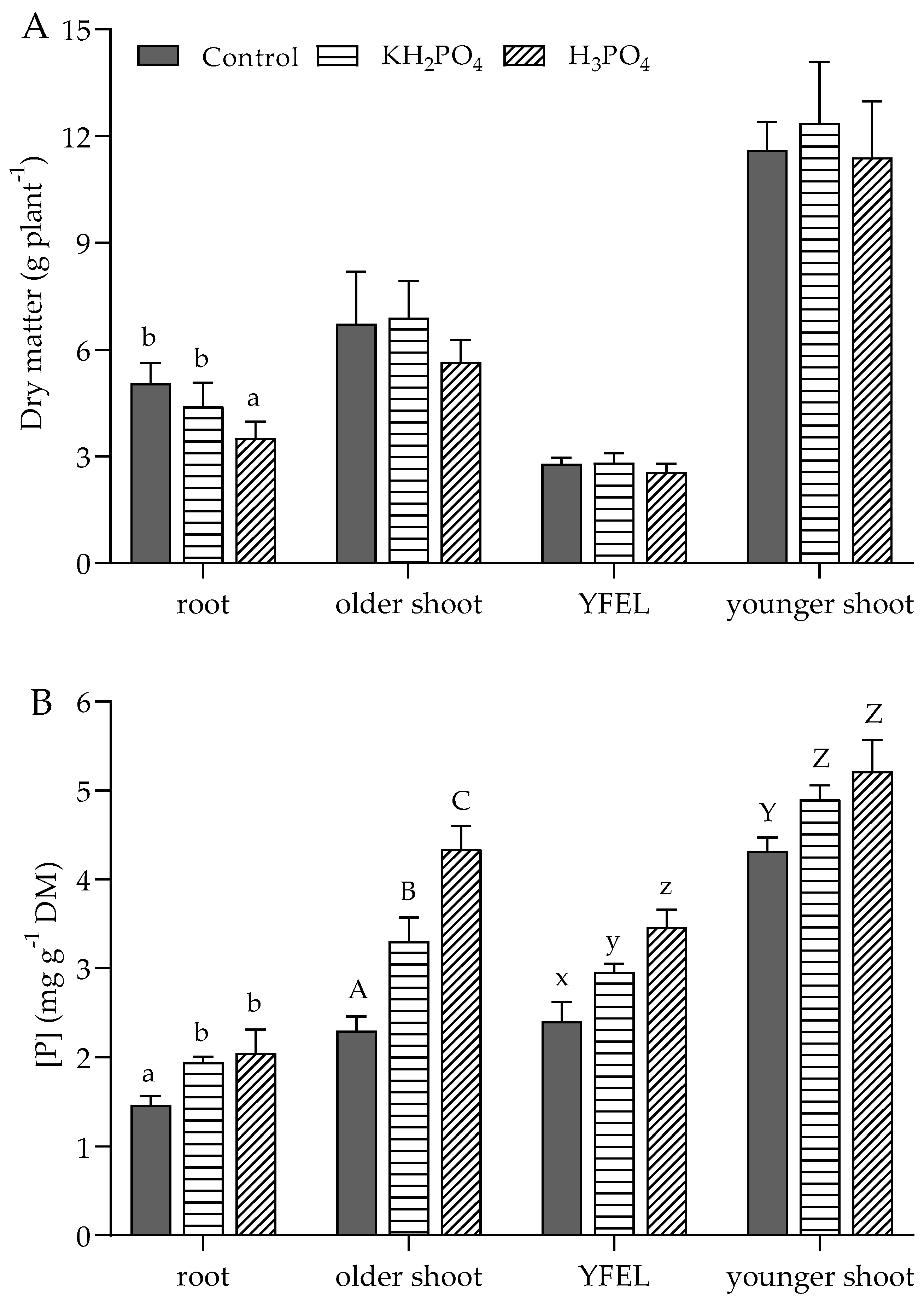
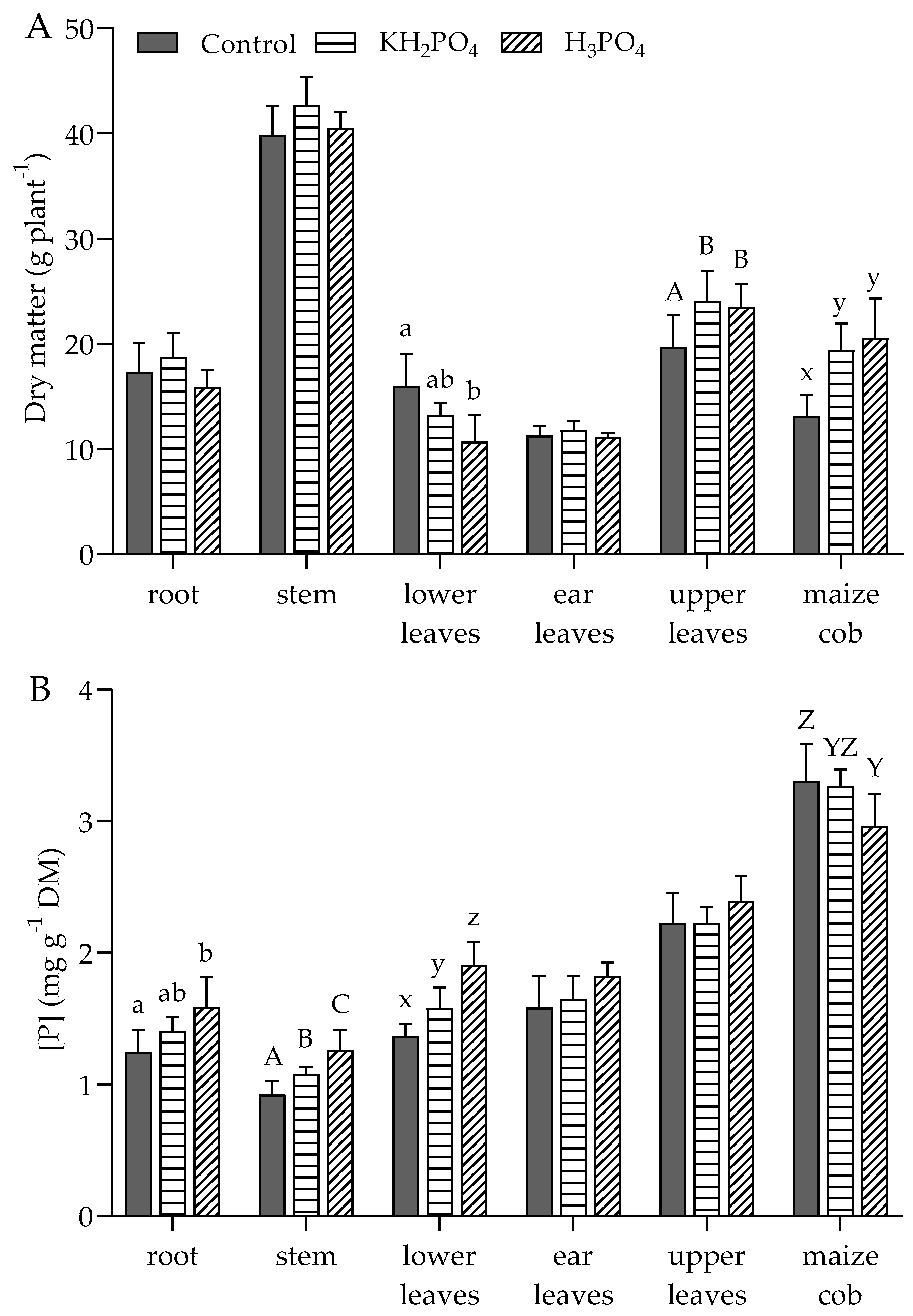
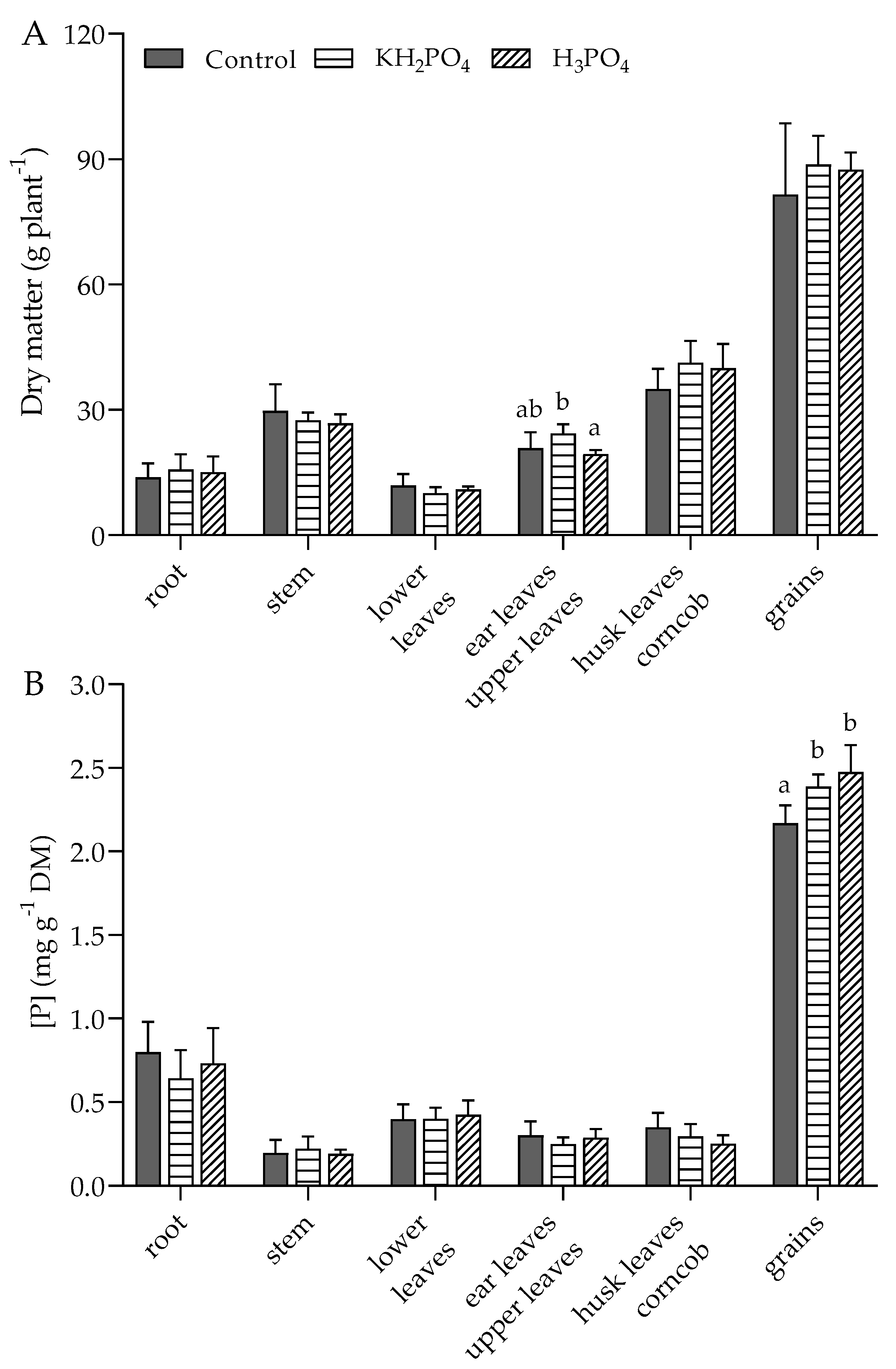
3.2. P Concentration in Various Parts of Plants at 6 Leaf Stage, Flowering, and Maturity
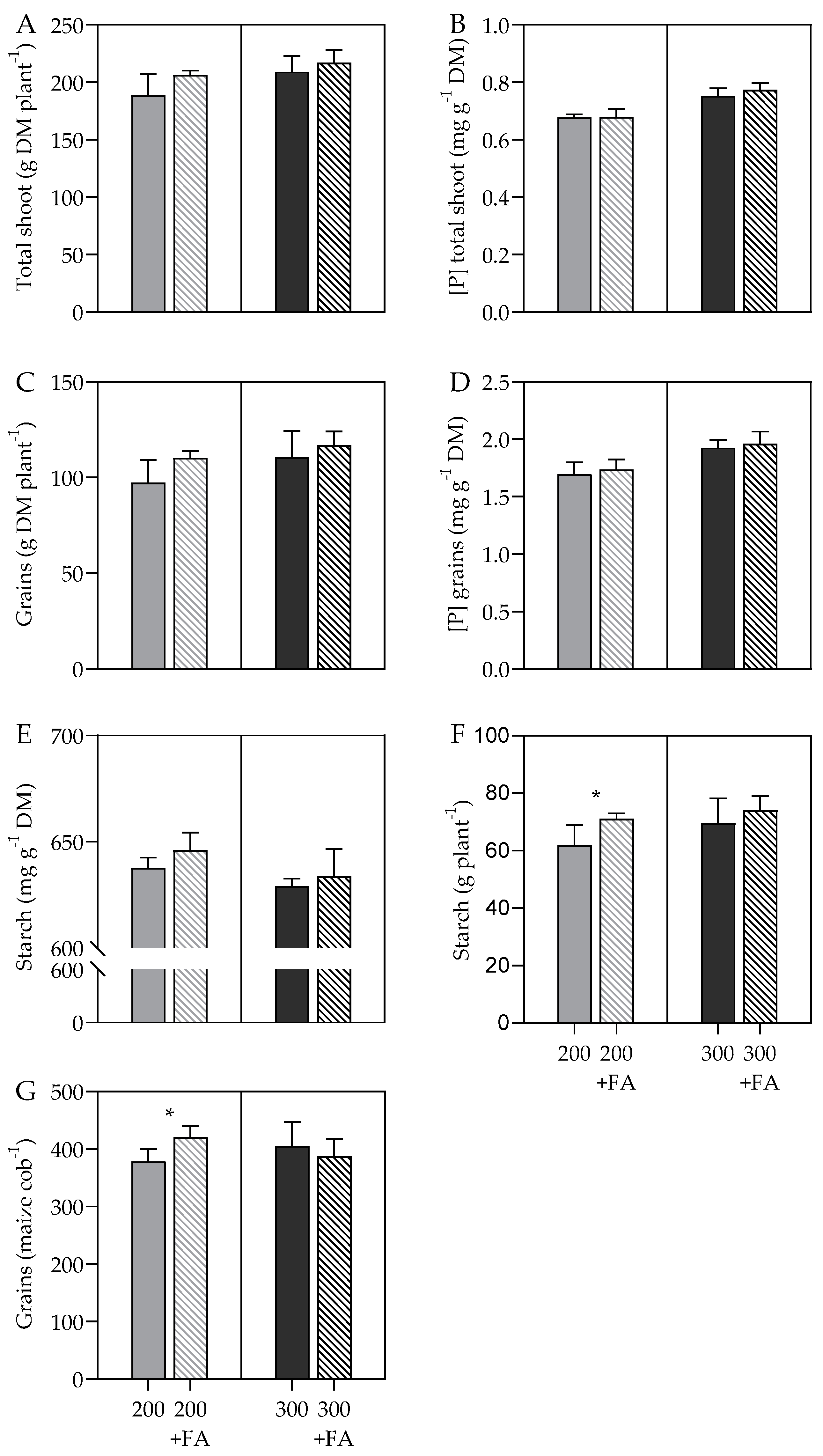
3.3. Dry Matter and P Concentration according to P Soil and Foliar Fertilization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schachtman, D.P.; Reid, R.J.; Ayling, S. Phosphorus Uptake by Plants: From Soil to Cell. Plant Physiol. 1998, 116, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.A.; Flaten, D.N.; Tomasiewicz, D.J.; Sheppard, S.C. The importance of early season phosphorus nutrition. Can. J. Plant Sci. 2001, 81, 211–224. [Google Scholar] [CrossRef]
- Maathuis, F.J. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Raghothama, K.G.; Karthikeyan, A.S. Phosphate Acquisition. Plant Soil 2005, 274, 37–49. [Google Scholar] [CrossRef]
- Rodriguez, D.; Andrade, F.; Goudriaan, J. Effects of phosphorus nutrition on tiller emergence in wheat. Plant Soil 1999, 209, 283–295. [Google Scholar] [CrossRef]
- Barry, D.A.J.; Miller, M.H. Phosphorus Nutritional Requirement of Maize Seedlings for Maximum Yield. Agron. J. 1989, 81, 95–99. [Google Scholar] [CrossRef]
- Plénet, D.; Etchebest, S.; Mollier, A.; Pellerin, S. Growth analysis of maize field crops under phosphorus deficiency. I. Leaf Growth. Plant Soil 2000, 223, 119–132. [Google Scholar] [CrossRef]
- Assuero, S.G.; Mollier, A.; Pellerin, S. The decrease in growth of phosphorus-deficient maize leaves is related to a lower cell production. Plant Cell Environ. 2004, 27, 887–895. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Chapter 6—Functions of Macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, H., Marschner, P., Eds.; Academic Press: Boston, MA, USA, 2012; pp. 135–189. ISBN 9780123849052. [Google Scholar]
- Noack, S.R.; McBeath, T.M.; McLaughlin, M.J. Potential for foliar phosphorus fertilisation of dryland cereal crops: A review. Crop Pasture Sci. 2010, 61, 659–669. [Google Scholar] [CrossRef]
- Bundesministerium für Ernährung und Landwirtschaft. Statistisches Jahrbuch über Ernährung, Landwirtschaft und Forsten der Bundesrepublik Deutschland 2018; Bundesministerium für Ernährung und Landwirtschaft: Berlin, Germany, 2018; ISBN 978-3-8308-1365-1. [Google Scholar]
- Statistisches Bundesamt. Anbauflächen, Hektarerträge und Erntemengen Ausgewählter Anbaukulturen im Zeitvergleich. Available online: https://www.destatis.de/DE/Themen/Branchen-Unternehmen/Landwirtschaft-Forstwirtschaft-Fischerei/Feldfruech-te-Gruenland/Tabellen/liste-feldfruechte-zeitreihe.html;jsessionid=E85220EAEAA06CA3538A26C0E2CD633F.internet711 (accessed on 11 March 2021).
- Stamp, P. Chilling stress in maize. In Breeding of Silage Maize; Dolstra, O., Miedema, P., Eds.; Pudoc: Wageningen, Germany, 1986; pp. 43–50. ISBN 90-220-0895-9. [Google Scholar]
- Imran, M.; Mahmood, A.; Römheld, V.; Neumann, G. Nutrient seed priming improves seedling development of maize exposed to low root zone temperatures during early growth. Eur. J. Agron. 2013, 49, 141–148. [Google Scholar] [CrossRef]
- Pregitzer, K.; King, J. Effects of Soil Temperature on Nutrient Uptake. In Nutrient Acquisition by Plants: An Ecological Perspective; Bassiri Rad, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 277–310. [Google Scholar]
- Sutton, C.D. Effect of low soil temperature on phosphate nutrition of plants—A review. J. Sci. Food Agric. 1969, 20, 1–3. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Bieleski, R.L. Phosphate Pools, Phosphate Transport, and Phosphate Availability. Annu. Rev. Plant Physiol. 1973, 24, 225–252. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef]
- Pedersen, I.F.; Rubæk, G.H.; Sørensen, P. Cattle slurry acidification and application method can improve initial phosphorus availability for maize. Plant Soil 2017, 414, 143–158. [Google Scholar] [CrossRef]
- Hart, M.R.; Quin, B.F.; Nguyen, M.L. Phosphorus Runoff from Agricultural Land and Direct Fertilizer Effects: A Review. J. Environ. Qual. 2004, 33, 1954–1972. [Google Scholar] [CrossRef] [PubMed]
- Sharpley, A.N.; McDowell, R.W.; Kleinman, P.J.A. Phosphorus loss from land to water: Integrating agricultural and environmental management. Plant Soil 2001, 237, 287–307. [Google Scholar] [CrossRef]
- Bundesanstalt für Landwirtschaft und Ernährung. Die Neue Düngeverordnung, 2nd ed.; Bundesanstalt für Landwirtschaft und Ernährung: Berlin, Germany, 2018; ISBN 978-3-8308-1323-1. [Google Scholar]
- Fernández, V.; Brown, P.H. From plant surface to plant metabolism: The uncertain fate of foliar-applied nutrients. Front. Plant Sci. 2013, 4, 289. [Google Scholar] [CrossRef] [PubMed]
- Girma, K.; Martin, K.L.; Freeman, K.W.; Mosali, J.; Teal, R.K.; Raun, W.R.; Moges, S.M.; Arnall, D.B. Determination of Optimum Rate and Growth Stage for Foliar-Applied Phosphorus in Corn. Commun. Soil Sci. Plant Anal. 2007, 38, 1137–1154. [Google Scholar] [CrossRef]
- Fernández, V.; Eichert, T. Uptake of Hydrophilic Solutes Through Plant Leaves: Current State of Knowledge and Perspectives of Foliar Fertilization. Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar] [CrossRef]
- Fernández, V.; Sotiropoulos, T.; Brown, P.H. Foliar Fertilization: Scientific Principles and Field Pratices; International Fertilizer Industry Association (IFA): Paris, France, 2013; ISBN 979-10-92366-00-6. [Google Scholar]
- Arif, M.; Chohan, M.A.; Ali, S.; Gul, R.; Khan, S. Response of wheat to foliar application of nutrients. Am. J. Agric. Biol. Sci. 2006, 1, 30–34. [Google Scholar]
- Mosali, J.; Desta, K.; Teal, R.K.; Freeman, K.W.; Martin, K.L.; Lawles, J.W.; Raun, W.R. Effect of Foliar Application of Phosphorus on Winter Wheat Grain Yield, Phosphorus Uptake, and Use Efficiency. J. Plant Nutr. 2006, 29, 2147–2163. [Google Scholar] [CrossRef]
- Fernández, V.; Guzmán, P.; Peirce, C.A.E.; McBeath, T.M.; Khayet, M.; McLaughlin, M.J. Effect of wheat phosphorus status on leaf surface properties and permeability to foliar-applied phosphorus. Plant Soil 2014, 384, 7–20. [Google Scholar] [CrossRef]
- Peirce, C.A.E.; McBeath, T.M.; Fernández, V.; McLaughlin, M.J. Wheat leaf properties affecting the absorption and subsequent translocation of foliar-applied phosphoric acid fertiliser. Plant Soil 2014, 384, 37–51. [Google Scholar] [CrossRef]
- Lancashire, P.D.; Bleiholder, H.; Van Den Boom, T.; Langelüddeke, P.; Stauss, R.; Weber, E.; Witzenberger, A. A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- Jezek, M.; Geilfus, C.-M.; Bayer, A.; Mühling, K.H. Photosynthetic capacity, nutrient status, and growth of maize (Zea mays L.) upon MgSO4 leaf-application. Front. Plant Sci. 2015, 5, 781. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.; Schuldt, A.; Mannerkorpi, P.; Vearasilp, T. Zur enzymatischen Stärkebestimmung im Darminhalt und Kot von Kühen mit hitzestabiler Amylase. Arch. Anim. Nutr. 1987, 37, 455. [Google Scholar]
- Peirce, C.A.E.; McBeath, T.M.; Priest, C.; McLaughlin, M.J. The Timing of Application and Inclusion of a Surfactant Are Important for Absorption and Translocation of Foliar Phosphoric Acid by Wheat Leaves. Front. Plant Sci. 2019, 10, 1532. [Google Scholar] [CrossRef] [PubMed]
- McBeath, T.M.; Facelli, E.; Peirce, C.A.E.; Arachchige, V.K.; McLaughlin, M.J. Assessment of foliar-applied phosphorus fertiliser formulations to enhance phosphorus nutrition and grain production in wheat. Crop Pasture Sci. 2020, 71, 795. [Google Scholar] [CrossRef]
- Fageria, N.K.; Filho, M.B.; Moreira, A.; Guimarães, C.M. Foliar Fertilization of Crop Plants. J. Plant Nutr. 2009, 32, 1044–1064. [Google Scholar] [CrossRef]
- Barel, D.; Black, C.A. Effect of neutralization and addition of urea, sucrose, and various glycols on phosphorus absorption and leaf damage from foliar-applied phosphate. Plant Soil 1979, 52, 515–525. [Google Scholar] [CrossRef]
- Reed, D.W.; Tukey, H.B. Effect of pH on foliar absorption of phosphorus compounds by chrysanthemum. J. Am. Soc. Hortic. Sci. 1978, 103, 337–340. [Google Scholar]
- Koontz, H.; Biddulph, O. Factors Affecting Absorption and Translocation of Foliar Applied Phosphorus. Plant Physiol. 1957, 32, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, W.D.; Kirkby, E.A.; Peuke, A.D.; Pate, J.S.; Hartung, W. Effects of P deficiency on assimilation and transport of nitrate and phosphate in intact plants of castor bean (Ricinus communis L.). J. Exp. Bot. 1997, 48, 75–91. [Google Scholar] [CrossRef]
- Görlach, B.M.; Mühling, K.H. Phosphate foliar application increases biomass and P concentration in P deficient maize. J. Plant Nutr. Soil Sci. 2021, 183, 1472. [Google Scholar] [CrossRef]
- Batten, G.; Wardlaw, I.; Aston, M. Growth and the distribution of phosphorus in wheat developed under various phosphorus and temperature regimes. Aust. J. Agric. Res. 1986, 37, 459–469. [Google Scholar] [CrossRef]
- Marshall, C.; Wardlaw, I. A Comparative Study of the Distribution and Speed of Movement of 14C Assimilates and Foliar-Applied 32P-Labelled Phosphate in Wheat. Aust. J. Biol. Sci. 1973, 26, 1–14. [Google Scholar] [CrossRef]
- McBeath, T.M.; McLaughlin, M.J.; Noack, S.R. And Wheat grain yield response to and translocation of foliar-applied phosphorus. Crop. Pasture Sci. 2011, 62, 58–65. [Google Scholar] [CrossRef]
- Leach, K.A.; Hameleers, A. The effects of a foliar spray containing phosphorus and zinc on the development, composition and yield of forage maize. Grass Forage Sci. 2001, 56, 311–315. [Google Scholar] [CrossRef]
- Froese, S.; Wiens, J.; Warkentin, T.; Schoenau, J. Response of canola, wheat, and pea to foliar phosphorus fertilization at a phosphorus-deficient site in eastern Saskatchewan. Can. J. Plant Sci. 2020, 100, 642–652. [Google Scholar] [CrossRef]
- Benbella, M.; Paulsen, G.M. Efficacy of Treatments for Delaying Senescence of Wheat Leaves: II. Senescence and Grain Yield under Field Conditions. Agron. J. 1998, 90, 332–338. [Google Scholar] [CrossRef]
- Sherchand, K.; Paulsen, G.M. Response of wheat to foliar phosphorus treatments under field and high temperature regimes. J. Plant Nutr. 1985, 8, 1171–1181. [Google Scholar] [CrossRef]


| Soil Experiment 1 | Soil Experiment 2 | |
|---|---|---|
| pH (CaCl2) | 5.9 | 6.1 |
| Phosphorus (mg 100 g−1 soil) | 7.5 | 5.0 |
| Phosphorus release rate (µg kg−1*10 min) | 825 | 519 |
| Potassium (mg 100 g−1 soil) | 8.6 | 7.9 |
| Magnesium (mg 100 g−1 soil) | 8.7 | 5.1 |
| Copper (mg 100 g−1 soil) | 1.3 | 0.9 |
| Manganese (mg 100 g−1 soil) | 23.6 | 145.5 |
| Zinc (mg 100 g−1 soil) | 1.4 | 1.3 |
| Boron (mg 100 g−1 soil) | 0.33 | 0.38 |
| Sulfur (mg 100 g−1 soil) | 5.7 | 5.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Görlach, B.M.; Henningsen, J.N.; Mackens, J.T.; Mühling, K.H. Evaluation of Maize Growth Following Early Season Foliar P Supply of Various Fertilizer Formulations and in Relation to Nutritional Status. Agronomy 2021, 11, 727. https://doi.org/10.3390/agronomy11040727
Görlach BM, Henningsen JN, Mackens JT, Mühling KH. Evaluation of Maize Growth Following Early Season Foliar P Supply of Various Fertilizer Formulations and in Relation to Nutritional Status. Agronomy. 2021; 11(4):727. https://doi.org/10.3390/agronomy11040727
Chicago/Turabian StyleGörlach, Bruno Maximilian, Jon Niklas Henningsen, Jens Torsten Mackens, and Karl Hermann Mühling. 2021. "Evaluation of Maize Growth Following Early Season Foliar P Supply of Various Fertilizer Formulations and in Relation to Nutritional Status" Agronomy 11, no. 4: 727. https://doi.org/10.3390/agronomy11040727
APA StyleGörlach, B. M., Henningsen, J. N., Mackens, J. T., & Mühling, K. H. (2021). Evaluation of Maize Growth Following Early Season Foliar P Supply of Various Fertilizer Formulations and in Relation to Nutritional Status. Agronomy, 11(4), 727. https://doi.org/10.3390/agronomy11040727







