Increasing Effective Use of Straw-Derived Nitrogen by Alternate Wetting/Drying Irrigation Combined with N Fertilization Addition in a Soil–Rice System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Site
2.2. Preparation of 15N-Labeled Wheat Straw
2.3. Experimental Design
2.4. Sampling and Analytical Measurements
2.5. Calculations and Statistical Analysis
3. Results
3.1. Straw-N Release
3.2. Straw-N Accumulation and Its Contribution to N Uptake in Rice Plants
3.3. Straw-N Residue in Soil
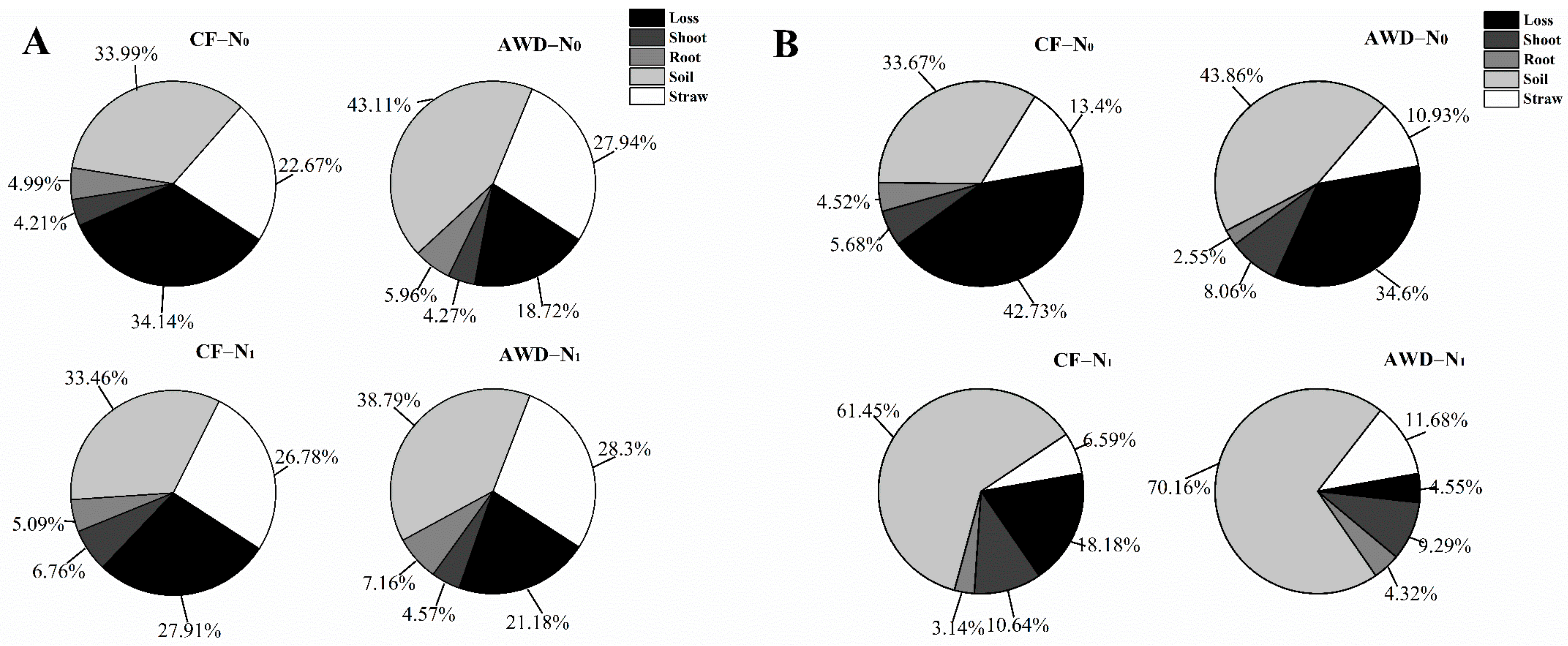
3.4. Distribution of Straw-N in the Soil–Plant System
4. Discussion
4.1. Utilization of Straw-N in the Soil–Rice System
4.2. Effect of Water Management on the Allocation of Straw-N in the Soil–Rice System
4.3. Effect of N Fertilization on the Allocation of Straw-N in the Soil–Rice System
4.4. Effect of Water Management Combined with N Fertilization on the Allocation of Straw-N in the Soil–Rice System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; FAO: Rome, Italy, 2012; p. 154. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations Data). Available online: http://www.fao.org/faostat/zh/#data/ (accessed on 23 October 2020).
- Dawe, D.; Frolking, S.; Li, C. Trends in rice–wheat area in China. Field Crop. Res. 2004, 87, 89–95. [Google Scholar] [CrossRef]
- Li, H.; Dai, M.; Dai, S.; Dong, X. Current status and environment impact of direct straw return in China’s cropland—A review. Ecotoxicol. Environ. Saf. 2018, 159, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Lam, S.K.; Wolf, B.; Kiese, R.; Chen, D.; Butterbach-Bahl, K. Trade-offs between soil carbon sequestration and reactive nitrogen losses under straw return in global agroecosystems. Glob. Chang. Biol. 2018, 24, 5919–5932. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zeng, Y.; Wu, J.; Shi, Q.; Pan, X. Effect of crop residue retention on rice yield in China: A meta-analysis. Field Crop. Res. 2013, 154, 188–194. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, C.-Y.; Tang, X.; Yang, S.-M.; Wang, J.-Y. Fate of Nitrogen from Organic and Inorganic Sources in Rice-Wheat Rotation Cropping System. Agric. Sci. China 2010, 9, 1017–1025. [Google Scholar] [CrossRef]
- Zheng, L.; Pei, J.; Jin, X.; Schaeffer, S.; An, T.; Wang, J. Impact of plastic film mulching and fertilizers on the distribution of straw-derived nitrogen in a soil-plant system based on 15 N–labeling. Geoderma 2018, 317, 15–22. [Google Scholar] [CrossRef]
- Xu, Y.; Ding, X.; Lal, R.; Gao, X.; Li, S.; Sun, L.; Wang, Y.; Li, M.; Bai, S.; Wang, J. Effect of soil fertility on the allocation of nitrogen derived from different maize residue parts in the soil-plant system. Geoderma 2020, 379, 114632. [Google Scholar] [CrossRef]
- Ueno, H.; Yamamuro, S. Fate of nitrogen derived from 15 N-labeled plant residues and composts in rice-planted paddy soil. Soil Sci. Plant. Nutr. 2001, 47, 747–754. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, W.; Li, T.; Cheng, X.; Liu, Q. Balancing straw returning and chemical fertilizers in China: Role of straw nutrient resources. Renew. Sustain. Energy Rev. 2018, 81, 2695–2702. [Google Scholar] [CrossRef]
- Fan, M.; Jiang, R.; Liu, X.; Zhang, F.; Lu, S.; Zeng, X.; Christie, P. Interactions between non-flooded mulching cultivation and varying nitrogen inputs in rice–wheat rotations. Field Crop. Res. 2005, 91, 307–318. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, D.; Zhang, G.; Wang, C. Effect of wheat straw application on ammonia volatilization from urea applied to a paddy field. Nutr. Cycl. Agroecosystems 2012, 94, 73–84. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, H.; Zhang, J.; Chen, G.; Zhu, H.; Zhou, S.; Xiao, H. Effects of wheat straw addition on dynamics and fate of nitrogen applied to paddy soils. Soil Tillage Res. 2018, 178, 92–98. [Google Scholar] [CrossRef]
- Lu, M.; Yang, Y.; Luo, Y.; Fang, C.; Zhou, X.; Chen, J.; Yang, X.; Li, B. Responses of ecosystem nitrogen cycle to nitrogen addition: A meta-analysis. New Phytol. 2010, 189, 1040–1050. [Google Scholar] [CrossRef]
- Fang, H.; Zhou, H.; Norton, G.J.; Price, A.H.; Raffan, A.C.; Mooney, S.J.; Peng, X.; Hallett, P.D. Interaction between contrasting rice genotypes and soil physical conditions induced by hydraulic stresses typical of alternate wetting and drying irrigation of soil. Plant. Soil 2018, 430, 233–243. [Google Scholar] [CrossRef] [Green Version]
- Bouman, B.; Tuong, T. Field water management to save water and increase its productivity in irrigated lowland rice. Agric. Water Manag. 2001, 49, 11–30. [Google Scholar] [CrossRef]
- Carrijo, D.R.; Lundy, M.E.; Linquist, B.A. Rice yields and water use under alternate wetting and drying irrigation: A meta-analysis. Field Crop. Res. 2017, 203, 173–180. [Google Scholar] [CrossRef]
- Cucu, M.A.; Said–Pullicino, D.; Maurino, V.; Bonifacio, E.; Romani, M.; Celi, L. Influence of redox conditions and rice straw incorporation on nitrogen availability in fertilized paddy soils. Biol. Fert. Soils 2013, 50, 755–764. [Google Scholar] [CrossRef]
- Shan, Y.; Cai, Z.; Han, Y.; Johnson, S.E.; Buresh, R.J. Organic acid accumulation under flooded soil conditions in relation to the incorporation of wheat and rice straws with different C:N ratios. Soil Sci. Plant. Nutr. 2008, 54, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Chu, G.; Wang, Z.; Zhang, H.; Liu, L.; Yang, J.; Zhang, J. Alternate wetting and moderate drying increases rice yield and reduces methane emission in paddy field with wheat straw residue incorporation. Food Energy Secur. 2015, 4, 238–254. [Google Scholar] [CrossRef]
- Yang, C.; Yang, L.; Yang, Y.; Ouyang, Z. Rice root growth and nutrient uptake as influenced by organic manure in continuously and alternately flooded paddy soils. Agric. Water Manag. 2004, 70, 67–81. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, Y.; Wang, Z.; Yang, J.; Zhang, J. An Alternate Wetting and Moderate Soil Drying Regime Improves Root and Shoot Growth in Rice. Crop. Sci. 2009, 49, 2246–2260. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Wan, Y.; Wang, B.; Waqas, M.A.; Cai, W.; Guo, C.; Zhou, S.; Su, R.; Qin, X.; et al. Combination of modified nitrogen fertilizers and water saving irrigation can reduce greenhouse gas emissions and increase rice yield. Geoderma 2018, 315, 1–10. [Google Scholar] [CrossRef]
- Liang, X.Q.; Chen, Y.X.; Nie, Z.Y.; Ye, Y.S.; Liu, J.; Tian, G.M.; Wang, G.H.; Tuong, T.P. Mitigation of nutrient losses via surface runoff from rice cropping systems with alternate wetting and drying irrigation and site-specific nutrient management practices. Environ. Sci. Pollut. Res. 2013, 20, 6980–6991. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, Z.; Wu, Y.; Mwiya, R. Response of Vertical Migration and Leaching of Nitrogen in Percolation Water of Paddy Fields under Water-Saving Irrigation and Straw Return Conditions. Water 2019, 11, 868. [Google Scholar] [CrossRef] [Green Version]
- Douglas, C.L.; Allmaras, R.R.; Rasmussen, P.E.; Ramig, R.E.; Roager, N.C. Wheat Straw Composition and Placement Effects on Decomposition in Dryland Agriculture of the Pacific Northwest. Soil Sci. Soc. Am. J. 1980, 44, 833–837. [Google Scholar] [CrossRef]
- Voroney, R.P.; Paul, E.A.; Anderson, D.W. Decomposition of Wheat Straw and Stabilization Of Microbial Products. Can. J. Soil Sci. 1989, 69, 63–77. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Yao, S.-H.; Hu, F. Microbial biomass dynamics and soil wettability as affected by the intensity and frequency of wetting and drying during straw decomposition. Eur. J. Soil Sci. 2007, 58, 1482–1492. [Google Scholar] [CrossRef]
- Wu, J.; Guo, X.S.; Wang, Y.Q.; Xu, Z.Y.; Lu, J.W. Decomposition characteristics of rapeseed and wheat straw under different water regimes and straw incorporating models. J. Food Agric. Environ. 2011, 9, 572–577. [Google Scholar]
- Benbi, D.K.; Khosa, M.K. Effects of Temperature, Moisture, and Chemical Composition of Organic Substrates on C Mineralization in Soils. Commun. Soil Sci. Plant. Anal. 2014, 45, 2734–2753. [Google Scholar] [CrossRef]
- Henriksen, T.M.; Breland, T.A. Nitrogen availability effects on carbon mineralization, fungal and bacterial growth, and enzyme activities during decomposition of wheat straw in soil. Soil. Biol. Biochem. 1999, 31, 1121–1134. [Google Scholar] [CrossRef]
- Hadas, A.; Kautsky, L.; Goek, M.; Kara, E.E. Rates of decomposition of plant residues and available nitrogen in soil, related to residue composition through simulation of carbon and nitrogen turnover. Soil Biol. Biochem. 2004, 36, 255–266. [Google Scholar] [CrossRef]
- Esther, O.J.; Guo, C.-H.; Tian, X.-H.; Li, H.-Y.; Zhou, Y.-X. The Effects of Three Mineral Nitrogen Sources and Zinc on Maize and Wheat Straw Decomposition and Soil Organic Carbon. J. Integr. Agric. 2014, 13, 2768–2777. [Google Scholar] [CrossRef]
- Guan, X.K.; Wei, L.; Turner, N.C.; Ma, S.C.; Yang, M.D.; Wang, T.C. Improved straw management practices promote in situ straw decomposition and nutrient release, and increase crop production. J. Clean Prod. 2020, 250, 119514. [Google Scholar] [CrossRef]
- Ding, W.C.; Li, S.T.; Huang, S.M. Bioavailability and Fate of Nitrogen from 15N–labeled Corn Straw as Affected by Nitrogen Management and Straw Microbial Inoculants. Sci. Agric. Sin. 2016, 49, 2725–2736. [Google Scholar]
- Xing, G.; Zhu, Z. An assessment of N loss from agricultural fields to the environment in China. Nutr. Cycl. Agroecosystems 2000, 57, 67–73. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, G.; Yan, D.; Zha, S. Nitrogen minerallzation of applied 15N labeled straw in paddy soils in the Taihu lake reglon. Acta Pedol. Sin. 2012, 49, 77–85. [Google Scholar]
- Xu, G.-W.; Lu, D.-K.; Wang, H.-Z.; Li, Y. Morphological and physiological traits of rice roots and their relationships to yield and nitrogen utilization as influenced by irrigation regime and nitrogen rate. Agric. Water Manag. 2018, 203, 385–394. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, W.; Beebout, S.S.; Zhang, H.; Liu, L.; Yang, J.; Zhang, J. Grain yield, water and nitrogen use efficiencies of rice as influenced by irrigation regimes and their interaction with nitrogen rates. Field Crop. Res. 2016, 193, 54–69. [Google Scholar] [CrossRef]
- Liang, Y.; Li, F.; Nong, M.; Luo, H.; Zhang, J. Microbial Activity in Paddy Soil and Water Use Efficiency of Rice as Affected by Irrigation Method and Nitrogen Level. Commun. Soil Sci. Plant. Anal. 2015, 47, 19–31. [Google Scholar] [CrossRef]
- Pan, F.; Li, Y.; Chapman, S.J.; Yao, H. Effect of rice straw application on microbial community and activity in paddy soil under different water status. Environ. Sci. Pollut. Res. 2015, 23, 5941–5948. [Google Scholar] [CrossRef]
- Dobbie, K.E.; Smith, K.A. Nitrous oxide emission factors for agricultural soils in Great Britain: The impact of soil water-filled pore space and other controlling variables. Glob. Chang. Biol. 2003, 9, 204–218. [Google Scholar] [CrossRef]
- Wang, J.; Jia, J.; Xiong, Z.; Khalil, M.; Xing, G. Water regime–nitrogen fertilizer–straw incorporation interaction: Field study on nitrous oxide emissions from a rice agroecosystem in Nanjing, China. Agric. Ecosyst. Environ. 2011, 141, 437–446. [Google Scholar] [CrossRef]
- Inselsbacher, E.; Umana, N.H.-N.; Stange, F.C.; Gorfer, M.; Schüller, E.; Ripka, K.; Zechmeister-Boltenstern, S.; Hood-Novotny, R.; Strauss, J.; Wanek, W. Short-term competition between crop plants and soil microbes for inorganic N fertilizer. Soil Biol. Biochem. 2010, 42, 360–372. [Google Scholar] [CrossRef]
- Zhang, F.; Cui, Z.; Fan, M.; Zhang, W.; Chen, X.; Jiang, R. Integrated Soil-Crop System Management: Reducing Environmental Risk while Increasing Crop Productivity and Improving Nutrient Use Efficiency in China. J. Environ. Qual. 2011, 40, 1051–1057. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, N.; Carranca, C.; Goufo, P.; Pereira, J.; Trindade, H.; Coutinho, J. Impact of agricultural practices, elevated temperature and atmospheric carbon dioxide concentration on nitrogen and pH dynamics in soil and floodwater during the seasonal rice growth in Portugal. Soil Tillage Res. 2015, 145, 198–207. [Google Scholar] [CrossRef]
- Näsholm, T.; Kielland, K.; Ganeteg, U. Uptake of organic nitrogen by plants. New Phytol. 2009, 182, 31–48. [Google Scholar] [CrossRef]
- Henry, H.A.L.; Jefferies, R.L. Interactions in the uptake of amino acids, ammonium and nitrate ions in the Arctic salt–marsh grass, Puccinellia phryganodes. Plant. Cell Environ. 2003, 26, 419–428. [Google Scholar] [CrossRef]
- Persson, J.; Gardeström, P.; Näsholm, T. Uptake, metabolism and distribution of organic and inorganic nitrogen sources by Pinus sylvestris. J. Exp. Bot. 2006, 57, 2651–2659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Chen, T.; Wang, Z.; Zhang, H.; Yang, J.; Zhang, J. Combination of site-specific nitrogen management and alternate wetting and drying irrigation increases grain yield and nitrogen and water use efficiency in super rice. Field Crop. Res. 2013, 154, 226–235. [Google Scholar] [CrossRef]
- Zhang, X.-B.; Ge, J.-L.; Dai, Q.-G.; Lu, Y.; Zhou, G.-S.; Wei, H.-H.; Meng, T.-Y.; Tao, Y.; Li, X.-Y.; Ding, E.-H. A dynamic model and its characteristics for nitrogen accumulation after transplanting in medium-maturity types of Yongyou japonica/indica hybrids. Acta Agron. Sin. 2020, 46, 798–807. [Google Scholar] [CrossRef]
- Qin, B.; Xu, P.; Wu, Q.; Luo, L.; Zhang, Y. Environmental issues of Lake Taihu, China. Hydrobiology 2007, 581, 3–14. [Google Scholar] [CrossRef]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nat. Cell Biol. 2015, 528, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Treatment | Initial | Panicle Initiation Stage (PI) | Mature Stage (MS) |
|---|---|---|---|
| CF–N0 | 304.20 | 44.10c | 40.77a |
| AWD–N0 | 304.20 | 66.47a | 33.25b |
| CF–N1 | 304.20 | 58.17b | 20.04c |
| AWD–N1 | 304.20 | 57.57b | 35.52b |
| Average a | |||
| CF | 304.20 | 51.14 | 30.40 |
| AWD | 304.20 | 62.02 | 34.38 |
| N0 | 304.20 | 55.29 | 37.01 |
| N1 | 304.20 | 57.87 | 27.78 |
| ANOVA analysis | F (p) | F (p) | |
| W | 59.51 (<0.001) | 13.22 (<0.01) | |
| N | 3.34 (0.11) | 71.22 (<0.001) | |
| W×N | 66.31 (<0.001) | 110.47 (<0.001) |
| Treatment | Panicle Initiation Stage (PI) | Mature Stage (MS) | |||||
|---|---|---|---|---|---|---|---|
| Root | Stem–Leaf | Whole Plant | Root | Stem–Leaf | Panicle | Whole Plant | |
| CF–N0 | 15.16c | 12.81b | 27.97c | 13.74a | 5.48c | 11.81c | 31.04b |
| AWD–N0 | 18.11b | 12.99b | 31.10b | 7.77c | 7.47b | 17.06b | 32.29b |
| CF–N1 | 15.48c | 20.55a | 36.03a | 9.55b | 10.14a | 22.22a | 41.91a |
| AWD–N1 | 21.75a | 13.90b | 35.65a | 13.14a | 9.73a | 18.52b | 41.39a |
| Average a | |||||||
| CF | 15.32 | 16.68 | 32.00 | 11.65 | 7.81 | 17.02 | 36.47 |
| AWD | 19.93 | 13.44 | 33.37 | 10.45 | 8.60 | 17.79 | 36.84 |
| N0 | 16.63 | 12.90 | 29.53 | 10.76 | 6.47 | 14.44 | 31.66 |
| N1 | 18.61 | 17.23 | 35.84 | 11.35 | 9.93 | 20.37 | 41.65 |
| ANOVA analysis | F (p) | F (p) | F (p) | F (p) | F (p) | F (p) | F (p) |
| W | 65.55 (<0.001) | 21.60 (0.002) | 2.85 (0.130) | 26.57 (0.001) | 5.52 (0.047) | 1.43 (0.266) | 0.24 (0.639) |
| N | 12.08 (0.008) | 38.67 (<0.001) | 59.79 (<0.001) | 6.52 (0.034) | 105.79 (<0.001) | 84.71 (<0.001) | 172.21 (<0.001) |
| W × N | 8.55 (0.019) | 24.12 (0.001) | 4.62 (0.064) | 428.33 (<0.001) | 12.64 (0.007) | 48.09 (<0.001) | 1.35 (0.278) |
| Treatment | Panicle Initiation Stage (PI) | Mature Stage (MS) | |||||
|---|---|---|---|---|---|---|---|
| Root | Stem–Leaf | Whole Plant | Root | Stem–Leaf | Panicle | Whole Plant | |
| CF–N0 | 18.41a | 4.07b | 7.06b | 13.76a | 2.72b | 3.10b | 4.54a |
| AWD–N0 | 16.31b | 4.44a | 7.70a | 10.44b | 3.59a | 4.06a | 4.59a |
| CF–N1 | 14.59c | 3.85c | 5.64c | 9.02c | 2.41b | 2.40c | 2.88b |
| AWD–N1 | 15.07c | 2.16d | 4.52d | 10.47b | 1.87c | 1.75d | 2.43c |
| Average a | |||||||
| CF | 16.50 | 3.96 | 6.35 | 11.39 | 2.56 | 2.75 | 3.71 |
| AWD | 15.69 | 3.30 | 6.11 | 10.46 | 2.73 | 2.90 | 3.51 |
| N0 | 17.36 | 4.25 | 7.38 | 12.10 | 3.15 | 3.58 | 4.57 |
| N1 | 14.83 | 3.00 | 5.08 | 9.74 | 2.14 | 2.07 | 2.65 |
| ANOVA analysis | F (p) | F (p) | F (p) | F (p) | F (p) | F (p) | F (p) |
| W | 5.27 (0.051) | 98.19 (<0.001) | 2.09 (0.186) | 5.04 (0.055) | 2.96 (0.124) | 7.33 (0.027) | 8.97 (0.017) |
| N | 5.61 (<0.001) | 349.81 (<0.001) | 196.14 (<0.001) | 32.21 (<0.001) | 111.64 (<0.001) | 689.64 (<0.001) | 801.96 (<0.001) |
| W × N | 13.35 (0.006) | 237.78 (<0.001) | 28.82 (<0.001) | 33.04 (<0.001) | 54.02 (<0.001) | 196.51 (<0.001) | 14.30 (0.005) |
| Treatment | Panicle Initiation Stage (PI) | Mature Stage (MS) | ||
|---|---|---|---|---|
| Straw-N (mg pot−1) | Ndfs (%) | Straw-N (mg pot−1) | Ndfs (%) | |
| CF–N0 | 103.33c | 0.62b | 102.42d | 0.63d |
| AWD–N0 | 131.05a | 0.75a | 133.41c | 0.84c |
| CF–N1 | 101.73c | 0.73a | 186.94b | 1.17b |
| AWD–N1 | 117.91b | 0.69ab | 213.44a | 1.36a |
| Average a | ||||
| CF | 102.53 | 0.67 | 144.68 | 0.90 |
| AWD | 124.48 | 0.72 | 173.43 | 1.10 |
| N0 | 117.19 | 0.69 | 117.92 | 0.73 |
| N1 | 109.82 | 0.71 | 200.19 | 1.26 |
| ANOVA analysis | F (p) | F (p) | F (p) | F (p) |
| W | 48.35 (<0.001) | 4.42 (0.069) | 59.47 (<0.001) | 68.41 (<0.001) |
| N | 5.46 (0.048) | 0.90 (0.371) | 487.18 (<0.001) | 470.03 (<0.001) |
| W × N | 3.34 (0.105) | 13.61 (0.006) | 0.37 (0.563) | 0.22 (0.649) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhou, Y.; Li, W.; Nadeem, M.Y.; Ding, Y.; Jiang, Y.; Chen, L.; Li, G. Increasing Effective Use of Straw-Derived Nitrogen by Alternate Wetting/Drying Irrigation Combined with N Fertilization Addition in a Soil–Rice System. Agronomy 2021, 11, 750. https://doi.org/10.3390/agronomy11040750
Zhang J, Zhou Y, Li W, Nadeem MY, Ding Y, Jiang Y, Chen L, Li G. Increasing Effective Use of Straw-Derived Nitrogen by Alternate Wetting/Drying Irrigation Combined with N Fertilization Addition in a Soil–Rice System. Agronomy. 2021; 11(4):750. https://doi.org/10.3390/agronomy11040750
Chicago/Turabian StyleZhang, Jianwei, Yan Zhou, Weiwei Li, Muhammad Y. Nadeem, Yanfeng Ding, Yu Jiang, Lin Chen, and Ganghua Li. 2021. "Increasing Effective Use of Straw-Derived Nitrogen by Alternate Wetting/Drying Irrigation Combined with N Fertilization Addition in a Soil–Rice System" Agronomy 11, no. 4: 750. https://doi.org/10.3390/agronomy11040750








