Effect of Soil Water Availability on Physiological Parameters, Yield, and Seed Quality in Four Quinoa Genotypes (Chenopodium quinoa Willd.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
2.3. Management of the Experiments
2.4. Evaluations
2.5. Statistical Analysis
3. Results
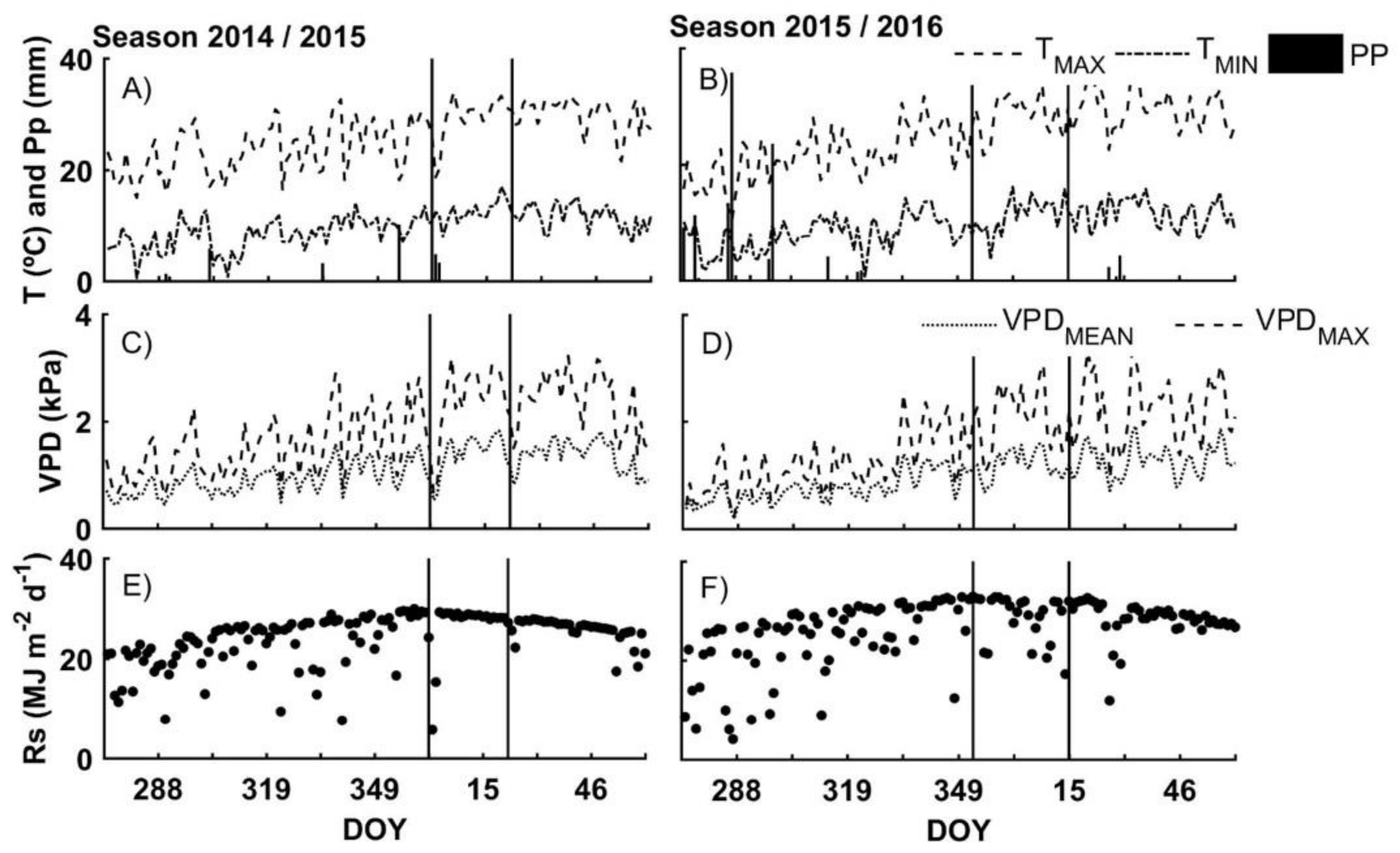
3.1. Environmental Conditions
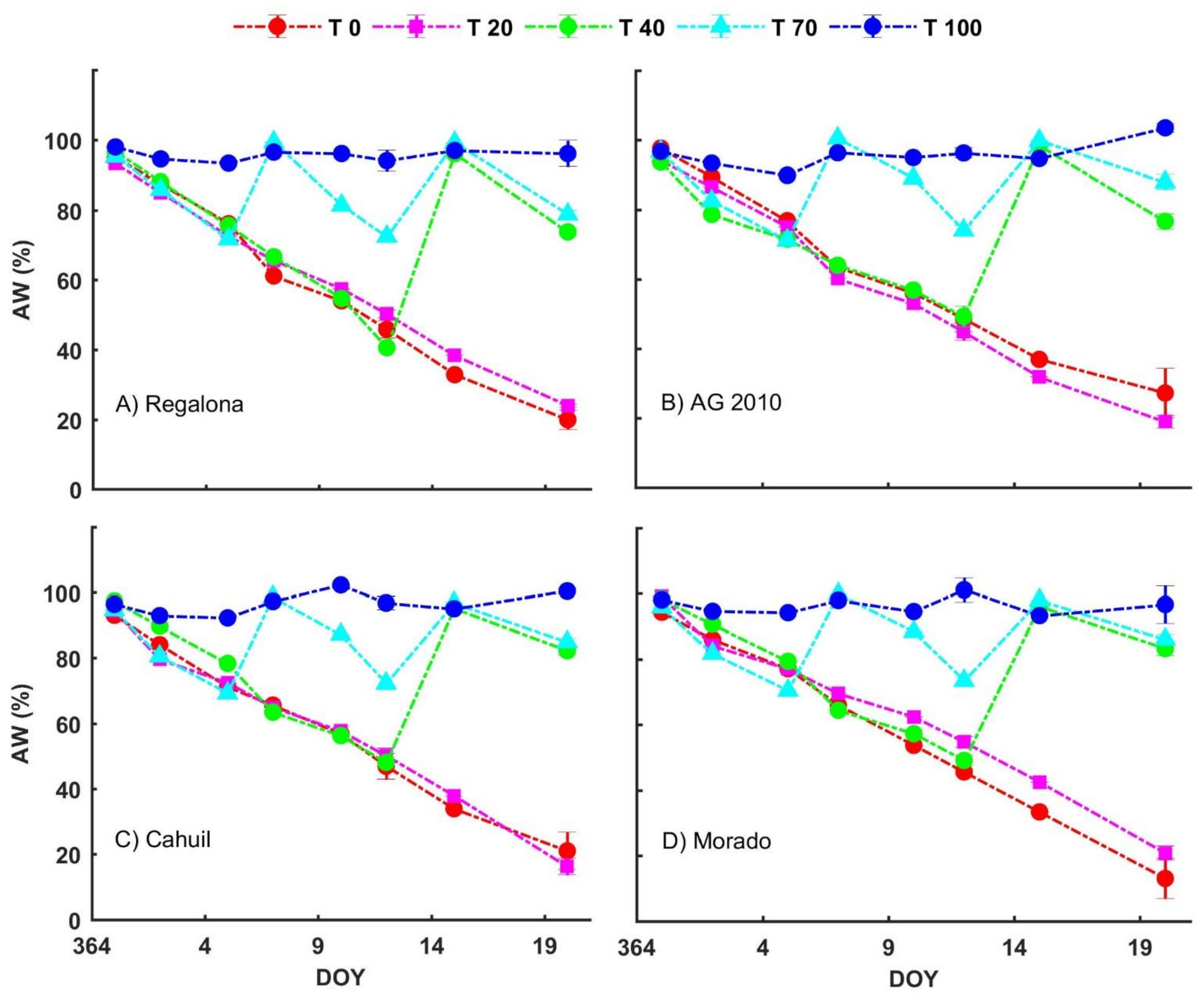
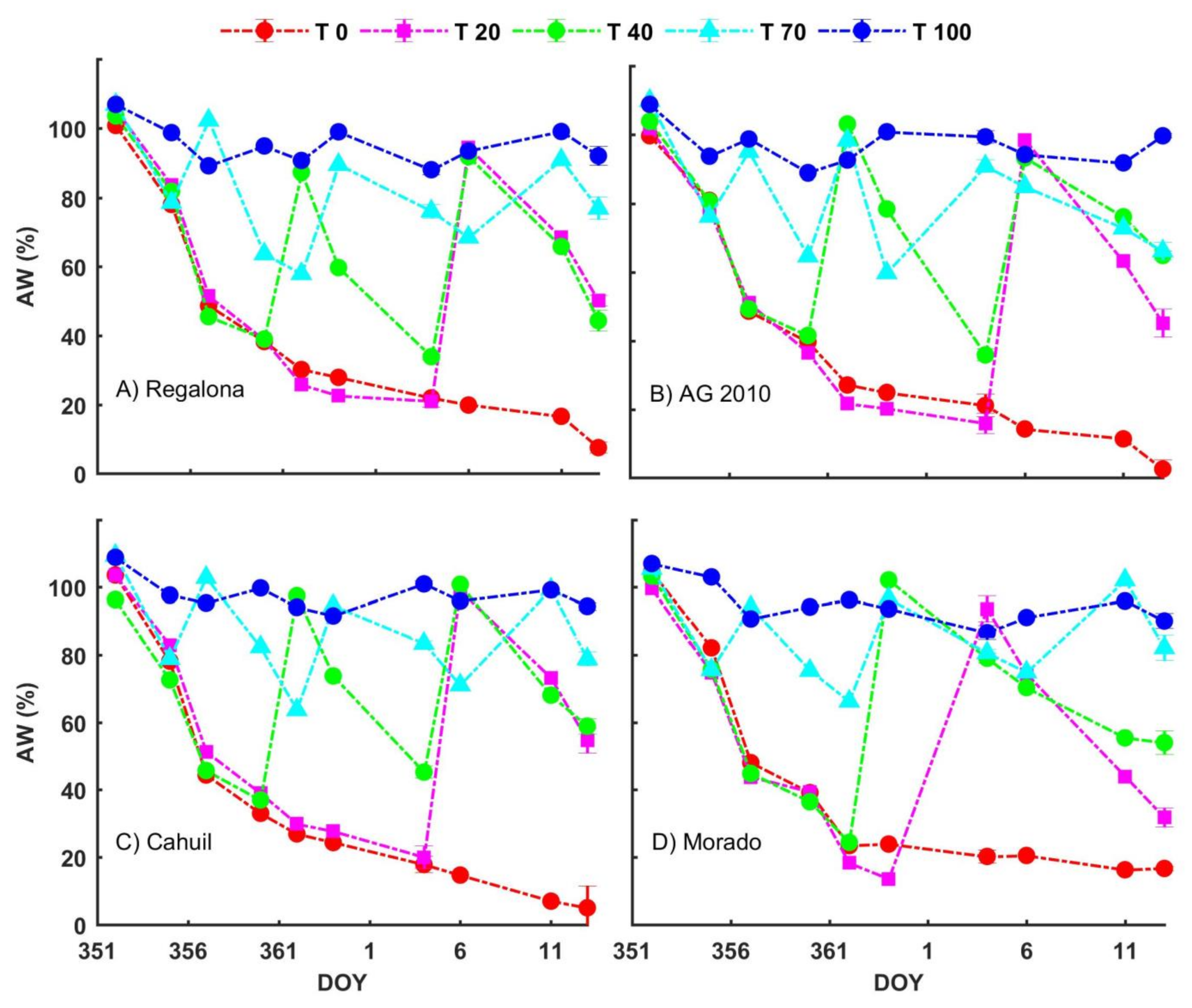
3.2. Water Availability Treatments
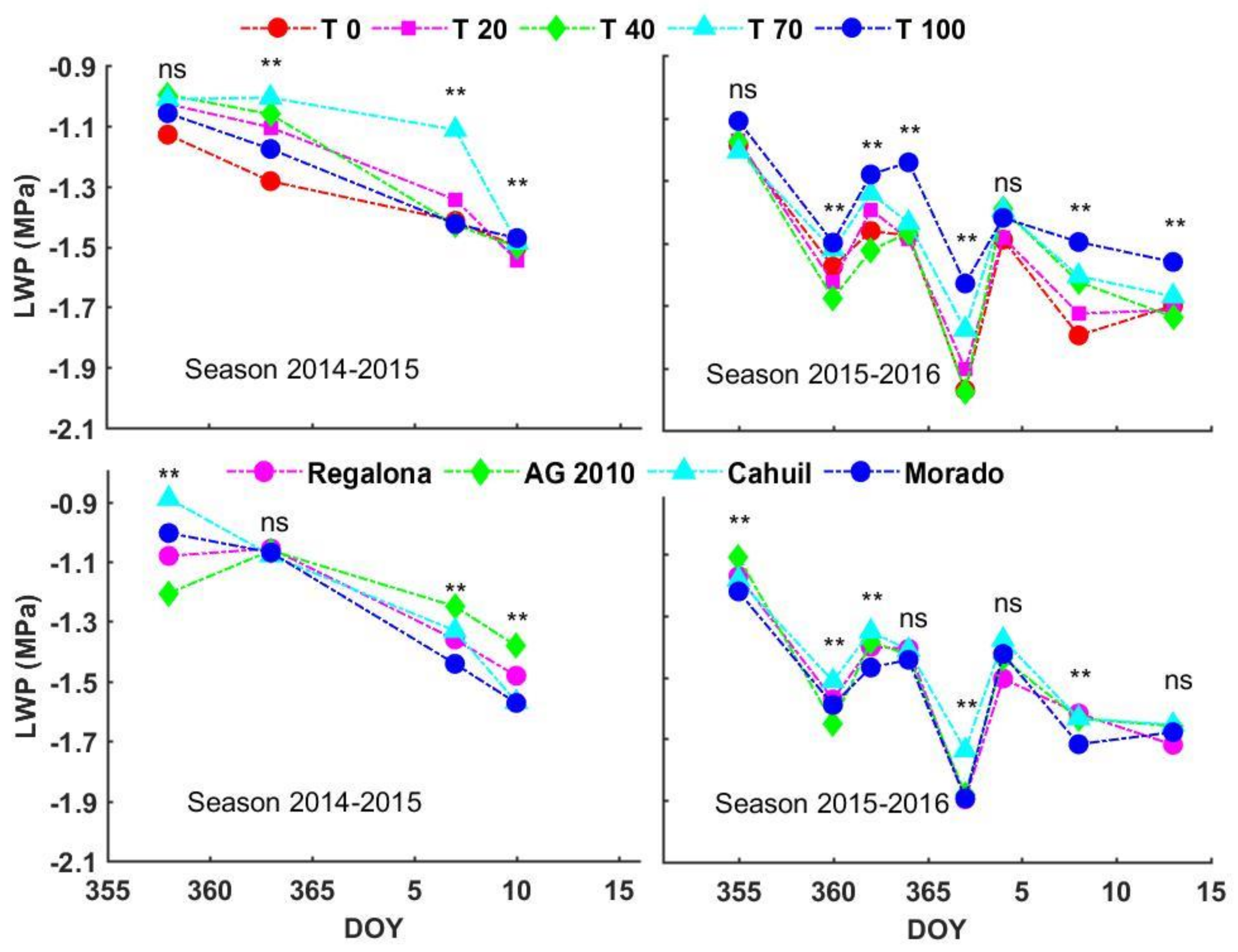
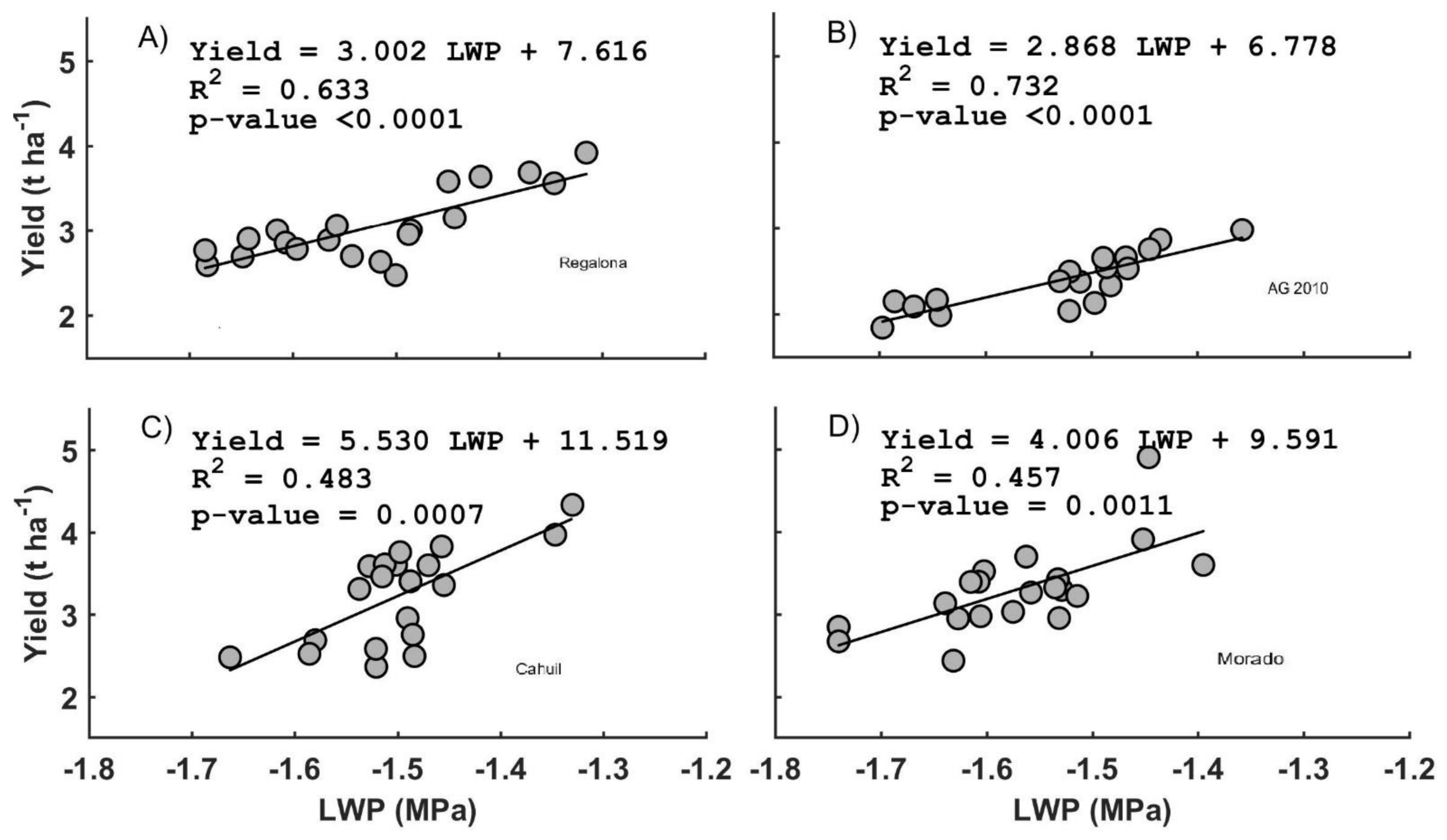
3.3. Leaf Water Potential (LWP)
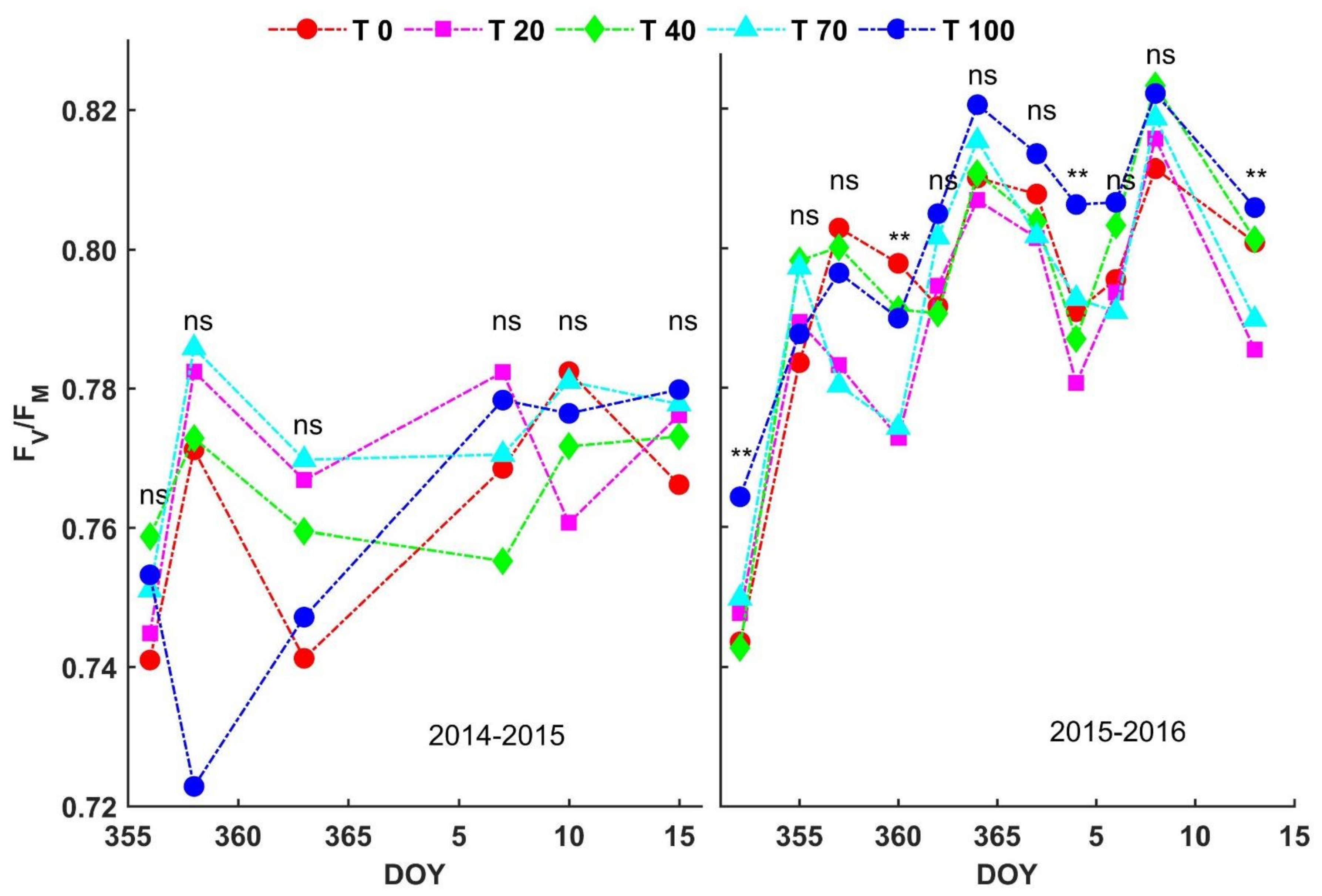
3.4. Chlorophyll Fluorescence
3.5. Seed Yield
3.6. Protein Content
4. Discussion
4.1. Leaf Water Potential
4.2. Chlorophyll Fluorescence
4.3. Seed Yield
4.4. Protein Content
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Huang, S.; Huang, Q.; Leng, G.; Guo, Y.; Wang, L.; Fang, W.; Li, P.; Zheng, X. Assessing agricultural drought risk and its dynamic evolution characteristics. Agric. Water Manag. 2020, 231, 106003. [Google Scholar] [CrossRef]
- Bhusal, N.; Han, S.-G.; Yoon, T.-M. Impact of drought stress on photosynthetic response, leaf water potential, and stem sap flow in two cultivars of bi-leader apple trees (Malus × domestica Borkh.). Sci. Hortic. 2019, 246, 535–543. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Zurita-Silva, A.; Jacobsen, S.-E.; Razzaghi, F.; Alvarez-Flores, R.; Ruiz, K.; Morales, A.; Silva, H. Quinoa Drought Responses and Adaptation. In State of the Art Report of Quinoa in the World in 2013; FAO & CIRAD: Rome, Italy, 2015; pp. 157–171. [Google Scholar]
- Bazile, D.; Pulvento, C.; Verniau, A.; Al-Nusairi, M.S.; Ba, D.; Breidy, J.; Hassan, L.; Mohammed, M.I.; Mambetov, O.; Otambekova, M.; et al. Worldwide Evaluations of Quinoa: Preliminary Results from Post International Year of Quinoa FAO Projects in Nine Countries. Front. Plant Sci. 2016, 7, 850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhargava, A.; Shukla, S.; Ohri, D. Chenopodium quinoa—An Indian perspective. Ind. Crop. Prod. 2006, 23, 73–87. [Google Scholar] [CrossRef]
- Bertero, H.; De La Vega, A.; Correa, G.; Jacobsen, S.; Mujica, A. Genotype and genotype-by-environment interaction effects for grain yield and grain size of quinoa (Chenopodium quinoa Willd.) as revealed by pattern analysis of international multi-environment trials. Field Crop. Res. 2004, 89, 299–318. [Google Scholar] [CrossRef]
- Jaikishun, S.; Li, W.; Yang, Z.; Song, S. Quinoa: In Perspective of Global Challenges. Agronomy 2019, 9, 176. [Google Scholar] [CrossRef] [Green Version]
- Hinojosa, L.; González, J.A.; Barrios-Masias, F.H.; Fuentes, F.; Murphy, K.M. Quinoa Abiotic Stress Responses: A Review. Plants 2018, 7, 106. [Google Scholar] [CrossRef] [Green Version]
- Stoleru, V.; Slabu, C.; Vitanescu, M.; Peres, C.; Cojocaru, A.; Covasa, M.; Mihalache, G. Tolerance of Three Quinoa Cultivars (Chenopodium quinoa Willd.) to Salinity and Alkalinity Stress During Germination Stage. Agronomy 2019, 9, 287. [Google Scholar] [CrossRef] [Green Version]
- Pulvento, C.; Riccardi, M.; Lavini, A.; Iafelice, G.; Marconi, E.; D’Andria, R. Yield and Quality Characteristics of Quinoa Grown in Open Field Under Different Saline and Non-Saline Irrigation Regimes. J. Agron. Crop. Sci. 2012, 198, 254–263. [Google Scholar] [CrossRef]
- Razzaghi, F.; Plauborg, F.; Jacobsen, S.-E.; Jensen, C.R.; Andersen, M.N. Effect of nitrogen and water availability of three soil types on yield, radiation use efficiency and evapotranspiration in field-grown quinoa. Agric. Water Manag. 2012, 109, 20–29. [Google Scholar] [CrossRef]
- Al-Naggar, A.M.M.; El-Salam, R.M.A.; Badran, A.E.E.; El-Moghazi, M.M.A. Genotype and Drought Effects on Morphological, Physiological and Yield Traits of Quinoa (Chenopodium quinoa Willd.). Asian J. Adv. Agric. Res. 2017, 3, 1–15. [Google Scholar] [CrossRef]
- Krause, G.H.; Weis, E. Chlorophyll Fluorescence and Photosynthesis: The Basics. Ann Rev Plant Physiol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Jensen, C.; Jacobsen, S.-E.; Andersen, M.; Núñez, N.; Andersen, S.; Rasmussen, L.; Mogensen, V. Leaf gas exchange and water relation characteristics of field quinoa (Chenopodium quinoa Willd.) during soil drying. Eur. J. Agron. 2000, 13, 11–25. [Google Scholar] [CrossRef]
- Jacobsen, S.-E.; Liu, F.; Jensen, C.R. Does root-sourced ABA play a role for regulation of stomata under drought in quinoa (Chenopodium quinoa Willd.). Sci. Hortic. 2009, 122, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Daryanto, S.; Wang, L.; Jacinthe, P.-A. Global synthesis of drought effects on cereal, legume, tuber and root crops production: A review. Agric. Water Manag. 2017, 179, 18–33. [Google Scholar] [CrossRef] [Green Version]
- Yang, A.; Akhtar, S.S.; Amjad, M.; Iqbal, S.; Jacobsen, S.-E. Growth and Physiological Responses of Quinoa to Drought and Temperature Stress. J. Agron. Crop. Sci. 2016, 202, 445–453. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; Volume 29, pp. 153–188. [Google Scholar] [CrossRef] [Green Version]
- Gámez, A.L.; Soba, D.; Zamarreño, Á.M.; García-Mina, J.M.; Aranjuelo, I.; Morales, F. Effect of Water Stress during Grain Filling on Yield, Quality and Physiological Traits of Illpa and Rainbow Quinoa (Chenopodium quinoa Willd.) Cultivars. Plants 2019, 8, 173. [Google Scholar] [CrossRef] [Green Version]
- Issa Ali, O.; Fghire, R.; Anaya, F.; Benlhabib, O.; Wahbi, S. Physiological and Morphological Responses of Two Quinoa Cul-tivars (Chenopodium Quinoa Willd.) to Drought Stress. Gesunde Pflanz 2019, 71, 123–133. [Google Scholar] [CrossRef]
- Fghire, R.; Anaya, F.; Ali, O.I.; Benlhabib, O.; Ragab, R.; Wahbi, S. Physiological and photosynthetic response of quinoa to drought stress. Chil. J. Agric. Res. 2015, 75, 174–183. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Liu, F.; Bendevis, M.; Shabala, S.; Jacobsen, S.-E. Sensitivity of Two Quinoa (Chenopodiumquinoa Willd.) Varieties to Progressive Drought Stress. J. Agron. Crop. Sci. 2013, 200, 12–23. [Google Scholar] [CrossRef]
- Martínez, E.A.; Veas, E.; Jorquera, C.; Martín, R.S.; Jara, P. Re-Introduction of Quínoa into Arid Chile: Cultivation of Two Lowland Races under Extremely Low Irrigation. J. Agron. Crop. Sci. 2009, 195, 1–10. [Google Scholar] [CrossRef]
- Geerts, S.; Raes, D.; Garcia, M.; Mendoza, J.; Huanca, R. Crop water use indicators to quantify the flexible phenology of quinoa (Chenopodium quinoa Willd.) in response to drought stress. Field Crop. Res. 2008, 108, 150–156. [Google Scholar] [CrossRef]
- Geerts, S.; Raes, D.; Garcia, M.; Condori, O.; Mamani, J.; Miranda, R.; Cusicanqui, J.; Taboada, C.; Yucra, E.; Vacher, J. Could deficit irrigation be a sustainable practice for quinoa (Chenopodium quinoa Willd.) in the Southern Bolivian Altiplano? Agric. Water Manag. 2008, 95, 909–917. [Google Scholar] [CrossRef]
- Geerts, S.; Raes, D.; Garcia, M.; Vacher, J.; Mamani, R.; Mendoza, J.; Huanca, R.; Morales, B.; Miranda, R.; Cusicanqui, J.; et al. Introducing deficit irrigation to stabilize yields of quinoa (Chenopodium quinoa Willd.). Eur. J. Agron. 2008, 28, 427–436. [Google Scholar] [CrossRef]
- Garcia, M.; Raes, D.; Jacobsen, S.-E. Evapotranspiration analysis and irrigation requirements of quinoa (Chenopodium quinoa) in the Bolivian highlands. Agric. Water Manag. 2003, 60, 119–134. [Google Scholar] [CrossRef]
- Fischer, S.; Wilckens, R.; Jara, J.; Aranda, M.; Valdivia, W.; Bustamante, L.; Graf, F.; Obal, I. Protein and antioxidant composition of quinoa (Chenopodium quinoa Willd.) sprout from seeds submitted to water stress, salinity and light conditions. Ind. Crop. Prod. 2017, 107, 558–564. [Google Scholar] [CrossRef]
- Stolpe, N. Descripciones de Los Principales Suelos de La VIII Región de Chile; Universidad de Concepción, Departamento de Suelos y Recursos Naturales: Chillán, Chile, 2006. [Google Scholar]
- Klute, A. Water Retention: Laboratory Methods. In Methods of Soil Analysis Part. 5-Mineralogical Methods; Wiley Online Library: Hoboken, NJ, USA, 2018; volume 5, pp. 635–662. [Google Scholar]
- AGRIMED; Tome, I.V. Center of Agriculture and Environment; University of Chile: Santiago, Chile, 2017. [Google Scholar]
- Bertero, H.D. Response of Developmental Processes to Temperature and Photoperiod in Quinoa (Chenopodium quinoa Willd.). Food Rev. Int. 2003, 19, 87–97. [Google Scholar] [CrossRef]
- Bunce, J.A. Variation in Yield Responses to Elevated CO2 and a Brief High Temperature Treatment in Quinoa. Plants 2017, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Razzaghi, F.; Ahmadi, S.H.; Adolf, V.I.; Jensen, C.R.; Jacobsen, S.-E.; Andersen, M.N. Water Relations and Transpiration of Quinoa (Chenopodium quinoa Willd.) Under Salinity and Soil Drying. J. Agron. Crop. Sci. 2011, 197, 348–360. [Google Scholar] [CrossRef]
- Jacobsen, S.-E.; Mujica, A.; Jensen, C.R. The Resistance of Quinoa (Chenopodium quinoa Willd.) to Adverse Abiotic Factors. Food Rev. Int. 2003, 19, 99–109. [Google Scholar] [CrossRef]
- Alghory, A.; Yazar, A. Evaluation of crop water stress index and leaf water potential for deficit irrigation management of sprinkler-irrigated wheat. Irrig. Sci. 2019, 37, 61–77. [Google Scholar] [CrossRef]
- Xue, Q.; Zhu, Z.; Musick, J.T.; Stewart, B.; Dusek, D.A. Physiological mechanisms contributing to the increased water-use efficiency in winter wheat under deficit irrigation. J. Plant Physiol. 2006, 163, 154–164. [Google Scholar] [CrossRef]
- Sanchez, H.B.; LeMeur, R.; Van Damme, P.; Jacobsen, S.-E. Ecophysiological Analysis Of Drought And Salinity Stress Of Quinoa (Chenopodium Quinoawilld.). Food Rev. Int. 2003, 19, 111–119. [Google Scholar] [CrossRef]
- Killi, D.; Haworth, M. Diffusive and Metabolic Constraints to Photosynthesis in Quinoa during Drought and Salt Stress. Plants 2017, 6, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkel, T.; Méthy, M.; Thenot, F. Radiation Use Efficiency, Chlorophyll Fluorescence, and Reflectance Indices Associated with Ontogenic Changes in Water-Limited Chenopodium quinoa Leaves. Photosynth 2002, 40, 227–232. [Google Scholar] [CrossRef]
- Fischer, S.; Wilckens, R.; Jara, J.; Aranda, M. Variation in antioxidant capacity of quinoa (Chenopodium quinoa Will) subjected to drought stress. Ind. Crop. Prod. 2013, 46, 341–349. [Google Scholar] [CrossRef]
- Fghire, R.; Wahbi, S.; Anaya, F.; Ali, O.I.; Benlhabib, O.; Ragab, R. Response of Quinoa to Different Water Management Strategies: Field Experiments and Saltmed Model Application Results. Irrig. Drain. 2015, 64, 29–40. [Google Scholar] [CrossRef]
- Becker, V.I.; Goessling, J.W.; Duarte, B.; Caçador, I.; Liu, F.; Rosenqvist, E.; Jacobsen, S.-E. Combined effects of soil salinity and high temperature on photosynthesis and growth of quinoa plants (Chenopodium quinoa). Funct. Plant Biol. 2017, 44, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa, L.; Matanguihan, J.B.; Murphy, K.M. Effect of high temperature on pollen morphology, plant growth and seed yield in quinoa (Chenopodium quinoa Willd.). J. Agron. Crop. Sci. 2018, 205, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Präger, A.; Munz, S.; Nkebiwe, P.M.; Mast, B.; Graeff-Hönninger, S. Yield and Quality Characteristics of Different Quinoa (Chenopodium quinoa Willd.) Cultivars Grown under Field Conditions in Southwestern Germany. Agronomy 2018, 8, 197. [Google Scholar] [CrossRef] [Green Version]
- Muchow, R.C.; Sinclair, T.R.; Bennett, J.M. Temperature and Solar Radiation Effects on Potential Maize Yield across Locations. Agron. J. 1990, 82, 338–343. [Google Scholar] [CrossRef]
- Navarro-Lisboa, R.; Herrera, C.; Zúñiga, R.; Enrione, J.; Guzmán, F.; Matiacevich, S.; Astudillo-Castro, C. Quinoa proteins (Chenopodium quinoa Willd.) fractionated by ultrafiltration using ceramic membranes: The role of pH on physicochemical and conformational properties. Food Bioprod. Process. 2017, 102, 20–30. [Google Scholar] [CrossRef]
- Föste, M.; Elgeti, D.; Brunner, A.-K.; Jekle, M.; Becker, T. Isolation of quinoa protein by milling fractionation and solvent extraction. Food Bioprod. Process. 2015, 96, 20–26. [Google Scholar] [CrossRef]
- Kozioł, M. Chemical composition and nutritional evaluation of quinoa (Chenopodium quinoa Willd.). J. Food Compos. Anal. 1992, 5, 35–68. [Google Scholar] [CrossRef]
- Bascuñán-Godoy, L.; Reguera, M.; Abdel-Tawab, Y.M.; Blumwald, E. Water deficit stress-induced changes in carbon and nitrogen partitioning in Chenopodium quinoa Willd. Planta 2015, 243, 591–603. [Google Scholar] [CrossRef]
- Bazile, D.; Martínez, E.A.; Fuentes, F. Diversity of Quinoa in a Biogeographical Island: A Review of Constraints and Potential from Arid to Temperate Regions of Chile. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Zurita-Silva, A.; Fuentes, F.; Zamora, P.; Jacobsen, S.-E.; Schwember, A.R. Breeding quinoa (Chenopodium quinoa Willd.): Potential and perspectives. Mol. Breed. 2014, 34, 13–30. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Biondi, S.; Oses, R.; Acuña-Rodríguez, I.S.; Antognoni, F.; Martinez-Mosqueira, E.A.; Coulibaly, A.; Canahua-Murillo, A.; Pinto, M.; Zurita-Silva, A.; et al. Quinoa biodiversity and sustainability for food security under climate change. A review. Agron. Sustain. Dev. 2013, 34, 349–359. [Google Scholar] [CrossRef] [Green Version]
- Fujihara, S.; Sasaki, H.; Aoyagi, Y.; Sugahara, T. Nitrogen-to-Protein Conversion Factors for Some Cereal Products in Japan. J. Food Sci. 2008, 73, C204–C209. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Ibuji, A.; Chen, Y.; Kawamura, Y.; Mitsunaga, T. Composition of Quinoa Protein Fractions. Nippon Shokuhin 762 Kagaku Kogaku Kaishi 2003, 50, 546–549. [Google Scholar] [CrossRef]
- McDowell, N.G. Mechanisms Linking Drought, Hydraulics, Carbon Metabolism, and Vegetation Mortality. Plant. Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parchin, R.A.; Shaban, M. Protein profile and seeds storage proteins changes in wheat genotypes under control and drought stress conditions. Sci. Agric. 2014, 1, 6–8. [Google Scholar] [CrossRef]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.E.; Salami, S.A.; Babalar, M. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Lee, H.; Adhikari, A.; Han, A.R.; Kim, H.S. Evaluation of morphological, physiological, and biochemical traits for assessing drought resistance in eleven tree species. Sci. Total. Environ. 2021, 779, 146466. [Google Scholar] [CrossRef]
- Blum, A. Drought resistance, water-use efficiency, and yield potential—are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Fábián, A.; Jäger, K.; Rakszegi, M.; Barnabás, B. Embryo and endosperm development in wheat (Triticum aestivum L.) kernels subjected to drought stress. Plant Cell Rep. 2011, 30, 551–563. [Google Scholar] [CrossRef]
- Farooq, M.; Bramley, H.; Palta, J.A.; Siddique, K.H. Heat Stress in Wheat during Reproductive and Grain-Filling Phases. Crit. Rev. Plant Sci. 2011, 30, 491–507. [Google Scholar] [CrossRef]
- Li, P.; Chen, J.; Wu, P. Agronomic Characteristics and Grain Yield of 30 Spring Wheat Genotypes under Drought Stress and Nonstress Conditions. Agron. J. 2011, 103, 1619–1628. [Google Scholar] [CrossRef] [Green Version]
- Yi, B.; Zhou, Y.-F.; Gao, M.-Y.; Zhang, Z.; Han, Y.; Yang, G.-D.; Xu, W.; Huang, R.-D. Effect of Drought Stress During Flowering Stage on Starch Accumulation and Starch Synthesis Enzymes in Sorghum Grains. J. Integr. Agric. 2014, 13, 2399–2406. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, Y.S.; Baek, K.H.; Jung, H.; Ha, S.-H.; Choi, Y.D.; Kim, M.; Reuzeau, C.; Kim, J.-K. Root-Specific Expression of OsNAC10 Improves Drought Tolerance and Grain Yield in Rice under Field Drought Conditions. Plant Physiol. 2010, 153, 185–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, J.; Zong, W.; Hu, H.; Li, X.; Xiao, J.; Xiong, L. A STRESS-RESPONSIVE NAC1-Regulated Protein Phosphatase Gene Rice Protein Phosphatase18 Modulates Drought and Oxidative Stress Tolerance through Abscisic Acid-Independent Reactive Oxygen Species Scavenging in Rice. Plant Physiol. 2014, 166, 2100–2114. [Google Scholar] [CrossRef] [Green Version]
- Arumingtyas, E.L.; Savitri, E.S.; Purwoningrahayu, R.D. Protein Profiles and Dehydrin Accumulation in Some Soybean Varieties (Glycine max L. Merr) in Drought Stress Conditions. Am. J. Plant Sci. 2013, 4, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Carjuzaa, P.; Castellión, M.; Distéfano, A.J.; Del Vas, M.; Maldonado, S. Detection and subcellular localization of dehydrin-like proteins in quinoa (Chenopodium quinoa Willd.) embryos. Protoplasma 2008, 233, 149–156. [Google Scholar] [CrossRef]
- Laino, P.; Shelton, D.; Finnie, C.; De Leonardis, A.M.; Mastrangelo, A.M.; Svensson, B.; Lafiandra, D.; Masci, S. Comparative proteome analysis of metabolic proteins from seeds of durum wheat (cv. Svevo) subjected to heat stress. Proteomics 2010, 10, 2359–2368. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef]
- Gambãn, B.; Borrás, L.; Gambín, B. Resource distribution and the trade-off between seed number and seed weight: A comparison across crop species. Ann. Appl. Biol. 2010, 156, 91–102. [Google Scholar] [CrossRef]
- Hand, S.C.; Menze, M.A.; Toner, M.; Boswell, L.; Moore, D. LEA Proteins During Water Stress: Not Just for Plants Anymore. Annu. Rev. Physiol. 2011, 73, 115–134. [Google Scholar] [CrossRef] [Green Version]
- Iizumi, T.; Yokozawa, M.; Sakurai, G.; Travasso, M.I.; Romanenkov, V.; Oettli, P.; Newby, T.; Ishigooka, Y.; Furuya, J. Historical changes in global yields: Major cereal and legume crops from 1982 to 2006. Glob. Ecol. Biogeogr. 2013, 23, 346–357. [Google Scholar] [CrossRef]

| Season 2014/2015 | |||||
|---|---|---|---|---|---|
| AW (%) | Genotype | ||||
| Regalona | AG 2010 | Cahuil | Morado | Average | |
| Grain Yield (t ha−1) | |||||
| T 0 | 0.56 | 0.50 | 0.81 | 0.41 | 0.57 d |
| T 20 | 1.32 | 1.21 | 1.11 | 0.66 | 1.07 c |
| T 40 | 1.50 | 1.59 | 1.25 | 1.12 | 1.37 c |
| T 70 | 1.79 | 1.94 | 2.09 | 1.32 | 1.78 b |
| T 100 | 2.47 | 2.33 | 2.81 | 1.79 | 2.35 a |
| Average | 1.53 a | 1.51 a | 1.61 a | 1.06 b | |
| Season 2015/2016 | |||||
| AW (%) | Genotype | ||||
| Regalona | AG 2010 | Cahuil | Morado | Average | |
| Grain yield (t ha−1) | |||||
| T 0 | 2.82 | 2.07 | 3.04 | 2.91 | 2.71 bc |
| T 20 | 2.40 | 2.07 | 2.99 | 2.95 | 2.60 c |
| T 40 | 2.69 | 2.60 | 3.57 | 3.58 | 3.11 ab |
| T 70 | 2.79 | 2.43 | 3.48 | 3.32 | 3.01 abc |
| T 100 | 3.68 | 2.61 | 3.66 | 3.69 | 3.41 a |
| Average | 2.88 b | 2.36 c | 3.35 a | 3.29 a | |
| Season 2014/2015 | ||||
| AW (%) | TP (g 100 g−1) | Alb (mg g−1) | Glob (mg g−1) | OP (mg g−1) |
| T 0 | 12.9b | 30.4a | 32.3a | 45.5b |
| T 20 | 12.5b | 30.4a | 32.9a | 50.7a |
| T 40 | 14.0a | 35.0a | 36.5a | 51.1a |
| T 70 | 13.6ab | 34.0a | 37.2a | 50.5a |
| T 100 | 13.8a | 35.1a | 32.5a | 50.5a |
| Genotype | ||||
| Regalona | 13.8a | 33.7a | 35.9a | 50.3a |
| AG 2010 | 12.4b | 32.3a | 31.3a | 48.8a |
| Cahuil | 13.6ab | 33.0a | 36.2a | 50.2a |
| Morado | 13.9a | 32.9a | 33.5a | 49.4a |
| Season 2015/2016 | ||||
| AW (%) | TP (g 100 g−1) | Alb (mg g−1) | Glob (mg g−1) | OP (mg g−1) |
| T 0 | 11.1a | 32.3c | 39.4a | 38.3a |
| T 20 | 10.5a | 35.7bc | 36.7a | 39.3a |
| T 40 | 10.8a | 41.5ab | 39.5a | 41.8a |
| T 70 | 10.8a | 40.3ab | 40.2a | 46.1a |
| T 100 | 11.2a | 43.5a | 44.1a | 43.5a |
| Genotype | ||||
| Regalona | 10.8ab | 41.3a | 38.6b | 41.6a |
| AG 2010 | 10.8ab | 37.0ab | 38.7b | 43.4a |
| Cahuil | 10.7b | 36.3b | 40.3ab | 39.7a |
| Morado | 11.2a | 40.1ab | 42.4a | 42.5a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdivia-Cea, W.; Bustamante, L.; Jara, J.; Fischer, S.; Holzapfel, E.; Wilckens, R. Effect of Soil Water Availability on Physiological Parameters, Yield, and Seed Quality in Four Quinoa Genotypes (Chenopodium quinoa Willd.). Agronomy 2021, 11, 1012. https://doi.org/10.3390/agronomy11051012
Valdivia-Cea W, Bustamante L, Jara J, Fischer S, Holzapfel E, Wilckens R. Effect of Soil Water Availability on Physiological Parameters, Yield, and Seed Quality in Four Quinoa Genotypes (Chenopodium quinoa Willd.). Agronomy. 2021; 11(5):1012. https://doi.org/10.3390/agronomy11051012
Chicago/Turabian StyleValdivia-Cea, Walter, Luis Bustamante, Jorge Jara, Susana Fischer, Eduardo Holzapfel, and Rosemarie Wilckens. 2021. "Effect of Soil Water Availability on Physiological Parameters, Yield, and Seed Quality in Four Quinoa Genotypes (Chenopodium quinoa Willd.)" Agronomy 11, no. 5: 1012. https://doi.org/10.3390/agronomy11051012
APA StyleValdivia-Cea, W., Bustamante, L., Jara, J., Fischer, S., Holzapfel, E., & Wilckens, R. (2021). Effect of Soil Water Availability on Physiological Parameters, Yield, and Seed Quality in Four Quinoa Genotypes (Chenopodium quinoa Willd.). Agronomy, 11(5), 1012. https://doi.org/10.3390/agronomy11051012






