Physiology of Nitrogen and Calcium Nutrition in Blueberry (Vaccinium sp.)
Abstract
:1. Introduction
2. Nitrogen Physiology in Blueberry
2.1. Nitrogen Acquisition in Blueberry: Organic N
2.2. Nitrogen Acquisition in Blueberry: Inorganic N
Inorganic N Acquisition Mechanisms
2.3. Nitrogen Translocation in Blueberry
2.4. Nitrogen Assimilation in Blueberry
2.5. Nitrogen Storage and Remobilization in Blueberry
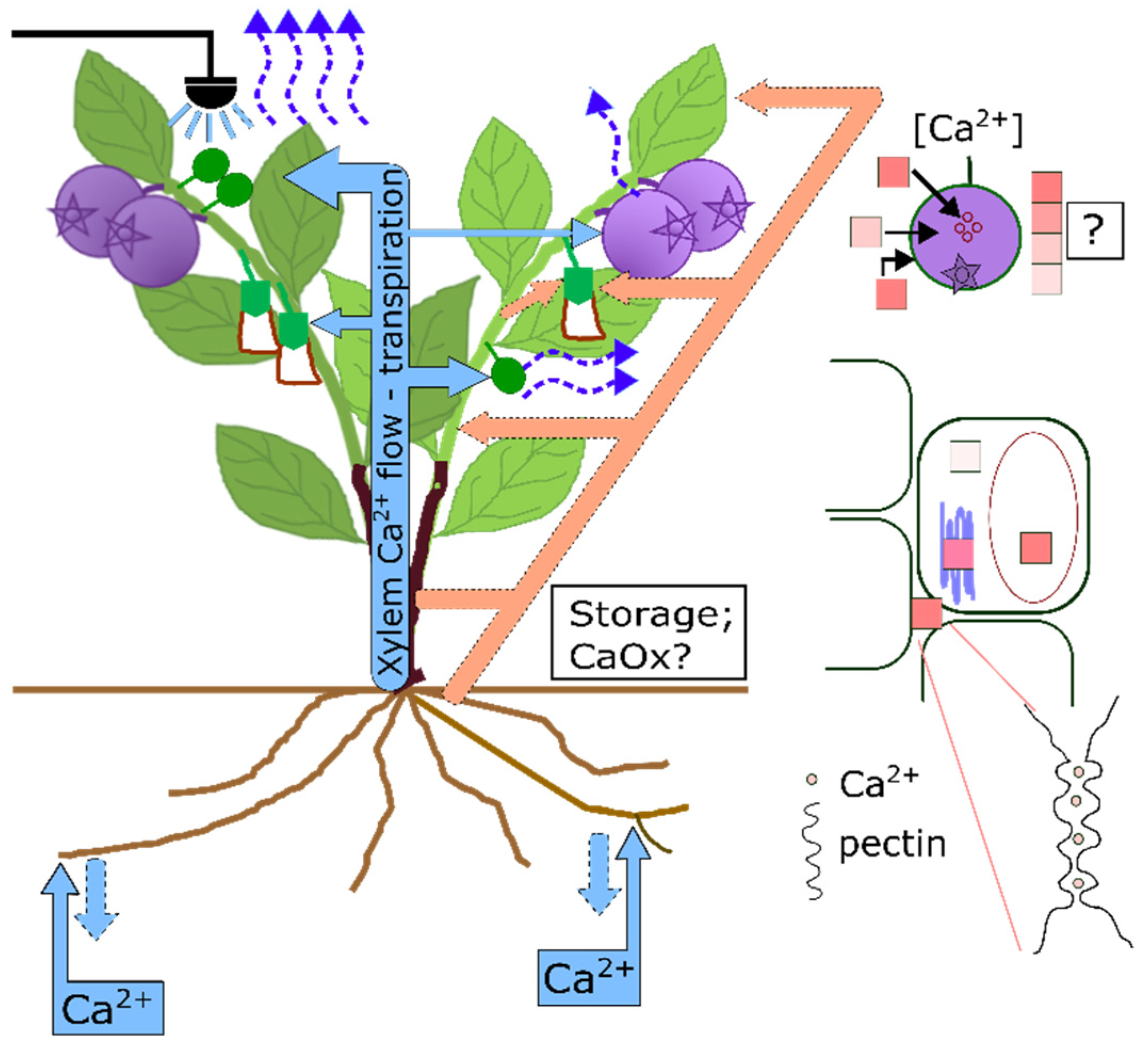
3. Calcium Physiology in Blueberry
3.1. Calcium Acquisition in Blueberry
3.2. Calcium Translocation in Blueberry
3.2.1. Calcium Transport to the Fruit and Its Distribution
3.2.2. Approaches to Improve Fruit [Ca2+]
3.3. Calcium Storage and Remobilization in Blueberry
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jeyabalan, J.; Aqil, F.; Munagala, R.; Annamalai, L.; Vadhanam, M.V.; Gupta, R.C. Chemopreventive and therapeutic activity of dietary blueberry against estrogen-mediated breast cancer. J. Agric. Food Chem. 2014, 62, 3963–3971. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Costa, E.M.; Veiga, M.; Morais, R.M.; Calhau, C.; Pintado, M. Health promoting properties of blueberries: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Davidson, K.T.; Zhu, Z.; Balabanov, D.; Zhao, L.; Wakefield, M.R.; Bai, Q.; Fang, Y. Beyond conventional medicine-a look at blueberry, a cancer-fighting superfruit. Pathol. Oncol. Res. 2018, 24, 733–738. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. 2019. Available online: http://www.fao.org/faostat/ (accessed on 15 December 2020).
- Gupta, V.; Estrada, A.D.; Blakley, I.; Reid, R.; Patel, K.; Meyer, M.D.; Andersen, S.U.; Brown, A.F.; Lila, M.A.; Loraine, A.E. RNA-Seq analysis and annotation of a draft blueberry genome assembly identifies candidate genes involved in fruit ripening, biosynthesis of bioactive compounds, and stage-specific alternative splicing. GigaScience 2015, 4, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colle, M.; Leisner, C.P.; Wai, C.M.; Ou, S.; Bird, K.A.; Wang, J.; Wisecaver, J.H.; Yocca, A.E.; Alger, E.I.; Tang, H. Haplotype-phased genome and evolution of phytonutrient pathways of tetraploid blueberry. GigaScience 2019, 8, giz012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korcak, R.F. Nutrition of blueberry and other calcifuges. Hortic. Rev. 1988, 10, 183–227. [Google Scholar]
- Retamales, J.; Hancock, J.F. Blueberries; CAB International: Boston, MA, USA, 2012. [Google Scholar]
- Townsend, L. Influence of form of nitrogen and pH on growth and nutrient levels in the leaves and roots of the lowbush blueberry. Can. J. Sci. 1969, 49, 333–338. [Google Scholar] [CrossRef]
- Rosen, C.J.; Allan, D.L.; Luby, J.J. Nitrogen form and solution pH influence growth and nutrition of two Vaccinium clones. J. Am. Soc. Hortic. Sci. 1990, 115, 83–89. [Google Scholar] [CrossRef]
- Sugiyama, N.; Hanawa, S. Growth responses of rabbiteye blueberry plants to N forms at constant pH in solution culture. J. Jpn. Soc. Hortic. Sci. 1992, 61, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Hanson, E.J.; Retamales, J.B. Effect of nitrogen source and timing on highbush blueberry performance. HortScience 1992, 27, 1265–1267. [Google Scholar] [CrossRef] [Green Version]
- Banados, M.P.; Strik, B.C.; Bryla, D.R.; Righetti, T.L.J.H. Response of highbush blueberry to nitrogen fertilizer during field establishment, I: Accumulation and allocation of fertilizer nitrogen and biomass. HortScience 2012, 47, 648–655. [Google Scholar] [CrossRef]
- Bryla, D.R.; Strik, B.C.; Banados, M.P.; Righetti, T.L. Response of highbush blueberry to nitrogen fertilizer during field establishment—II. Plant nutrient requirements in relation to nitrogen fertilizer supply. HortScience 2012, 47, 917–926. [Google Scholar] [CrossRef]
- Fang, Y.; Williamson, J.; Darnell, R.; Li, Y.; Liu, G. Optimizing Nitrogen Fertigation Rates for Young Southern Highbush Blueberry. Agronomy 2020, 10, 389. [Google Scholar] [CrossRef] [Green Version]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [Green Version]
- Birkhold, K.T.; Darnell, R.L. Contribution of storage and currently assimilated nitrogen to vegetative and reproductive growth of rabbiteye blueberry. J. Am. Soc. Hortic. Sci. 1993, 118, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Stribley, D.; Read, D. The biology of mycorrhiza in the Ericaceae: VII. The relationship between mycorrhizal infection and the capacity to utilize simple and complex organic nitrogen sources. New Phytol. 1980, 86, 365–371. [Google Scholar] [CrossRef]
- Näsholm, T.; Kielland, K.; Ganeteg, U. Uptake of organic nitrogen by plants. New Phytol. 2009, 182, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Näsholm, T.; Huss-Danell, K.; Högberg, P. Uptake of organic nitrogen in the field by four agriculturally important plant species. Ecology 2000, 81, 1155–1161. [Google Scholar] [CrossRef]
- Tegeder, M.; Rentsch, D. Uptake and partitioning of amino acids and peptides. Mol. Plant 2010, 3, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Nie, J.; Bai, R.; Sui, X. Amino acid transporters in plants: Identification and function. Plants 2020, 9, 972. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Foster, J.; Chen, J.; Voll, L.M.; Weber, A.P.; Tegeder, M. AAP1 transports uncharged amino acids into roots of Arabidopsis. Plant J. 2007, 50, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Svennerstam, H.; Ganeteg, U.; Näsholm, T. Root uptake of cationic amino acids by Arabidopsis depends on functional expression of amino acid permease 5. New Phytol. 2008, 180, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bush, D.R. LHT1, a lysine-and histidine-specific amino acid transporter in arabidopsis. Plant Physiol. 1997, 115, 1127–1134. [Google Scholar] [CrossRef] [Green Version]
- Hirner, A.; Ladwig, F.; Stransky, H.; Okumoto, S.; Keinath, M.; Harms, A.; Frommer, W.B.; Koch, W. Arabidopsis LHT1 is a high-affinity transporter for cellular amino acid uptake in both root epidermis and leaf mesophyll. Plant Cell 2006, 18, 1931–1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perchlik, M.; Foster, J.; Tegeder, M. Different and overlapping functions of Arabidopsis LHT6 and AAP1 transporters in root amino acid uptake. J. Exp. Bot. 2014, 65, 519–5204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grallath, S.; Weimar, T.; Meyer, A.; Gumy, C.; Suter-Grotemeyer, M.; Neuhaus, J.-M.; Rentsch, D. The AtProT family. Compatible solute transporters with similar substrate specificity but differential expression patterns. Plant Physiol. 2005, 137, 117–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, S.; Gumy, C.; Blatter, E.; Boeffel, S.; Fricke, W.; Rentsch, D. In planta function of compatible solute transporters of the AtProT family. J. Exp. Bot. 2011, 62, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Komarova, N.Y.; Thor, K.; Gubler, A.; Meier, S.; Dietrich, D.; Weichert, A.; Grotemeyer, M.S.; Tegeder, M.; Rentsch, D. AtPTR1 and AtPTR5 transport dipeptides in planta. Plant Physiol. 2008, 148, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, D.; Hammes, U.; Thor, K.; Suter-Grotemeyer, M.; Flückiger, R.; Slusarenko, A.J.; Ward, J.M.; Rentsch, D. AtPTR1, a plasma membrane peptide transporter expressed during seed germination and in vascular tissue of Arabidopsis. Plant J. 2004, 40, 488–499. [Google Scholar] [CrossRef]
- Paungfoo-Lonhienne, C.; Lonhienne, T.G.; Rentsch, D.; Robinson, N.; Christie, M.; Webb, R.I.; Gamage, H.K.; Carroll, B.J.; Schenk, P.M.; Schmidt, S. Plants can use protein as a nitrogen source without assistance from other organisms. Proc. Natl. Acad. Sci. USA 2008, 105, 4524–4529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payá-Milans, M.; Nunez, G.H.; Olmstead, J.W.; Rinehart, T.A.; Staton, M. Regulation of gene expression in roots of the pH-sensitive Vaccinium corymbosum and the pH-tolerant Vaccinium arboreum in response to near neutral pH stress using RNA-Seq. BMC Genom. 2017, 18, 580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Read, D. The structure and function of the ericoid mycorrhizal root. Ann. Bot. 1996, 77, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Scagel, C.F. Inoculation with ericoid mycorrhizal fungi alters fertilizer use of highbush blueberry cultivars. HortScience 2005, 40, 786–794. [Google Scholar] [CrossRef] [Green Version]
- Stribley, D.; Read, D. The biology of mycorrhiza in the Ericaceae IV. The effect of mycorrhizal infection on uptake of 15N from labelled soil by Vaccinium macrocarpon ait. New Phytol. 1974, 73, 1149–1155. [Google Scholar] [CrossRef]
- Kosola, K.R.; Workmaster, B.A.A.; Spada, P.A. Inoculation of cranberry (Vaccinium macrocarpon) with the ericoid mycorrhizal fungus Rhizoscyphus ericae increases nitrate influx. New Phytol. 2007, 176, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Näsholm, T.; Ekblad, A.; Nordin, A.; Giesler, R.; Högberg, M.; Högberg, P. Boreal forest plants take up organic nitrogen. Nature 1998, 392, 914–916. [Google Scholar] [CrossRef]
- Bajwa, R.; Read, D. The biology of mycorrhiza in the Ericaceae: IX. Peptides as nitrogen sources for the ericoid endophyte and for mycorrhizal and non-mycorrhizal plants. New Phytol. 1985, 101, 459–467. [Google Scholar] [CrossRef]
- Scagel, C.F.; Yang, W.Q. Cultural variation and mycorrhizal status of blueberry plants in NW Oregon commercial production fields. Int. J. Fruit Sci. 2005, 5, 85–111. [Google Scholar] [CrossRef]
- Sadowsky, J.J.; Hanson, E.J.; Schilder, A.M.C. Root colonization by ericoid mycorrhizae and dark septate endophytes in organic and conventional blueberry fields in Michigan. Int. J. Fruit Sci. 2012, 12, 169–187. [Google Scholar] [CrossRef]
- Li, J.; Mavrodi, O.V.; Hou, J.; Blackmon, C.; Babiker, E.M.; Mavrodi, D.V. Comparative analysis of rhizosphere microbiomes of southern highbush blueberry (Vaccinium corymbosum L.), Darrow’s blueberry (V. darrowii Camp), and rabbiteye blueberry (V. virgatum Aiton). Front. Microbiol. 2020, 11, 370. [Google Scholar] [CrossRef] [Green Version]
- Powell, C.L.; Bates, P.M. Ericoid mycorrhizas stimulate fruit yield of blueberry. HortScience 1981, 16, 655–656. [Google Scholar]
- Haynes, R.; Swift, R. Growth and nutrient uptake by highbush blueberry plants in a peat medium as influenced by pH, applied micronutrients and mycorrhizal inoculation. Sci. Hortic. 1985, 27, 285–294. [Google Scholar] [CrossRef]
- Boudsocq, S.; Lata, J.-C.; Mathieu, J.; Abbadie, L.; Barot, S. Modelling approach to analyse the effects of nitrification inhibition on primary production. Funct. Ecol. 2009, 23, 220–230. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. Ecological significance and complexity of N-source preference in plants. Ann. Bot. 2013, 112, 957–963. [Google Scholar] [CrossRef] [Green Version]
- Haynes, R.; Goh, K.M. Ammonium and nitrate nutrition of plants. Biol. Rev. 1978, 53, 465–510. [Google Scholar] [CrossRef]
- Cain, J.C. A comparison of ammonium and nitrate nitrogen for blueberries. Proc. Am. Soc. Hortic. Sci. 1952, 59, 161–166. [Google Scholar]
- Herath, H.; Eaton, G. Some effects of water table, pH, and nitrogen fertilization upon growth and nutrient-element content of high bush blueberry plants. Proc. Am. Soc. Hortic. Sci. 1968, 92, 274–283. [Google Scholar]
- Townsend, L. Effect of nitrate and ammonium nitrogen on the growth of the lowbush blueberry. Can. J. Plant Sci. 1966, 46, 209–210. [Google Scholar] [CrossRef]
- Townsend, L. Effect of ammonium nitrogen and nitrate nitrogen, separately and in combination, on the growth of the highbush blueberry. Can. J. Plant Sci. 1967, 47, 555–562. [Google Scholar] [CrossRef]
- Osorio, R.; Cáceres, C.; Covarrubias, J.I. Vegetative and physiological responses of “Emerald” blueberry to Ammoniacal sources with a nitrification inhibitor. J. Soil Sci. Plant Nutr. 2019, 20, 507–515. [Google Scholar] [CrossRef]
- Poonnachit, U.; Darnell, R. Effect of ammonium and nitrate on ferric chelate reductase and nitrate reductase in Vaccinium species. Ann. Bot. 2004, 93, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Merhaut, D.J.; Darnell, R.L. Ammonium and nitrate accumulation in containerized southern highbush blueberry plants. HortScience 1995, 30, 1378–1381. [Google Scholar] [CrossRef]
- Oertli, J. Effect of form of nitrogen and pH on growth of blueberry plants. Agron. J. 1963, 55, 305–307. [Google Scholar] [CrossRef] [Green Version]
- Alt, D.S.; Doyle, J.W.; Malladi, A. Nitrogen-source preference in blueberry (Vaccinium sp.): Enhanced shoot nitrogen assimilation in response to direct supply of nitrate. J. Plant Physiol. 2017, 216, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Takamizo, T.; Sugiyama, N. Growth responses to N forms in rabbiteye and highbush blueberries. J. Jpn. Soc. Hortic. Sci. 1991, 60, 41–45. [Google Scholar] [CrossRef]
- Claussen, W.; Lenz, F. Effect of ammonium or nitrate nutrition on net photosynthesis, growth, and activity of the enzymes nitrate reductase and glutamine synthetase in blueberry, raspberry and strawberry. Plant Soil 1999, 208, 95–102. [Google Scholar] [CrossRef]
- Hammett, L.; Ballinger, W. A nutrient solution-sand culture system for studying the influence of N form on highbush blueberries. HortScience 1972, 7, 498–500. [Google Scholar]
- Hayatsu, M.; Tago, K.; Saito, M. Various players in the nitrogen cycle: Diversity and functions of the microorganisms involved in nitrification and denitrification. Soil Sci. Plant Nutr. 2008, 54, 33–45. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat. Plants 2017, 3, 17074. [Google Scholar] [CrossRef]
- Hu, H.-W.; Xu, Z.-H.; He, J.-Z. Ammonia-oxidizing archaea play a predominant role in acid soil nitrification. Adv. Agron. 2014, 125, 261–302. [Google Scholar]
- Könneke, M.; Bernhard, A.E.; José, R.; Walker, C.B.; Waterbury, J.B.; Stahl, D.A. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 2005, 437, 543–546. [Google Scholar] [CrossRef]
- Leininger, S.; Urich, T.; Schloter, M.; Schwark, L.; Qi, J.; Nicol, G.W.; Prosser, J.I.; Schuster, S.; Schleper, C. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 2006, 442, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Lehtovirta-Morley, L.E.; Stoecker, K.; Vilcinskas, A.; Prosser, J.I.; Nicol, G.W. Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil. Proc. Natl. Acad. Sci. USA 2011, 108, 15892–15897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Throop, P.A.; Hanson, E.J. Nitrification and utilization of fertilizer nitrogen by highbush blueberry. J. Plant Nutr. 1998, 21, 1731–1742. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Ito, O.; Sahrawat, K.L.; Berry, W.L.; Nakahara, K.; Ishikawa, T.; Watanabe, T.; Suenaga, K.; Rondon, M.; Rao, I.M. Scope and Strategies for Regulation of Nitrification in Agricultural Systems—Challenges and Opportunities. Crit. Rev. Plant Sci. 2006, 25, 303–335. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Alcántara, B.; Quiñones, A.; Polo, C.; Primo-Millo, E.; Legaz, F. Use of nitrification inhibitor DMPP to improve nitrogen uptake efficiency in citrus trees. J. Agric. Sci. 2013, 5, 1–18. [Google Scholar] [CrossRef]
- Covarrubias, J.I.; Pisi, A.; Rombolà, A.D. Evaluation of sustainable management techniques for preventing iron chlorosis in the grapevine. Aust. J. Grape Wine Res. 2014, 20, 149–159. [Google Scholar] [CrossRef]
- Martínez, F.; Palencia, P.; Weiland, C.; Alonso, D.; Oliveira, J. Influence of nitrification inhibitor DMPP on yield, fruit quality and SPAD values of strawberry plants. Sci. Hortic. 2015, 185, 233–239. [Google Scholar] [CrossRef]
- Sun, L.; Lu, Y.; Yu, F.; Kronzucker, H.J.; Shi, W. Biological nitrification inhibition by rice root exudates and its relationship with nitrogen-use efficiency. New Phytol. 2016, 212, 646–656. [Google Scholar] [CrossRef] [Green Version]
- Subbarao, G.; Rondon, M.; Ito, O.; Ishikawa, T.; Rao, I.M.; Nakahara, K.; Lascano, C.; Berry, W. Biological nitrification inhibition (BNI)—Is it a widespread phenomenon? Plant Soil 2007, 294, 5–18. [Google Scholar] [CrossRef]
- Tanaka, J.P.; Nardi, P.; Wissuwa, M. Nitrification inhibition activity, a novel trait in root exudates of rice. AoB Plants 2010, 2010, plq014. [Google Scholar]
- Siddiqi, M.Y.; Glass, A.D.; Ruth, T.J.; Rufty, T.W. Studies of the uptake of nitrate in barley: I. Kinetics of 13NO3− influx. Plant Physiol. 1990, 93, 1426–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kronzucker, H.J.; Siddiqi, M.Y.; Glass, A.D. Kinetics of NO3− influx in spruce. Plant Physiol. 1995, 109, 319–326. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Siddiqi, M.Y.; Glass, A.D. Kinetics of NH4+ influx in spruce. Plant Physiol. 1996, 110, 773–779. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.Y.; Siddiqi, M.Y.; Ruth, T.J.; Glass, A.D. Ammonium uptake by rice roots (I. Fluxes and subcellular distribution of 13NH4+). Plant Physiol. 1993, 103, 1249–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslam, M.; Travis, R.L.; Huffaker, R.C. Comparative kinetics and reciprocal inhibition of nitrate and nitrite uptake in roots of uninduced and induced barley (Hordeum vulgare L.) seedlings. Plant Physiol. 1992, 99, 1124–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, N.M.; Glass, A.D. Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci. 1998, 3, 389–395. [Google Scholar] [CrossRef]
- Tischner, R. Nitrate uptake and reduction in plants. J. Crop Improv. 2006, 15, 53–95. [Google Scholar] [CrossRef]
- Miller, A.J.; Fan, X.; Orsel, M.; Smith, S.J.; Wells, D.M. Nitrate transport and signalling. J. Exp. Bot. 2007, 58, 2297–2306. [Google Scholar] [CrossRef]
- Sugiyama, N.; Ishigaki, K. Uptake of nitrate-nitrogen by blueberry plants. J. Plant Nutr. 1994, 17, 1975–1982. [Google Scholar] [CrossRef]
- Wang, M.Y.; Siddiqi, M.Y.; Ruth, T.J.; Glass, A.D. Ammonium uptake by rice roots (II. Kinetics of 13NH4+ influx across the plasmalemma). Plant Physiol. 1993, 103, 1259–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youngdahl, L.; Pacheco, R.; Street, J.; Vlek, P. The kinetics of ammonium and nitrate uptake by young rice plants. Plant Soil 1982, 69, 225–232. [Google Scholar] [CrossRef]
- Sugiyama, N.; Hirooka, M. Uptake of ammonium-nitrogen by blueberry plants. J. Plant Nutr. 1993, 16, 1975–1981. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Siddiqi, M.Y.; Glass, A.D. Conifer root discrimination against soil nitrate and the ecology of forest succession. Nature 1997, 385, 59–61. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Hsu, P.-K.; Tsay, Y.-F. Uptake, allocation and signaling of nitrate. Trends Plant Sci. 2012, 17, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Cheng, Y.-H.; Chen, K.-E.; Tsay, Y.-F. Nitrate transport, signaling, and use efficiency. Annu. Rev. Plant Biol. 2018, 69, 85–122. [Google Scholar] [CrossRef] [PubMed]
- Krapp, A.; David, L.C.; Chardin, C.; Girin, T.; Marmagne, A.; Leprince, A.-S.; Chaillou, S.; Ferrario-Méry, S.; Meyer, C.; Daniel-Vedele, F. Nitrate transport and signalling in Arabidopsis. J. Exp. Bot. 2014, 65, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Okamoto, M.; Crawford, N.M.; Siddiqi, M.Y.; Glass, A.D. Dissection of the AtNRT2. 1: AtNRT2. 2 inducible high-affinity nitrate transporter gene cluster. Plant Physiol. 2007, 143, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.H.; Tsay, Y.F. Switching between the two action modes of the dual-affinity nitrate transporter CHL1 by phosphorylation. EMBO J. 2003, 22, 1005–1013. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.-H.; Lin, S.-H.; Hu, H.-C.; Tsay, Y.-F. CHL1 functions as a nitrate sensor in plants. Cell 2009, 138, 1184–1194. [Google Scholar] [CrossRef] [Green Version]
- Huang, N.-C.; Liu, K.-H.; Lo, H.-J.; Tsay, Y.-F. Cloning and functional characterization of an Arabidopsis nitrate transporter gene that encodes a constitutive component of low-affinity uptake. Plant Cell 1999, 11, 1381–1392. [Google Scholar] [CrossRef] [Green Version]
- Ludewig, U.; Neuhäuser, B.; Dynowski, M. Molecular mechanisms of ammonium transport and accumulation in plants. FEBS Lett. 2007, 581, 2301–2308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiba, T.; Krapp, A. Plant nitrogen acquisition under low availability: Regulation of uptake and root architecture. Plant Cell Physiol. 2014, 57, 707–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loqué, D.; Yuan, L.; Kojima, S.; Gojon, A.; Wirth, J.; Gazzarrini, S.; Ishiyama, K.; Takahashi, H.; Von Wirén, N. Additive contribution of AMT1; 1 and AMT1; 3 to high-affinity ammonium uptake across the plasma membrane of nitrogen-deficient Arabidopsis roots. Plant J. 2006, 48, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Segonzac, C.; Boyer, J.-C.; Ipotesi, E.; Szponarski, W.; Tillard, P.; Touraine, B.; Sommerer, N.; Rossignol, M.; Gibrat, R. Nitrate efflux at the root plasma membrane: Identification of an Arabidopsis excretion transporter. Plant Cell 2007, 19, 3760–3777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Britto, D.T.; Kronzucker, H.J. Futile cycling at the plasma membrane: A hallmark of low-affinity nutrient transport. Trends Plant Sci. 2006, 11, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-H.; Kuo, H.-F.; Canivenc, G.; Lin, C.-S.; Lepetit, M.; Hsu, P.-K.; Tillard, P.; Lin, H.-L.; Wang, Y.-Y.; Tsai, C.-B. Mutation of the Arabidopsis NRT1. 5 nitrate transporter causes defective root-to-shoot nitrate transport. Plant Cell 2008, 20, 2514–2528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [Green Version]
- Britto, D.T.; Siddiqi, M.Y.; Glass, A.D.; Kronzucker, H.J. Futile transmembrane NH4+ cycling: A cellular hypothesis to explain ammonium toxicity in plants. Proc. Natl. Acad. Sci. USA 2001, 98, 4255–4258. [Google Scholar] [CrossRef] [Green Version]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef] [Green Version]
- Howitt, S.M.; Udvardi, M.K. Structure, function and regulation of ammonium transporters in plants. Biochim. Biophys. Acta (BBA) Biomembr. 2000, 1465, 152–170. [Google Scholar] [CrossRef] [Green Version]
- Tobin, A.K.; Yamaya, T. Cellular compartmentation of ammonium assimilation in rice and barley. J. Exp. Bot. 2001, 52, 591–604. [Google Scholar] [CrossRef]
- Schjoerring, J.K.; Husted, S.; Mäck, G.; Mattsson, M. The regulation of ammonium translocation in plants. J. Exp. Bot. 2002, 53, 883–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darnell, R.L.; Hiss, S.A. Uptake and assimilation of nitrate and iron in two Vaccinium species as affected by external nitrate concentration. J. Am. Soc. Hortic. Sci. 2006, 131, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Cruz, C.; Soares, M.; Martins-Loucao, M.; Lips, S. Nitrate reduction in seedlings of carob (Ceratonia siliqua L.). New Phytol. 1991, 119, 413–419. [Google Scholar] [CrossRef]
- Cruz, C.; Lips, S.H.; Martins-Loução, M.A. Nitrogen assimilation and transport in carob plants. Physiol. Plant. 1993, 89, 524–531. [Google Scholar] [CrossRef]
- Min, X.; Siddiqi, M.; Guy, R.; Glass, A.; Kronzucker, H. Induction of nitrate uptake and nitrate reductase activity in trembling aspen and lodgepole pine. Plant Cell Environ. 1998, 21, 1039–1046. [Google Scholar] [CrossRef] [Green Version]
- Min, X.; Siddiqi, Y.M.; Guy, R.; Glass, A.; Kronzucker, H. A comparative study of fluxes and compartmentation of nitrate and ammonium in early-successional tree species. Plant Cell Environ. 1999, 22, 821–830. [Google Scholar] [CrossRef]
- Lea, P.; Miflin, B. Alternative route for nitrogen assimilation in higher plants. Nature 1974, 251, 614–616. [Google Scholar] [CrossRef]
- Funayama, K.; Kojima, S.; Tabuchi-Kobayashi, M.; Sawa, Y.; Nakayama, Y.; Hayakawa, T.; Yamaya, T. Cytosolic glutamine synthetase1; 2 is responsible for the primary assimilation of ammonium in rice roots. Plant Cell Physiol. 2013, 54, 934–943. [Google Scholar] [CrossRef]
- Smirnoff, N.; Stewart, G. Nitrate assimilation and translocation by higher plants: Comparative physiology and ecological consequences. Physiol. Plant. 1985, 64, 133–140. [Google Scholar] [CrossRef]
- Pate, J. Transport and partitioning of nitrogenous solutes. Annu. Rev. Plant Physiol. 1980, 31, 313–340. [Google Scholar] [CrossRef]
- Smirnoff, N.; Todd, P.; Stewart, G. The occurrence of nitrate reduction in the leaves of woody plants. Ann. Bot. 1984, 54, 363–374. [Google Scholar] [CrossRef]
- Dirr, M.; Barker, A.; Maynard, D. Nitrate reductase activity in the leaves of the highbush blueberry and other plants. J. Am. Soc. Hortic. Sci. 1972, 97, 329–331. [Google Scholar]
- Aslam, M.; Rosichan, J.L.; Huffaker, R.C. Comparative induction of nitrate reductase by nitrate and nitrite in barley leaves. Plant Physiol. 1987, 83, 579–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslam, M.; Huffaker, R.C. Role of nitrate and nitrite in the induction of nitrite reductase in leaves of barley seedlings. Plant Physiol. 1989, 91, 1152–1156. [Google Scholar] [CrossRef]
- Crawford, N.M.; Arst Jr, H.N. The molecular genetics of nitrate assimilation in fungi and plants. Annu. Rev. Genet. 1993, 27, 115–146. [Google Scholar] [CrossRef]
- Darnell, R.L.; Cruz-Huerta, N. Uptake and assimilation of nitrate and iron in cultivated and wild Vaccinium species. Int. J. Fruit Sci. 2011, 11, 136–150. [Google Scholar] [CrossRef]
- Titus, J.S.; Kang, S.-M. Nitrogen metabolism, translocation, and recycling in apple trees. Hortic. Rev. 1982, 4, 204–246. [Google Scholar]
- Tromp, J. Nutrient reserves in roots of fruit trees, in particular carbohydrates and nitrogen. Plant Soil 1983, 71, 401–413. [Google Scholar] [CrossRef]
- Millard, P.; Grelet, G.-A. Nitrogen storage and remobilization by trees: Ecophysiological relevance in a changing world. Tree Physiol. 2010, 30, 1083–1095. [Google Scholar] [CrossRef] [Green Version]
- Carranca, C.; Brunetto, G.; Tagliavini, M. Nitrogen nutrition of fruit trees to reconcile productivity and environmental concerns. Plants 2018, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Fuchigami, L.H. Growth of young apple trees in relation to reserve nitrogen and carbohydrates. Tree Physiol. 2002, 22, 1297–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Ma, F.; Ranwala, D. Nitrogen storage and its interaction with carbohydrates of young apple trees in response to nitrogen supply. Tree Physiol. 2004, 24, 91–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grelet, G.A.; Alexander, I.J.; Proe, M.F.; Frossard, J.S.; Millard, P. Leaf habit influences nitrogen remobilization in Vaccinium species. J. Exp. Bot. 2001, 52, 993–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagliavini, M.; Millard, P.; Quartieri, M.; Marangoni, B. Timing of nitrogen uptake affects winter storage and spring remobilisation of nitrogen in nectarine (Prunus persica var. nectarina) trees. Plant Soil 1999, 211, 149–153. [Google Scholar] [CrossRef]
- Niederholzer, F.; DeJong, T.; Saenz, J.-L.; Muraoka, T.; Weinbaum, S. Effectiveness of fall versus spring soil fertilization of field-grown peach trees. J. Am. Soc. Hortic. Sci. 2001, 125, 644–648. [Google Scholar] [CrossRef]
- Rosecrance, R.C.; Weinbaum, S.A.; Brown, P.H. Alternate bearing affects nitrogen, phosphorus, potassium and starch storage pools in mature pistachio trees. Ann. Bot. 1998, 82, 463–470. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Bangerth, F. Calcium-related physiological disorders of plants. Annu. Rev. Phytopathol. 1979, 17, 97–122. [Google Scholar] [CrossRef]
- Hart, J.M.; Strik, B.; White, L.; Yang, W. Nutrient Management for Blueberries in Oregon; Oregon State University Extension Service Publication: Corvallis, OR, USA, 2006. [Google Scholar]
- Dayod, M.; Tyerman, S.D.; Leigh, R.A.; Gilliham, M. Calcium storage in plants and the implications for calcium biofortification. Protoplasma 2010, 247, 215–231. [Google Scholar] [CrossRef]
- Raleigh, S.; Chucka, J. Effect of nutrient ratio and concentration on growth and composition of tomato plants and on the occurence of blossom-end rot of the fruit. Plant Physiol. 1944, 19, 671–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, L.; Adams, P.; Li, X.; Shen, H.; Andrews, J.; Xu, Z. Responses of Ca-efficient and Ca-inefficient tomato cultivars to salinity in plant growth, calcium accumulation and blossom-end rot. J. Hortic. Sci. 1995, 70, 909–918. [Google Scholar] [CrossRef]
- Taylor, M.D.; Locascio, S.J. Blossom-end rot: A calcium deficiency. J. Plant Nutr. 2004, 27, 123–139. [Google Scholar] [CrossRef]
- del Amor, F.M.; Rubio, J.S. Effects of antitranspirant spray and potassium: Calcium: Magnesium ratio on photosynthesis, nutrient and water uptake, growth, and yield of sweet pepper. J. Plant Nutr. 2009, 32, 97–111. [Google Scholar] [CrossRef]
- Briggs, G.; Robertson, R. Apparent free space. Annu. Rev. Plant Physiol. 1957, 8, 11–30. [Google Scholar] [CrossRef]
- Sattelmacher, B. The apoplast and its significance for plant mineral nutrition. New Phytol. 2001, 149, 167–192. [Google Scholar] [CrossRef]
- Gilliham, M.; Dayod, M.; Hocking, B.J.; Xu, B.; Conn, S.J.; Kaiser, B.N.; Leigh, R.A.; Tyerman, S.D. Calcium delivery and storage in plant leaves: Exploring the link with water flow. J. Exp. Bot. 2011, 62, 2233–2250. [Google Scholar] [CrossRef] [PubMed]
- Hocking, B.; Tyerman, S.D.; Burton, R.A.; Gilliham, M. Fruit calcium: Transport and physiology. Front. Plant Sci. 2016, 7, 569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAinsh, M.R.; Pittman, J.K. Shaping the calcium signature. New Phytol. 2009, 181, 275–294. [Google Scholar] [CrossRef]
- White, P.J. The pathways of calcium movement to the xylem. J. Exp. Bot. 2001, 52, 891–899. [Google Scholar] [CrossRef]
- Karley, A.J.; White, P.J. Moving cationic minerals to edible tissues: Potassium, magnesium, calcium. Curr. Opin. Plant Biol. 2009, 12, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, U.M.; Manohar, M.; Gaur, V.S. Calcium transport from source to sink: Understanding the mechanism (s) of acquisition, translocation, and accumulation for crop biofortification. Acta Physiol. Plant. 2015, 37, 1722. [Google Scholar] [CrossRef]
- Demidchik, V.; Shabala, S.; Isayenkov, S.; Cuin, T.A.; Pottosin, I. Calcium transport across plant membranes: Mechanisms and functions. New Phytol. 2018, 220, 49–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conn, S.; Gilliham, M. Comparative physiology of elemental distributions in plants. Ann. Bot. 2010, 105, 1081–1102. [Google Scholar] [CrossRef] [Green Version]
- Thor, K. Calcium—Nutrient and messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef]
- Baxter, I.; Hosmani, P.S.; Rus, A.; Lahner, B.; Borevitz, J.O.; Muthukumar, B.; Mickelbart, M.V.; Schreiber, L.; Franke, R.B.; Salt, D.E. Root suberin forms an extracellular barrier that affects water relations and mineral nutrition in Arabidopsis. PLoS Genet. 2009, 5, e1000492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cholewa, E.; Peterson, C.A. Evidence for symplastic involvement in the radial movement of calcium in onion roots. Plant Physiol. 2004, 134, 1793–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Höfer, R.; Briesen, I.; Beck, M.; Pinot, F.; Schreiber, L.; Franke, R. The Arabidopsis cytochrome P450 CYP86A1 encodes a fatty acid ω-hydroxylase involved in suberin monomer biosynthesis. J. Exp. Bot. 2008, 59, 2347–2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina, I.; Li-Beisson, Y.; Beisson, F.; Ohlrogge, J.B.; Pollard, M. Identification of an Arabidopsis feruloyl-coenzyme A transferase required for suberin synthesis. Plant Physiol. 2009, 151, 1317–1328. [Google Scholar] [CrossRef] [Green Version]
- Ho, L.C. Environmental effects on the diurnal accumulation of 45Ca by young fruit and leaves of tomato plants. Ann. Bot. 1989, 63, 281–288. [Google Scholar] [CrossRef]
- Adams, P.; Holder, R. Effects of humidity, Ca and salinity on the accumulation of dry matter and Ca by the leaves and fruit of tomato (Lycopersicon esculentum). J. Hortic. Sci. 1992, 67, 137–142. [Google Scholar] [CrossRef]
- Frantz, J.M.; Ritchie, G.; Cometti, N.N.; Robinson, J.; Bugbee, B. Exploring the limits of crop productivity: Beyond the limits of tipburn in lettuce. J. Am. Soc. Hortic. Sci. 2004, 129, 331–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guichard, S.; Gary, C.; Leonardi, C.; Bertin, N. Analysis of growth and water relations of tomato fruits in relation to air vapor pressure deficit and plant fruit load. J. Plant Growth Regul. 2005, 24, 201–213. [Google Scholar] [CrossRef]
- Schon, M.K. Effects of foliar antitranspirant or calcium nitrate applications on yield and blossom-end rot occurrence in greenhouse-grown peppers. J. Plant Nutr. 1993, 16, 1137–1149. [Google Scholar] [CrossRef]
- De Freitas, S.T.; McElrone, A.J.; Shackel, K.A.; Mitcham, E.J. Calcium partitioning and allocation and blossom-end rot development in tomato plants in response to whole-plant and fruit-specific abscisic acid treatments. J. Exp. Bot. 2014, 65, 235–247. [Google Scholar] [CrossRef]
- Saure, M.C. Calcium translocation to fleshy fruit: Its mechanism and endogenous control. Sci. Hortic. 2005, 105, 65–89. [Google Scholar] [CrossRef]
- Guttridge, C.; Bradfield, E.; Holder, R. Dependence of calcium transport into strawberry leaves on positive pressure in the xylem. Ann. Bot. 1981, 48, 473–480. [Google Scholar] [CrossRef]
- Montanaro, G.; Dichio, B.; Xiloyannis, C. Significance of fruit transpiration on calcium nutrition in developing apricot fruit. J. Plant Nutr. Soil Sci. 2010, 173, 618–622. [Google Scholar] [CrossRef]
- Montanaro, G.; Dichio, B.; Lang, A.; Mininni, A.N.; Xiloyannis, C. Fruit calcium accumulation coupled and uncoupled from its transpiration in kiwifruit. J. Plant Physiol. 2015, 181, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Banuelos, G.S.; Bangerth, F.; Marschner, H. Relationship between polar basipetal auxin transport and acropetal Ca2+ transport into tomato fruits. Physiol. Plant. 1987, 71, 321–327. [Google Scholar] [CrossRef]
- Ho, L.C.; White, P.J. A cellular hypothesis for the induction of blossom-end rot in tomato fruit. Ann. Bot. 2005, 95, 571–581. [Google Scholar] [CrossRef] [Green Version]
- De Freitas, S.T.; Mitcham, E.I. Factors involved in fruit calcium deficiency disorders. Hortic. Rev. 2012, 40, 107–146. [Google Scholar]
- Marcelis, L.; Ho, L. Blossom-end rot in relation to growth rate and calcium content in fruits of sweet pepper (Capsicum annuum L.). J. Exp. Bot. 1999, 50, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Heuvelink, E.; Körner, O. Parthenocarpic fruit growth reduces yield fluctuation and blossom-end rot in sweet pepper. Ann. Bot. 2001, 88, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Perring, M. The effects of environment and cultural practices on calcium concentration in the apple fruit. Commun. Soil Sci. Plant Anal. 1979, 10, 279–293. [Google Scholar] [CrossRef]
- Tromp, J. The intake curve for calcium into apple fruits under various environmental conditions. Commun. Soil Sci. Plant Anal. 1979, 10, 325–335. [Google Scholar] [CrossRef]
- Xiloyannis, C.; Celano, G.; Montanaro, G.; Dichio, B.; Sebastiani, L.; Minnocci, A. Water relations, calcium and potassium, concentration in fruits and leaves during annual growth in mature kiwifruit plants. Acta Hortic. 2001, 564, 129–134. [Google Scholar] [CrossRef]
- Bernadac, A.; Jean-Baptiste, I.; Bertoni, G.; Morard, P. Change in calcium contents during melon (Cucumis melo L.) fruit development. Sci. Hortic. 1996, 66, 181–189. [Google Scholar]
- Stückrath, R.; Quevedo, R.; de la Fuente, L.; Hernández, A.; Sepúlveda, V. Effect of foliar application of calcium on the quality of blueberry fruits. J. Plant Nutr. 2008, 31, 1299–1312. [Google Scholar] [CrossRef]
- Yang, F.-H.; DeVetter, L.W.; Strik, B.C.; Bryla, D.R. Stomatal functioning and its influence on fruit calcium accumulation in northern highbush blueberry. HortScience 2020, 55, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Mayorga-Gómez, A.; Nambeesan, S.U.; Coolong, T.; Díaz-Pérez, J.C. Temporal relationship between calcium and fruit growth and development in bell pepper (Capsicum annuum L.). HortScience 2020, 55, 906–913. [Google Scholar] [CrossRef]
- Song, W.; Yi, J.; Kurniadinata, O.F.; Wang, H.; Huang, X. Linking fruit Ca uptake capacity to fruit growth and pedicel anatomy, a cross-species study. Front. Plant Sci. 2018, 9, 575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, L.; Belda, R.; Brown, M.; Andrews, J.; Adams, P. Uptake and transport of calcium and the possible causes of blossom-end rot in tomato. J. Exp. Bot. 1993, 44, 509–518. [Google Scholar] [CrossRef]
- Dražeta, L.; Lang, A.; Hall, A.J.; Volz, R.K.; Jameson, P.E. Causes and effects of changes in xylem functionality in apple fruit. Ann. Bot. 2004, 93, 275–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dichio, B.; Remorini, D.; Lang, S. Developmental changes in xylem functionality in kiwifruit fruit: Implications for fruit calcium accumulation. Acta Hortic. 2003, 610, 191–195. [Google Scholar] [CrossRef]
- Montanaro, G.; Dichio, B.; Lang, A.; Mininni, A.N.; Nuzzo, V.; Clearwater, M.J.; Xiloyannis, C. Internal versus external control of calcium nutrition in kiwifruit. J. Plant Nutr. Soil Sci. 2014, 177, 819–830. [Google Scholar] [CrossRef]
- Angeletti, P.; Castagnasso, H.; Miceli, E.; Terminiello, L.; Concellón, A.; Chaves, A.; Vicente, A.R. Effect of preharvest calcium applications on postharvest quality, softening and cell wall degradation of two blueberry (Vaccinium corymbosum) varieties. Postharvest Biol. Technol. 2010, 58, 98–103. [Google Scholar] [CrossRef]
- Hanson, E.J.; Berkheimer, S.F. Effect of soil calcium applications on blueberry yield and quality. Small Fruits Rev. 2004, 3, 133–139. [Google Scholar] [CrossRef]
- Hanson, E.J. Preharvest calcium sprays do not improve highbush blueberry (Vaccinium corymbosum L.) quality. HortScience 1995, 30, 977–978. [Google Scholar] [CrossRef] [Green Version]
- Vance, A.J.; Jones, P.; Strik, B.C. Foliar calcium applications do not improve quality or shelf life of strawberry, raspberry, blackberry, or blueberry fruit. HortScience 2017, 52, 382–387. [Google Scholar] [CrossRef]
- Manzi, M.; Lado, J. Foliar applications of calcium do not impact on fruit and leaf nutrient concentration or quality of ‘O’Neal’blueberry. J. Hortic. Sci. Biotechnol. 2019, 94, 676–684. [Google Scholar] [CrossRef]
- Smith, E.D. The effect of foliar calcium treatments on fruit weight and firmness of rabbiteye blueberry (Vaccinium virgatum Aiton). J. Am. Pomol. Soc. 2016, 70, 74–81. [Google Scholar]
- Arrington, M.; DeVetter, L.W. Foliar applications of calcium and boron do not increase fruit set or yield in northern highbush blueberry (Vaccinium corymbosum). HortScience 2017, 52, 1259–1264. [Google Scholar] [CrossRef]
- Blodgett, A.; Caldwell, R.; McManus, P. Effects of calcium salts on the cranberry fruit rot disease complex. Plant Dis. 2002, 86, 747–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.-H.; Bryla, D.R.; Strik, B.C. Critical temperatures and heating times for fruit damage in northern highbush blueberry. HortScience 2019, 54, 2231–2239. [Google Scholar] [CrossRef] [Green Version]
- Lobos, T.; Retamales, J.; Escobar, A.L.; Hanson, E. Timing of foliar calcium sprays improves fruit firmness and antioxidants in “Liberty” blueberries. J. Soil Sci. Plant Nutr. 2020. [Google Scholar] [CrossRef]
- Gerbrandt, E.M.; Mouritzen, C.; Sweeney, M. Foliar calcium corrects a deficiency causing green fruit drop in ‘Draper’ highbush blueberry (Vaccinium corymbosum L.). Agriculture 2019, 9, 63. [Google Scholar] [CrossRef] [Green Version]
- Hanson, E.J.; Beggs, J.L.; Beaudry, R.M. Applying calcium chloride postharvest to improve highbush blueberry firmness. HortScience 1993, 28, 1033–1034. [Google Scholar] [CrossRef] [Green Version]
- Tang, R.-J.; Luan, S. Regulation of calcium and magnesium homeostasis in plants: From transporters to signaling network. Curr. Opin. Plant Biol. 2017, 39, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Maillard, A.; Diquélou, S.; Billard, V.; Laîné, P.; Garnica, M.; Prudent, M.; Garcia-Mina, J.-M.; Yvin, J.-C.; Ourry, A. Leaf mineral nutrient remobilization during leaf senescence and modulation by nutrient deficiency. Front. Plant Sci. 2015, 6, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malone, M.; White, P.; Morales, M.A. Mobilization of calcium in glasshouse tomato plants by localized scorching. J. Exp. Bot. 2002, 53, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Paiva, E.A.S. Are calcium oxalate crystals a dynamic calcium store in plants? New Phytol. 2019, 223, 1707–1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, V.R.; Nakata, P.A. Calcium oxalate in plants: Formation and function. Annu. Rev. Plant Biol. 2005, 56, 41–71. [Google Scholar] [CrossRef]
- Gallaher, R.N.; Jones, J.B.J. Total, extractable and oxalate calcium and other elements in normal and mouse ear pecan tree tissues. J. Am. Soc. Hortic. Sci. 1976, 101, 692–696. [Google Scholar]
- Gallaher, R.N.; Perkins, H.F.; Jones, J.B.J. Calcium concentration and distribution in healthy and decline peach tree tissues. HortScience 1975, 10, 134–137. [Google Scholar]
- Storey, R.; Jones, R.G.W.; Schachtman, D.P.; Treeby, M.T. Calcium-accumulating cells in the meristematic region of grapevine root apices. Funct. Plant Biol. 2003, 30, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Volk, G.; Lynch-Holm, V.; Kostman, T.; Goss, L.; Franceschi, V. The role of druse and raphide calcium oxalate crystals in tissue calcium regulation in Pistia stratiotes leaves. Plant Biol. 2002, 4, 34–45. [Google Scholar] [CrossRef]

| Plant Part | N Concentration (% Dry Weight) | Notes | Source |
|---|---|---|---|
| Leaves | 1.2–2.1 | 8 | |
| 1.85–2.95 | 13 z | ||
| 1.7–2.7 | between leaf senescence and fruit harvest | 15 y | |
| Vegetative growth | 2.1 | typical period of fruit harvest | 14 x |
| 1.1–4.7 | During growing season | 13 | |
| 1.5–2.5 | between anthesis and ~80 d after anthesis | 17 w | |
| Woody canes | 0.4–0.75 | highest before anthesis; lowest during fruit development | 17 |
| 0.8–1.7 | highest at dormancy; low at fruit harvest | 14 | |
| 0.76–1.58 | highest at dormancy; lowest at fruit harvest | 13 | |
| Flower | 2.1–2.2 | anthesis | 17 |
| 5 | 14 | ||
| 5.3 | 13 | ||
| Fruit | 1–1.1 | at harvest | 17 |
| 1.37 | at harvest | 13, 14 | |
| Crown | 1.2–1.75 | at fruit harvest and at dormancy | 14 |
| 0.95–1.73 | highest at dormancy and lowest during mid-fruit development | 13 | |
| Root | 1.1–1.6 | Increasing towards end of fruit development | 17 |
| 1.2–1.6 | At dormancy and at fruit harvest | 14 | |
| 0.95–2.3 | Low during early fruit development and highest during dormancy (following year) | 13 |
| Species | N Concentration (mM) | N-Source Preference | Suggested Mechanism/Notes | Study |
|---|---|---|---|---|
| V. corymbosum ‘Jersey’ | 25 mM (250 mL per week); 2–8 mM as NH4NO3 or NH4Cl | NH4+ | pH: > 6.0; < 5.2 | 48 |
| V. corymbosum ‘Bluecrop’ | 5 mM | None | pH: 4.0, 6.0 and 8.0 | 55 |
| V. angustifolium | 1 mM and 10 mM | NH4+ | pH: 4.9 | 50 |
| V. corymbosum ‘Berkley’ | 1.5 mM | NH4+ | pH: 4.5; NH4NO3 displayed intermediate effects | 51 |
| V. angustifolium | 2 mM | NH4+ at pH 4.5 | pH: 4.5 and 6.0; pH and N-source may have independent effects | 9 |
| V. corymbosum ‘Wolcott’ | 0.44 mM to 1.75 mM (combinations of NH4+ and NO3− | None | pH: 5.8–6.2; pH of eluent decreased with increasing NH4+ | 59 |
| Interspecific hybrid clone of V. corymbosum and V. angustifolium ‘Northblue’ | 2 mM | None | pH: 4.5 and 6.5; Plants displayed higher growth at lower pH; plants displayed similar N uptake rates regardless of N-source | 10 |
| V. virgatum ‘Tifblue’ and V. corymbosum ‘Jersey | 1 mM to 4 mM (combinations of NH4+ and NO3−; final N: 4 mM) | None | pH: 5.5, continually corrected; leaves accumulated greater free NH4+ with higher NH4+ supply | 57 |
| V. virgatum ‘Tifblue’ | 1 mM | NH4+ (pH: 3.0; 4.0) | pH: 3.0, 4.0, 5.0 | 11 |
| V. corymbosum ‘Sharpblue’ | Soil drench of 7.5 mmol 15N in 500 mL | NH4+ | pH: 6.5; Uptake rates higher for NH4+; translocation of N to shoots higher for NH4+ | 54 |
| V. corymbosum ‘13-16-A’ | 6 mM | NH4+ | pH: 3.5–4.2 for NH4-N and 6.6-7.2 for NO3-N | 58 |
| V. arboreum (Va) and V. corymbosum (Vc) ‘Misty’ | 5 mM | NH4+ in Vc | pH: 5.5 | 53 |
| V. virgatum ‘Alapaha’ and V. corymbosum ‘Sweetcrisp’ | 5 mM | None: based on N uptake rates | pH: 5.0 | 56 |
| V. corymbosum ‘Emerald’ | 17.86 mmol N per week | NH4+ | NH4NO3 displayed marginally better performance | 52 |
| Type of Application | Application Details | Concentration of Applied Ca | Leaf [Ca] and Treatment Effect | Fruit [Ca] and Treatment Effect | Source |
|---|---|---|---|---|---|
| Soil | Calcitic lime; CaSO4; V. corymbosum; | 1100 and 550 kg ha−1 per year; four-year applications | 0.2–0.45%; increased during later years | 0.03–0.07%; inconsistent increase | 184 |
| CaSO4; previous season application; V. corymbosum | 600 kg ha−1 | NA | Cell wall [Ca2+] increased by >10%; firmness increased | 183 | |
| Foliar | CaCl2; Nutrical; V. corymbosum | 1–24.2 kg ha−1; | 0.25–0.44%; increased at higher rates | 0.03–0.04%; NS | 185 |
| CaCl2; Ca silicate; Ca chelate; Ca acetate; V. corymbosum | 0.34–0.67 kg ha−1 | 0.6–1.8%; NS | 0.02–0.06%; NS | 186 | |
| Ca(NO3)2; chelate Ca-oxide; V. corymbosum | 0.36–0.78 kg ha−1; applied four times during fruit development | 0.64% | 0.11% (at harvest); NS; firmness increased but inconsistent | 187 | |
| Ca(NO3)2; neutralized CaCo3; chelated Ca; V. virgatum | 0. 65 kg ha−1; 0.1 kg ha−1; 0.56 kg ha−1; applied twice | Inconsistent change (18% increase and 26% decrease) | NS; inconsistent change in firmness | 188 | |
| CaCl2 and CaSO4; V. corymbosum | 750–1500 ppm; 150 ppm, respectively; applied six times during fruit development | 0.6–0.9%; NS | 0.04–0.06%; NS | 189 | |
| CaCl2; Ca phosphite; Ca thiosulfate solution; V. corymbosum | 0.63 kg ha−1; 0.2 kg ha−1; and 0.42 kg ha−1; up to 2.5 kg ha−1 and up to 3 times | 0.57–0.75% | negative correlation with fruit drop; 0.11–0.19% in early fruit and 0.04–0.06% in ripe fruit; increased with high rates | 193 | |
| CaCl2; V. corymbosum | 0.4–0.8 kg ha−1 | Increase in firmness with increasing [Ca2+] | 192 | ||
| Postharvest dip | CaCl2 immersion; V. corymbosum | 0–4% | NA | Linear increase in fruit firmness with increasing [Ca2+] in dip; objectionable taste | 194 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doyle, J.W.; Nambeesan, S.U.; Malladi, A. Physiology of Nitrogen and Calcium Nutrition in Blueberry (Vaccinium sp.). Agronomy 2021, 11, 765. https://doi.org/10.3390/agronomy11040765
Doyle JW, Nambeesan SU, Malladi A. Physiology of Nitrogen and Calcium Nutrition in Blueberry (Vaccinium sp.). Agronomy. 2021; 11(4):765. https://doi.org/10.3390/agronomy11040765
Chicago/Turabian StyleDoyle, John W., Savithri U. Nambeesan, and Anish Malladi. 2021. "Physiology of Nitrogen and Calcium Nutrition in Blueberry (Vaccinium sp.)" Agronomy 11, no. 4: 765. https://doi.org/10.3390/agronomy11040765
APA StyleDoyle, J. W., Nambeesan, S. U., & Malladi, A. (2021). Physiology of Nitrogen and Calcium Nutrition in Blueberry (Vaccinium sp.). Agronomy, 11(4), 765. https://doi.org/10.3390/agronomy11040765





