Using UAV Borne, Multi-Spectral Imaging for the Field Phenotyping of Shoot Biomass, Leaf Area Index and Height of West African Sorghum Varieties under Two Contrasted Water Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Design and Field Trials
2.3. Data Collection and Processing
2.3.1. Data Collection
2.3.2. Extraction of Vegetation INDICES and Height Estimation at Plot Scale
2.4. Statistical Analyses
3. Results
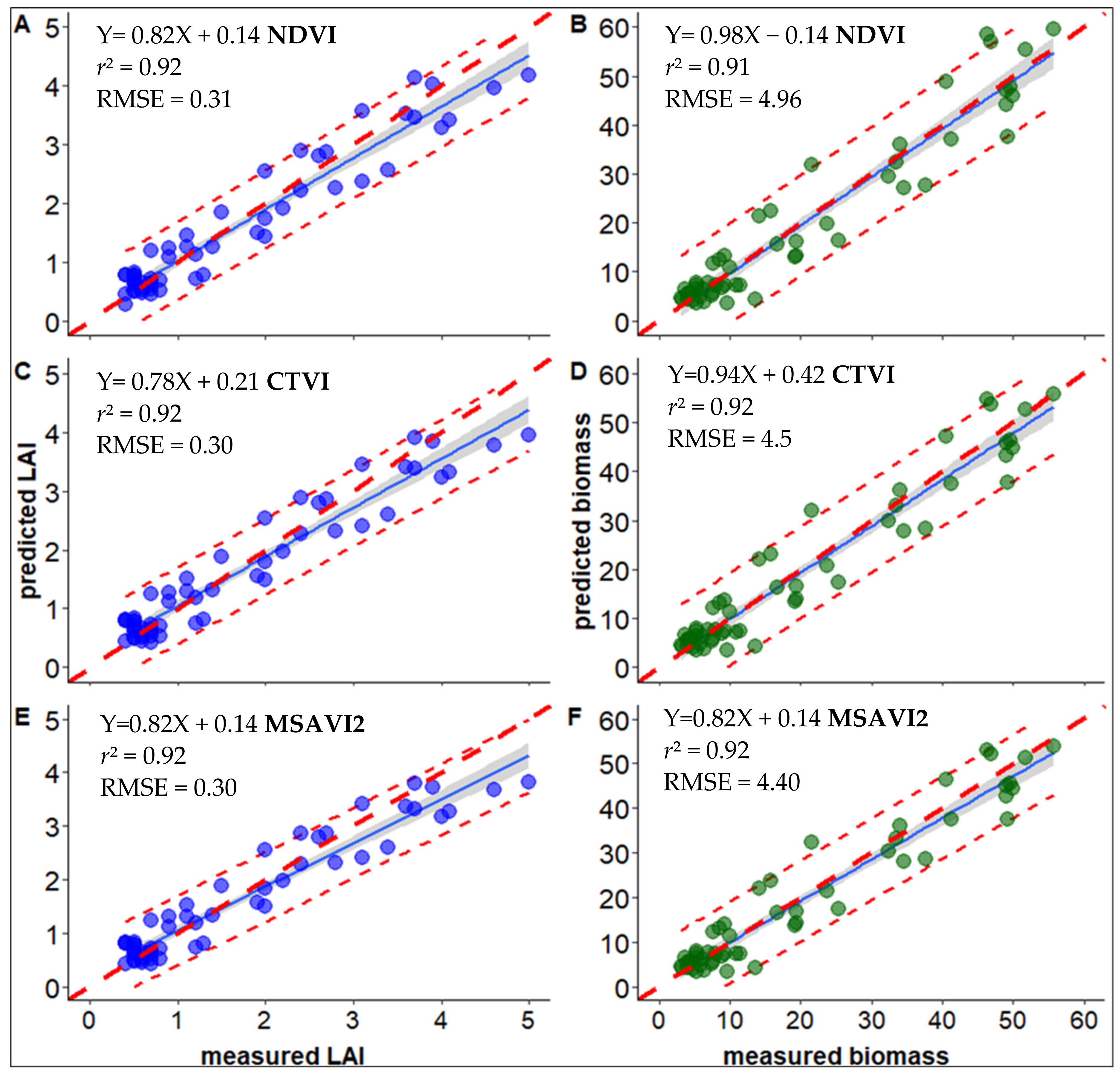
3.1. Calibration of LAI and Biomass Using UAV-Derived Spectral Indices
3.2. Validation LAI and Shoot Biomass Predictive Models
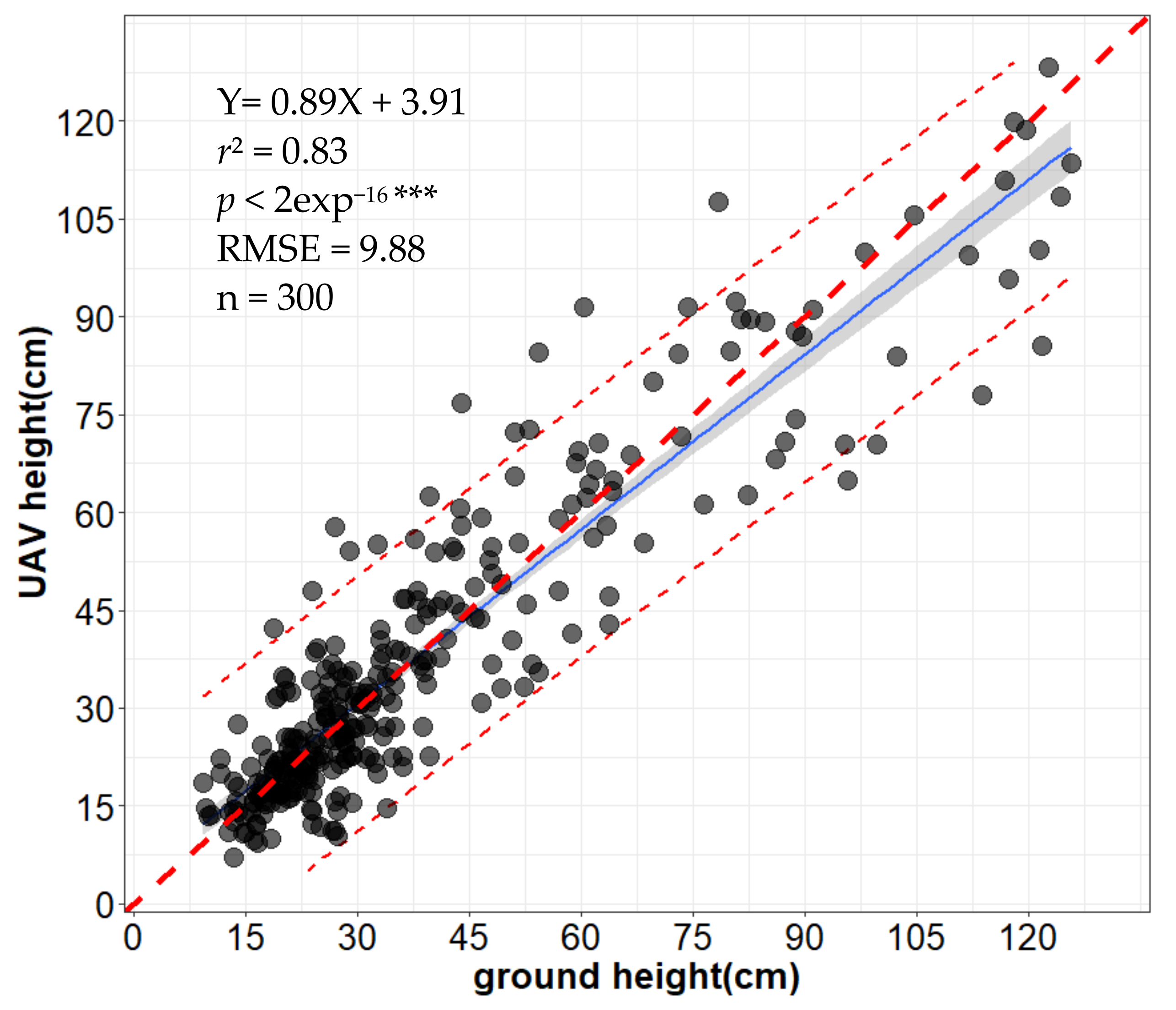
3.3. Assessment of UAV-Based Plant Height
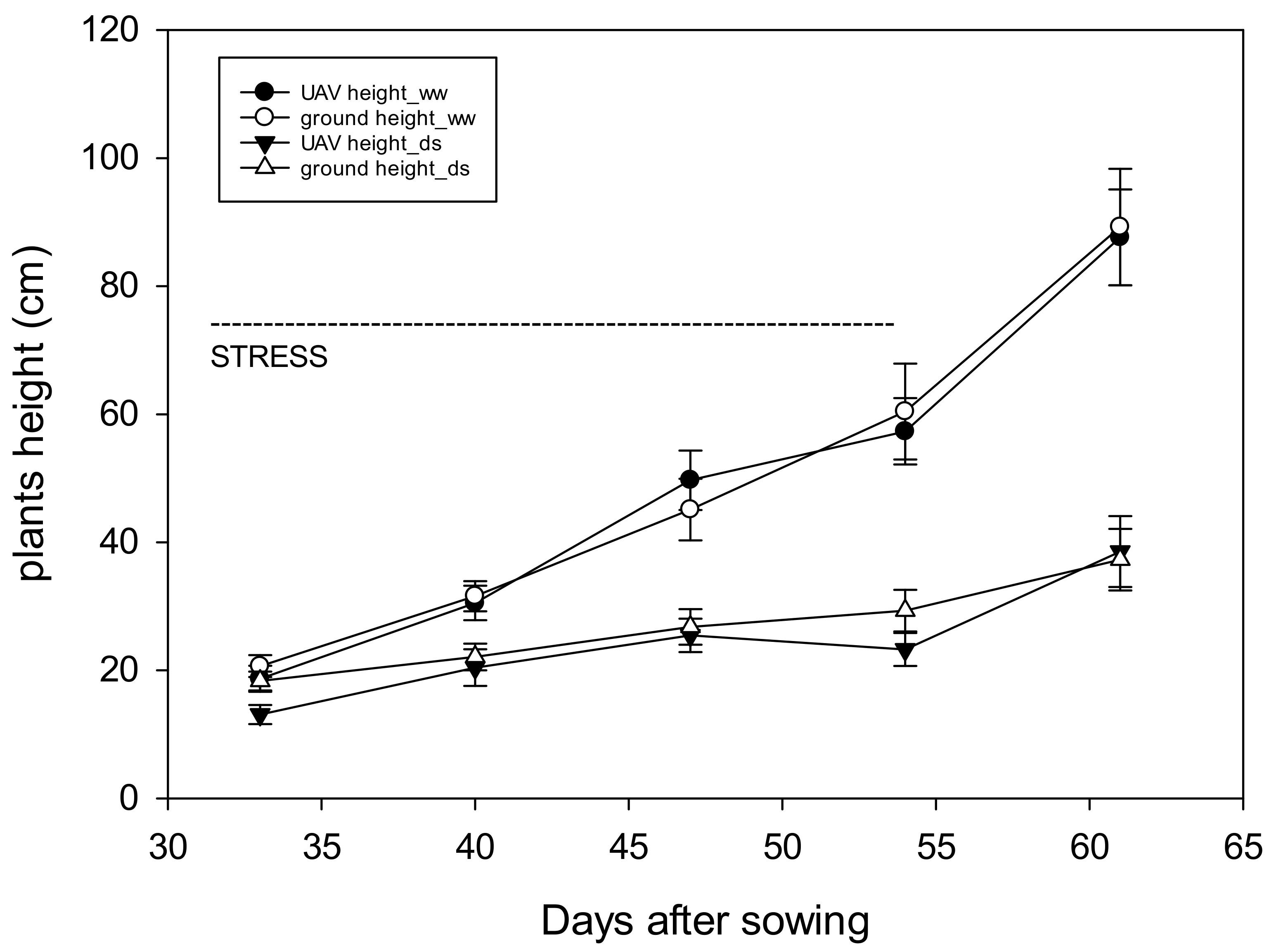
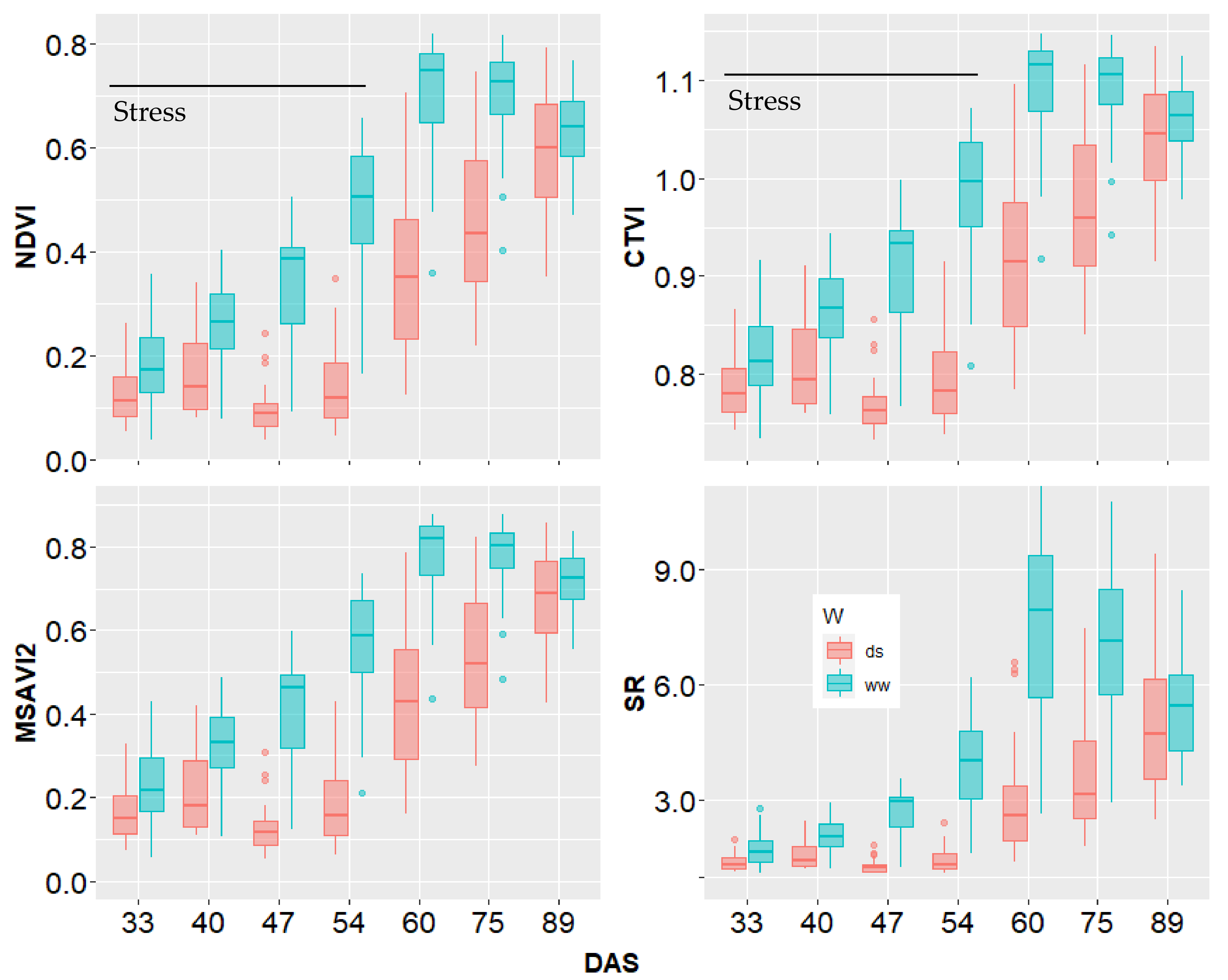
3.4. Monitoring Plant Development by UAV Based Vegetation Indices (VIs)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunter, M.C.; Smith, R.G.; Schipanski, M.E.; Atwood, L.W.; Mortensen, D.A. Agriculture in 2050: Recalibrating targets for sustainable intensification. Bioscience 2017, 67, 386–391. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I. The adaptation and mitigation potential of traditional agriculture in a changing climate. Clim. Chang. 2013, 140, 33–45. [Google Scholar] [CrossRef]
- Tari, I.; Laskay, G.; Takács, Z.; Poór, P. Response of sorghum to abiotic stresses: A review. J. Agron. Crop Sci. 2013, 199, 264–274. [Google Scholar] [CrossRef]
- Hadebe, S.T.; Modi, A.T.; Mabhaudhi, T. Drought Tolerance and Water Use of Cereal Crops: A Focus on Sorghum as a Food Security Crop in Sub-Saharan Africa. J. Agron. Crop Sci. 2017, 203, 177–191. [Google Scholar] [CrossRef]
- Billot, C.; Ramu, P.; Bouchet, S.; Chantereau, J.; Deu, M.; Gardes, L.; Noyer, J.L.; Rami, J.F.; Rivallan, R.; Li, Y.; et al. Massive Sorghum Collection Genotyped with SSR Markers to Enhance Use of Global Genetic Resources. PLoS ONE 2013, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Romana, K.K.; Chander, G.; Deshpande, S.; Gupta, R. Genomic-assisted enhancement in stress tolerance for productivity improvement in sorghum. Biotechnol. Crop Improv. 2018, 3, 265–288. [Google Scholar] [CrossRef]
- Bodian, A.; Ouattara, B.; Sarr, A.; Gano, B.; Sall, M.-N.; Ndir, K.-N.; Cissé, N.; Falalou, H. Genetic structure and diversity in Sorghum bicolor (L.) Moench landraces from marginal sorghum production lands in Senegal, based on SSR markers. J. Plant Breed. Genet. 2020, 7, 134–144. [Google Scholar] [CrossRef]
- Perrier, L.; Rouan, L.; Jaffuel, S.; Clément-Vidal, A.; Roques, S.; Soutiras, A.; Baptiste, C.; Bastianelli, D.; Fabre, D.; Dubois, C.; et al. Plasticity of sorghum stem biomass accumulation in response to water deficit: A multiscale analysis from internode tissue to plant level. Front. Plant Sci. 2017, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Belko, N.; Zaman-Allah, M.; Cisse, N.; Diop, N.N.; Zombre, G.; Ehlers, J.D.; Vadez, V. Lower soil moisture threshold for transpiration decline under water deficit correlates with lower canopy conductance and higher transpiration efficiency in drought-tolerant cowpea. Funct. Plant Biol. 2012, 39, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Neilson, E.H.; Edwards, A.M.; Blomstedt, C.K.; Berger, B.; Møller, B.L.; Gleadow, R.M. Utilization of a high-throughput shoot imaging system to examine the dynamic phenotypic responses of a C4 cereal crop plant to nitrogen and water deficiency over time. J. Exp. Bot. 2015, 66, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Gholipoor, M.; Sinclair, T.R.; Prasad, P.V.V. Genotypic variation within sorghum for transpiration response to drying soil. Plant Soil 2012, 357, 35–40. [Google Scholar] [CrossRef]
- Yan, G.; Hu, R.; Luo, J.; Weiss, M.; Jiang, H.; Mu, X.; Xie, D.; Zhang, W. Review of indirect optical measurements of leaf area index: Recent advances, challenges, and perspectives. Agric. For. Meteorol. 2019, 265, 390–411. [Google Scholar] [CrossRef]
- Larue, F.; Fumey, D.; Rouan, L.; Soulié, J.-C.; Roques, S.; Beurier, G.; Luquet, D. Modelling tiller growth and mortality as a sink-driven process using Ecomeristem: Implications for biomass sorghum ideotyping. Ann. Bot. 2019, 124, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, N.; Lee, S.; Mockler, T.C. High throughput phenotyping to accelerate crop breeding and monitoring of diseases in the field. Curr. Opin. Plant Biol. 2017, 38, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Eggen, M.; Ozdogan, M.; Zaitchik, B.; Ademe, D.; Foltz, J.; Simane, B. Vulnerability of sorghum production to extreme, sub-seasonal weather under climate change. Environ. Res. Lett. 2019, 14. [Google Scholar] [CrossRef]
- Borrell, A.K.; Mullet, J.E.; George-jaeggli, B.; Van Oosterom, E.J. Drought adaptation of stay-green sorghum is associated with canopy development, leaf anatomy, root growth, and water uptake. J. Exp. Bot. 2014, 65, 6251–6263. [Google Scholar] [CrossRef] [PubMed]
- Borrell, A.K.; Van Oosterom, E.J.; Mullet, J.E.; George-Jaeggli, B.; Jordan, D.R.; Klein, P.E.; Hammer, G.L. Stay-green alleles individually enhance grain yield in sorghum under drought by modifying canopy development and water uptake patterns. New Phytol. 2014, 203, 817–830. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Jin, Y.; Du, Y.L.; Wang, T.; Turner, N.C.; Yang, R.P.; Siddique, K.H.M.; Li, F.M. Genotypic variation in yield, yield components, root morphology and architecture, in soybean in relation to water and phosphorus supply. Front. Plant Sci. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Weiss, M.; Baret, F.; Smith, G.J.; Jonckheere, I.; Coppin, P. Review of methods for in situ leaf area index (LAI) determination Part II. Estimation of LAI, errors and sampling. Agric. For. Meteorol. 2004, 121, 37–53. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, X.; Zhu, X.; Dong, Z.; Guo, W. Estimation of biomass in wheat using random forest regression algorithm and remote sensing data. Crop J. 2016, 4, 212–219. [Google Scholar] [CrossRef]
- Hensgen, F.; Bühle, L.; Wachendorf, M. The effect of harvest, mulching and low-dose fertilization of liquid digestate on above ground biomass yield and diversity of lower mountain semi-natural grasslands. Agric. Ecosyst. Environ. 2016, 216, 283–292. [Google Scholar] [CrossRef]
- Huang, J.; Sedano, F.; Huang, Y.; Ma, H.; Li, X.; Liang, S.; Tian, L.; Zhang, X.; Fan, J.; Wu, W. Assimilating a synthetic Kalman filter leaf area index series into the WOFOST model to improve regional winter wheat yield estimation. Agric. For. Meteorol. 2016, 216, 188–202. [Google Scholar] [CrossRef]
- Barrero Farfan, I.D.; Murray, S.C.; Labar, S.; Pietsch, D. A multi-environment trial analysis shows slight grain yield improvement in Texas commercial maize. Field Crops Res. 2013, 149, 167–176. [Google Scholar] [CrossRef]
- Feng, W.; Guo, B.; Zhang, H.; He, L.; Zhang, Y.; Wang, Y.; Zhu, Y.; Guo, T. Remote estimation of above ground nitrogen uptake during vegetative growth in winter wheat using hyperspectral red-edge ratio data. Field Crops Res. 2015, 180, 197–206. [Google Scholar] [CrossRef]
- Li, J.; Shi, Y.; Veeranampalayam-Sivakumar, A.N.; Schachtman, D.P. Elucidating sorghum biomass, nitrogen and chlorophyll contents with spectral and morphological traits derived from unmanned aircraft system. Front. Plant Sci. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chapman, S.C.; Merz, T.; Chan, A.; Paul, J.; Hrabar, S.; Dreccer, M.F.; Holland, E.; Zheng, B.; Ling, T.J.; Jimenez-Berni, J. Pheno-Copter: A Low-Altitude, Autonomous Remote-Sensing Robotic Helicopter for High-Throughput Field-Based Phenotyping. Agronomy 2014, 4, 279–301. [Google Scholar] [CrossRef]
- Araus, L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19. [Google Scholar] [CrossRef]
- Ghanem, M.E.; Marrou, H.; Sinclair, T.R. Physiological phenotyping of plants for crop improvement. Trends Plant Sci. 2015, 20, 139–144. [Google Scholar] [CrossRef]
- Su, W.; Zhang, M.; Bian, D.; Liu, Z.; Huang, J.; Wang, W.; Wu, J.; Guo, H. Phenotyping of corn plants using unmanned aerial vehicle (UAV) images. Remote Sens. 2019, 11, 2021. [Google Scholar] [CrossRef]
- Han, X.; Thomasson, J.A.; Bagnall, G.C.; Pugh, N.A.; Horne, D.W.; Rooney, W.L.; Jung, J.; Chang, A.; Malambo, L.; Popescu, S.C.; et al. Measurement and Calibration of Plant-Height from Fixed-Wing UAV Images. Sensors 2018, 18, 4092. [Google Scholar] [CrossRef] [PubMed]
- Potgieter, A.B.; George-Jaeggli, B.; Chapman, S.C.; Laws, K.; Cadavid, L.A.S.; Wixted, J.; Watson, J.; Eldridge, M.; Jordan, D.R.; Hammer, G.L. Multi-Spectral Imaging from an Unmanned Aerial Vehicle Enables the Assessment of Seasonal Leaf Area Dynamics of Sorghum Breeding. Front. Plant Sci. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Bendig, J.; Bolten, A.; Bennertz, S.; Broscheit, J.; Eichfuss, S.; Bareth, G. Estimating biomass of barley using crop surface models (CSMs) derived from UAV-based RGB imaging. Remote Sens. 2014, 6, 10395–10412. [Google Scholar] [CrossRef]
- Shafian, S.; Rajan, N.; Schnell, R.; Bagavathiannan, M.; Valasek, J.; Shi, Y.; Olsenholler, J. Unmanned aerial systems-based remote sensing for monitoring sorghum growth and development. PLoS ONE 2018, 13, 1–15. [Google Scholar] [CrossRef]
- Viña, A.; Gitelson, A.A.; Nguy-Robertson, A.L.; Peng, Y. Comparison of different vegetation indices for the remote assessment of green leaf area index of crops. Remote Sens. Environ. 2011, 115, 3468–3478. [Google Scholar] [CrossRef]
- Haghighattalab, A.; González Pérez, L.; Mondal, S.; Singh, D.; Schinstock, D.; Rutkoski, J.; Ortiz-Monasterio, I.; Singh, R.P.; Goodin, D.; Poland, J. Application of unmanned aerial systems for high throughput phenotyping of large wheat breeding nurseries. Plant Methods 2016, 12, 1–15. [Google Scholar] [CrossRef]
- Ndiaye, M.; Adam, M.; Ganyo, K.K.; Guissé, A.; Cissé, N.; Muller, B. Genotype-environment interaction: Trade-Offs between the Agronomic Performance and Stability of Dual-Purpose Sorghum (Sorghum bicolor L. Moench) genotypes in Senegal. Agronomy 2019, 9, 867. [Google Scholar] [CrossRef]
- Ndiaye, M.; Adam, M.; Muller, B.; Guissé, A.; Cissé, N. Performances agronomiques et stabilité phénotypique de génotypes de Sorgho (Sorghum bicolor (L.) Moench) au Sénégal: Une étude des interactions. J. Appl. Biosci. 2018, 125, 12617–12629. [Google Scholar] [CrossRef][Green Version]
- Dembele, J.S.B.; Gano, B.; Vaksmann, M.; Dembele, L.L.; Doumbia, M.; Teme, N.; Diouf, D.; Audebert, A. Response of eight sorghum varieties to plant density and nitrogen fertilization in the Sudano-Sahelian zone in Mali. Afr. J. Agric. Res. 2020, 16, 1401–1410. [Google Scholar] [CrossRef]
- Gano, B.; Dembele, J.S.B.; Tovignan, T.K.; Sine, B.; Vadez, V.; Diouf, D.; Audebert, A. Adaptation Responses to Early Drought Stress of West Africa Sorghum Varieties. Agronomy 2021, 11, 443. [Google Scholar] [CrossRef]
- Iqbal, F.; Lucieer, A.; Barry, K. Simplified radiometric calibration for UAS-mounted multispectral sensor. Eur. J. Remote Sens. 2018, 51, 301–313. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2010. [Google Scholar]
- Zhang, Z.; Masjedi, A.; Zhao, J.; Crawford, M.M. Prediction of sorghum biomass based on image based features derived from time series of UAV images. In Proceedings of the International Geoscience and Remote Sensing Symposium (IGARSS), Fort Worth, TX, USA, 23–28 July 2017; pp. 6154–6157. [Google Scholar]
- Steven, M.D.; Malthus, T.J.; Baret, F. Toward Standardization of Vegetation Indices. In Remotely Sensed Data Characterization, Classification, and Accuracies; Thenkabail, P.S., Ed.; Taylor & Francis Group, LLC: Oxford, UK, 2015; pp. 175–193. ISBN 9781482217872. [Google Scholar]
- Shi, Y.; Murray, S.C.; Rooney, W.L.; Valasek, J.; Olsenholler, J.; Pugh, N.A.; Henrickson, J.; Bowden, E.; Zhang, D.; Thomasson, J.A. Corn and Sorghum Phenotyping Using a Fixed-Wing UAV-Based Remote Sensing System. In Autonomous Air and Ground Sensing Systems for Agricultural Optimization and Phenotyping; Valasek, J., Thomasson, J.A., Eds.; SPIE Digital Library: Bellingham, WA, USA, 2016; Volume 9866, ISBN 9781510601079. [Google Scholar]
- Rouse, J.W.; Hass, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. In Proceedings of the Third Earth Resources Technology Satellite (ERTS) Symposium, Washington, DC, USA, 10–14 December 1973; Volume 1, pp. 309–317. [Google Scholar]
- Perry, C.R.; Lautenschlager, L.F. Functional equivalence of spectral vegetation indices. Remote Sens. Environ. 1984, 14, 169–182. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote sensing of chlorophyll concentration in higher plant leaves. Adv. Sp. Res. 1998, 22, 689–692. [Google Scholar] [CrossRef]
- Qi, J.; Chehbouni, A.; Huete, A.R.; Kerr, Y.H.; Sorooshian, S. A modified soil adjusted vegetation index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Birth, G.S.; McVey, G.R. Measuring the Color of Growing Turf with a Reflectance Spectrophotometer. Agron. J. 1968, 60, 640–643. [Google Scholar] [CrossRef]
- Madec, S.; Baret, F.; De Solan, B.; Thomas, S.; Dutartre, D.; Jezequel, S.; Hemmerlé, M.; Colombeau, G.; Comar, A. High-throughput phenotyping of plant height: Comparing unmanned aerial vehicles and ground lidar estimates. Front. Plant Sci. 2017, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mclntosh, M.S. Analysis of combined experiments. Agron. J. 1983, 75, 153–155. [Google Scholar] [CrossRef]
- Bartlett, M.S. Properties of sufficiency and statistical tests. Proc. R. Soc. Lond. Ser. A 1937, 160, 268–282. [Google Scholar] [CrossRef]
- Fahlgren, N.; Gehan, M.A.; Baxter, I. ScienceDirect Lights, camera, action: High-throughput plant phenotyping is ready for a close-up. Curr. Opin. Plant Biol. 2015, 24, 93–99. [Google Scholar] [CrossRef]
- Reynolds, M.; Langridge, P. Physiological breeding. Curr. Opin. Plant Biol. 2016, 31, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Chapman, S.C.; Wang, X.; Potgieter, A.; Duan, T.; Jordan, D.; Guo, Y.; Zheng, B. Estimation of plant height using a high throughput phenotyping platform based on unmanned aerial vehicle and self-calibration: Example for sorghum breeding. Eur. J. Agron. 2018, 95, 24–32. [Google Scholar] [CrossRef]
- Yu, N.; Li, L.; Schmitz, N.; Tian, L.F.; Greenberg, J.A.; Diers, B.W. Development of methods to improve soybean yield estimation and predict plant maturity with an unmanned aerial vehicle based platform. Remote Sens. Environ. 2016, 187, 91–101. [Google Scholar] [CrossRef]
- Du, M.; Noguchi, N. Monitoring of wheat growth status and mapping of wheat yield’s within-field spatial variations using color images acquired from UAV-camera System. Remote Sens. 2017, 9, 289. [Google Scholar] [CrossRef]
- Shi, Y.; Alex Thomasson, J.; Murray, S.C.; Ace Pugh, N.; Rooney, W.L.; Shafian, S.; Rajan, N.; Rouze, G.; Morgan, C.L.S.; Neely, H.L.; et al. Unmanned aerial vehicles for high-throughput phenotyping and agronomic research. PLoS ONE 2016, 11, 1–26. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Myneni, R.B.; Hoffman, S.; Knyazikhin, Y.; Privette, J.L.; Glassy, J.; Tian, Y.; Wang, Y.; Song, X.; Zhang, Y.; Smith, G.R.; et al. Global products of vegetation leaf area and fraction absorbed PAR from year one of MODIS data. Remote Sens. Environ. 2002, 83, 214–231. [Google Scholar] [CrossRef]
- Huang, J.; Ma, H.; Su, W.; Zhang, X.; Huang, Y.; Fan, J.; Wu, W. Jointly Assimilating MODIS LAI and et Products into the SWAP Model for Winter Wheat Yield Estimation. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2015, 8, 4060–4071. [Google Scholar] [CrossRef]
- Mutanga, O.; Skidmore, A.K. Narrow band vegetation indices overcome the saturation problem in biomass estimation. Int. J. Remote Sens. 2004, 25, 3999–4014. [Google Scholar] [CrossRef]
- Singh, A.; Ganapathysubramanian, B.; Singh, A.K.; Sarkar, S. Machine Learning for High-Throughput Stress Phenotyping in Plants. Trends Plant Sci. 2016, 21, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhang, L.; Member, S.; Du, B.; Member, S.; Li, W.; Lai, Y.M. Band selection using improved sparse subspace clustering for hyperspectral imagery classification. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2015, 8, 2784–2797. [Google Scholar] [CrossRef]
- Varela, S.; Assefa, Y.; Vara Prasad, P.V.; Peralta, N.R.; Griffin, T.W.; Sharda, A.; Ferguson, A.; Ciampitti, I.A. Spatio-temporal evaluation of plant height in corn via unmanned aerial systems. J. Appl. Remote Sens. 2017, 11, 1. [Google Scholar] [CrossRef]
- Yue, J.; Yang, G.; Li, C.; Li, Z.; Wang, Y.; Feng, H.; Xu, B. Estimation of winter wheat above-ground biomass using unmanned aerial vehicle-based snapshot hyperspectral sensor and crop height improved models. Remote Sens. 2017, 9. [Google Scholar] [CrossRef]
- Chen, D.; Neumann, K.; Friedel, S.; Kilian, B.; Chen, M.; Altmann, T.; Klukas, C. Dissecting the phenotypic components of crop plant growthand drought responses based on high-throughput image analysisW open. Plant Cell 2014, 26, 4636–4655. [Google Scholar] [CrossRef]
- Seelig, H.D.; Hoehn, A.; Stodieck, L.S.; Klaus, D.M.; Adams, W.W.; Emery, W.J. The assessment of leaf water content using leaf reflectance ratios in the visible, near-, and short-wave-infrared. Int. J. Remote Sens. 2008, 29, 3701–3713. [Google Scholar] [CrossRef]
- Ramu, P.; Billot, C.; Rami, J.F.; Senthilvel, S.; Upadhyaya, H.D.; Ananda Reddy, L.; Hash, C.T. Assessment of genetic diversity in the sorghum reference set using EST-SSR markers. Theor. Appl. Genet. 2013, 126, 2051–2064. [Google Scholar] [CrossRef] [PubMed]
- Malambo, L.; Popescu, S.C.; Murray, S.C.; Putman, E.; Pugh, N.A.; Horne, D.W.; Richardson, G.; Sheridan, R.; Rooney, W.L.; Avant, R.; et al. Multitemporal field-based plant height estimation using 3D point clouds generated from small unmanned aerial systems high-resolution imagery. Int. J. Appl. Earth Obs. Geoinf. 2018, 64, 31–42. [Google Scholar] [CrossRef]
- Niederheiser, R.; Rutzinger, M.; Bremer, M.; Wichmann, V. Dense image matching of terrestrial imagery for deriving high-resolution topographic properties of vegetation locations in alpine terrain. Int. J. Appl. Earth Obs. Geoinf. 2018, 66, 146–158. [Google Scholar] [CrossRef]
- Moeckel, T.; Dayananda, S.; Nidamanuri, R.R.; Nautiyal, S.; Hanumaiah, N.; Buerkert, A.; Wachendorf, M. Estimation of vegetable crop parameter by multi-temporal UAV-borne images. Remote Sens. 2018, 10, 805. [Google Scholar] [CrossRef]
- Khan, Z.; Chopin, J.; Cai, J.; Eichi, V.R.; Haefele, S.; Miklavcic, S.J. Quantitative estimation of wheat phenotyping traits using ground and aerial imagery. Remote Sens. 2018, 10, 950. [Google Scholar] [CrossRef]
- Watanabe, K.; Guo, W.; Arai, K.; Takanashi, H.; Kajiya-kanegae, H. High-Throughput Phenotyping of Sorghum Plant Height Using an Unmanned Aerial Vehicle and Its Application to Genomic Prediction Modeling. Front. Plant Sci. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Hassan, M.A.; Yang, M.; Fu, L.; Rasheed, A.; Zheng, B.; Xia, X.; Xiao, Y.; He, Z. Accuracy assessment of plant height using an unmanned aerial vehicle for quantitative genomic analysis in bread wheat. Plant Methods 2019, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Magney, T.S.; Eitel, J.U.H.; Huggins, D.R.; Vierling, L.A. Proximal NDVI derived phenology improves in-season predictions of wheat quantity and quality. Agric. For. Meteorol. 2016, 217, 46–60. [Google Scholar] [CrossRef]
- Foster, A.J.; Kakani, V.G.; Mosali, J. Estimation of bioenergy crop yield and N status by hyperspectral canopy reflectance and partial least square regression. Precis. Agric. 2017, 18, 192–209. [Google Scholar] [CrossRef]
- Samborski, S.M.; Gozdowski, D.; Walsh, O.S.; Lamb, D.W.; Stępień, M.; Gacek, E.S.; Drzazga, T. Winter wheat genotype effect on canopy reflectance: Implications for using NDVI for in-season nitrogen topdressing recommendations. Agron. J. 2015, 107, 2097–2106. [Google Scholar] [CrossRef]
- Babar, M.A.; Reynolds, M.P.; Van Ginkel, M.; Klatt, A.R.; Raun, W.R.; Stone, M.L. Spectral reflectance indices as a potential indirect selection criteria for wheat yield under irrigation. Crop Sci. 2006, 46, 578–588. [Google Scholar] [CrossRef]
- Mulla, D.J. Twenty five years of remote sensing in precision agriculture: Key advances and remaining knowledge gaps. Biosyst. Eng. 2013, 114, 358–371. [Google Scholar] [CrossRef]
- Laudien, R.; Bareth, G.; Doluschitz, R. Multitemporal Hyperspectral Data Analysis for Regional Detection of Plant Stress by Using an Airborne-and Tractor-Based Spectrometer—Case Study: Sugar Beet Disease Rhizoctonia Solani. In Proceedings of the Analysis and Applications, International Society for Photogrammetry and Remote Sensing (ISPRS), Beijing, China, 17–19 October 2005; pp. 1–5. [Google Scholar]
- Sankaran, S.; Khot, L.R.; Espinoza, C.Z.; Jarolmasjed, S.; Sathuvalli, V.R.; Vandemark, G.J.; Miklas, P.N.; Carter, A.H.; Pumphrey, M.O.; Knowles, R.R.N.; et al. Low-altitude, high-resolution aerial imaging systems for row and field crop phenotyping: A review. Eur. J. Agron. 2015, 70, 112–123. [Google Scholar] [CrossRef]
- Yang, G.; Liu, J.; Zhao, C.; Li, Z.; Huang, Y. Unmanned Aerial Vehicle Remote Sensing for Field-Based Crop Phenotyping: Current Status and Perspectives. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
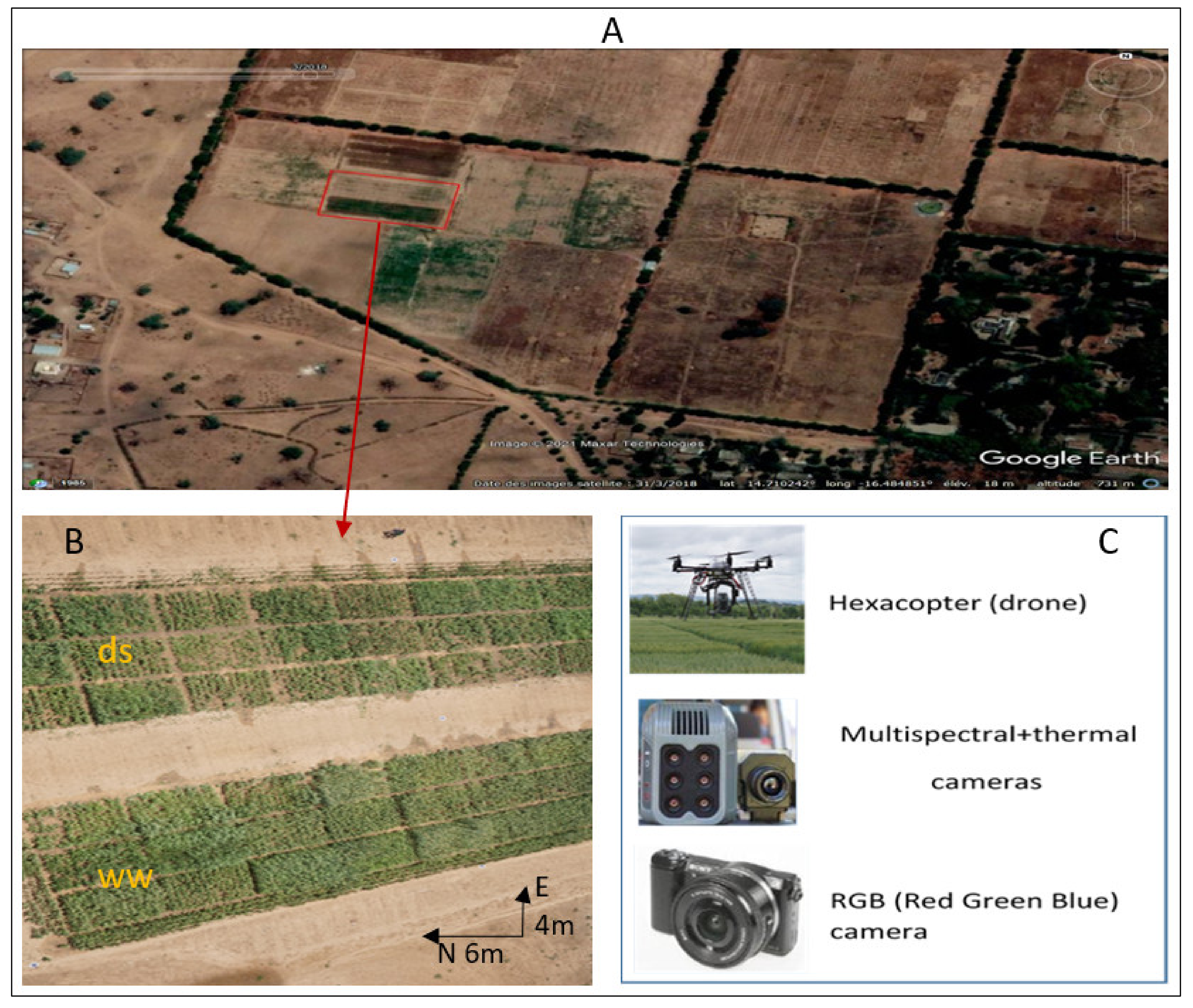



| Variety | Code | Type | Maturity (days) | Height (cm) | Potential Yield (t/ha) | Panicle Form | Photoperiod- Sensitivity | Isohyet (mm) | Origin |
|---|---|---|---|---|---|---|---|---|---|
| Fadda | V1 | Guinea (hybrid) | 128 | 200–300 | 4.5 | non compact | moderate | 700–1000 | Mali (IER/ICRISAT) |
| NIELENI | V2 | Guinea (hybrid) | 115 | 300 | 4 | semi compact | low | 700–800 | Mali (IER/ICRISAT) |
| IS15401 | V3 | Guinea-Caudatum | 115 | 400–450 | 2 | semi compact | high | 900–1200 | Mali (IER/ICRISAT) |
| PABLO | V4 | Guinea (hybrid) | 125 | 400 | 4 | non compact | moderate | 700–1000 | Mali (IER/ICRISAT) |
| CSM63E | V5 | Guinea | 90 | 400 | 2 | non compact | low | 600–1000 | Mali (IER/ICRISAT) |
| SK5912 | V6 | Caudatum | 170 | 200 | 2.5–3.5 | semi compact | high | 700–900 | Nigeria |
| GRINKAN | V7 | Caudatum | 90 | 120 | 4 | semi compact | non | 500–800 | Mali (IER/ICRISAT) |
| SOUMBA | V8 | Caudatum | 115 | 250 | 2.5 | semi compact | low | 600–1000 | Mali (IER/ICRISAT) |
| 621B | V9 | Caudatum | 105 | 175 | 2.5–3 | semi compact | non | 600–900 | Senegal (ISRA) |
| F2-20 | V10 | Caudatum | 110 | 210 | 3–5.3 | semi compact | low | 600–900 | Senegal (ISRA) |
| Vegetation Indices | Formulas | Ref. |
|---|---|---|
| Normalized Difference Vegetation Index | NDVI = (ρNIR − ρRed)/(ρNIR + ρRed) | [45] |
| The Corrected Transformed Vegetation Index | CTVI = [(NDVI + 0.5)/ABS(NDVI + 0.5)] × [√ABS(NDVI + 0.5)] | [46] |
| Green Normalized Difference Vegetation Index | GNDVI = (ρNIR − ρGreen)/(ρNIR + ρGreen) | [47] |
| Modified Soil-Adjusted Vegetation Index 2 model | MSAVI2 = [(2 × ρNIR + 1 − √((2 × ρNIR+1)2 − 8(ρNIR − ρRed))]/2 | [48] |
| Simple Ratio | SR = ρNIR/ρRed | [49] |
| Vegetation Indices | Regression Models | r | r2 | p-Value |
|---|---|---|---|---|
| NDVI | LAI = 0.3732 × exp (2.9648 × NDVI) | 0.91 | 0.83 | <0.001 |
| CTVI | LAI = 0.0069 × exp (5.5322 × CTVI) | 0.9 | 0.82 | <0.001 |
| MSAVI2 | LAI = 0.3392 × exp (2.7498 × MSAVI2) | 0.9 | 0.82 | <0.001 |
| GNDVI | LAI = 0.1841 × exp (4.9761 × GNDVI) | 0.87 | 0.76 | <0.001 |
| SR | LAI = 0.4438 × SR + 0.0126 | 0.87 | 0.77 | <0.001 |
| NDVI | Biomass = 3.1153 × exp (3.7021 × NDVI) | 0.77 | 0.6 | <0.001 |
| CTVI | Biomass = 0.0206 × exp (6.9446 × CTVI) | 0.77 | 0.6 | <0.001 |
| MSAVI2 | Biomass = 2.7357 × exp (3.4587 × GNDVI) | 0.77 | 0.6 | <0.001 |
| GNDVI | Biomass = 1.3612 × exp (6.0606 × MSAVI2) | 0.72 | 0.52 | <0.001 |
| SR | Biomass = 6.0128 × SR + 0.8947 | 0.68 | 0.47 | <0.001 |
| LAI | Biomass | |||||
|---|---|---|---|---|---|---|
| Vegetation Indices | r2 | RMSE | Signif. | r2 | RMSE | Signif. |
| NDVI | 0.92 | 0.31 | *** | 0.91 | 4.96 | *** |
| CTVI | 0.92 | 0.30 | *** | 0.92 | 4.50 | *** |
| MSAVI2 | 0.92 | 0.30 | *** | 0.92 | 4.40 | *** |
| GNDVI | 0.80 | 0.44 | *** | 0.77 | 7.06 | *** |
| SR | 0.89 | 0.37 | *** | 0.88 | 5.26 | *** |
| NDVI | CTVI | MSAVI2 | SR | |||||
|---|---|---|---|---|---|---|---|---|
| V | ww | ds | ww | ds | ww | ds | ww | ds |
| V1 | 0.63 | 0.38 | 1.06 | 0.92 | 0.71 | 0.46 | 6.83 | 3.00 |
| V2 | 0.68 | 0.37 | 1.08 | 0.91 | 0.76 | 0.44 | 6.77 | 3.00 |
| V3 | 0.73 | 0.59 | 1.11 | 0.94 | 0.81 | 0.66 | 7.97 | 4.02 |
| V4 | 0.70 | 0.33 | 1.09 | 0.89 | 0.77 | 0.40 | 7.53 | 2.71 |
| V5 | 0.71 | 0.39 | 1.10 | 0.94 | 0.79 | 0.47 | 7.91 | 2.85 |
| V6 | 0.62 | 0.36 | 1.05 | 0.89 | 0.70 | 0.42 | 5.69 | 3.24 |
| V7 | 0.74 | 0.28 | 1.11 | 0.88 | 0.81 | 0.35 | 8.59 | 2.20 |
| V8 | 0.74 | 0.45 | 1.11 | 0.94 | 0.81 | 0.53 | 8.17 | 4.46 |
| V9 | 0.70 | 0.40 | 1.09 | 0.94 | 0.78 | 0.49 | 6.99 | 2.92 |
| V10 | 0.67 | 0.20 | 1.08 | 0.83 | 0.75 | 0.25 | 7.33 | 1.74 |
| Mean | 0.69 | 0.37 | 1.08 | 0.90 | 0.76 | 0.44 | 7.37 | 3.01 |
| V | ** | ** | *** | * | ||||
| W | *** | *** | *** | *** | ||||
| V × W | * | * | * | * | ||||
| LAI_NDVI | LAI_CTVI | LAI_m | ||||
|---|---|---|---|---|---|---|
| V | ww | ds | ww | ds | ww | ds |
| V1 | 1.50 | 0.62 | 1.53 | 0.62 | 1.90 | 0.60 |
| V2 | 1.56 | 0.61 | 1.59 | 0.61 | 1.90 | 0.80 |
| V3 | 2.09 | 0.70 | 2.12 | 0.72 | 1.57 | 0.90 |
| V4 | 1.60 | 0.58 | 1.63 | 0.57 | 1.40 | 0.45 |
| V5 | 1.95 | 0.56 | 1.97 | 0.56 | 2.23 | 0.60 |
| V6 | 1.39 | 0.70 | 1.43 | 0.70 | 1.63 | 0.57 |
| V7 | 2.01 | 0.53 | 2.03 | 0.52 | 1.90 | 0.47 |
| V8 | 1.93 | 0.68 | 1.96 | 0.69 | 2.63 | 0.80 |
| V9 | 1.78 | 0.64 | 1.81 | 0.65 | 1.53 | 0.67 |
| V10 | 1.74 | 0.48 | 1.77 | 0.47 | 1.57 | 0.53 |
| Mean | 1.75 | 0.61 | 1.78 | 0.61 | 1.83 | 0.64 |
| V | * | * | ** | |||
| W | *** | *** | *** | |||
| V × W | * | * | * | |||
| Biomass_MSAVI2 | Biomass_SR | Biomass_m | ||||
|---|---|---|---|---|---|---|
| V | ww | ds | ww | ds | ww | ds |
| V1 | 17.62 | 5.53 | 21.12 | 9.69 | 18.30 | 15.70 |
| V2 | 18.27 | 5.38 | 22.08 | 9.54 | 22.55 | 10.79 |
| V3 | 25.85 | 6.65 | 27.56 | 10.50 | 19.11 | 12.36 |
| V4 | 18.73 | 4.96 | 22.38 | 9.01 | 17.38 | 7.90 |
| V5 | 23.94 | 4.73 | 26.10 | 8.93 | 29.82 | 11.84 |
| V6 | 15.77 | 6.62 | 20.49 | 10.49 | 23.87 | 11.39 |
| V7 | 24.47 | 4.35 | 28.25 | 8.57 | 35.72 | 8.00 |
| V8 | 23.59 | 6.32 | 26.84 | 10.36 | 27.32 | 9.21 |
| V9 | 21.52 | 5.76 | 24.51 | 9.87 | 22.49 | 18.88 |
| V10 | 21.00 | 3.75 | 24.55 | 7.91 | 18.28 | 5.86 |
| Mean | 21.08 | 5.42 | 24.39 | 9.50 | 23.48 | 11.31 |
| V | * | * | *** | |||
| W | *** | *** | *** | |||
| V × W | * | ** | *** | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gano, B.; Dembele, J.S.B.; Ndour, A.; Luquet, D.; Beurier, G.; Diouf, D.; Audebert, A. Using UAV Borne, Multi-Spectral Imaging for the Field Phenotyping of Shoot Biomass, Leaf Area Index and Height of West African Sorghum Varieties under Two Contrasted Water Conditions. Agronomy 2021, 11, 850. https://doi.org/10.3390/agronomy11050850
Gano B, Dembele JSB, Ndour A, Luquet D, Beurier G, Diouf D, Audebert A. Using UAV Borne, Multi-Spectral Imaging for the Field Phenotyping of Shoot Biomass, Leaf Area Index and Height of West African Sorghum Varieties under Two Contrasted Water Conditions. Agronomy. 2021; 11(5):850. https://doi.org/10.3390/agronomy11050850
Chicago/Turabian StyleGano, Boubacar, Joseph Sékou B. Dembele, Adama Ndour, Delphine Luquet, Gregory Beurier, Diaga Diouf, and Alain Audebert. 2021. "Using UAV Borne, Multi-Spectral Imaging for the Field Phenotyping of Shoot Biomass, Leaf Area Index and Height of West African Sorghum Varieties under Two Contrasted Water Conditions" Agronomy 11, no. 5: 850. https://doi.org/10.3390/agronomy11050850
APA StyleGano, B., Dembele, J. S. B., Ndour, A., Luquet, D., Beurier, G., Diouf, D., & Audebert, A. (2021). Using UAV Borne, Multi-Spectral Imaging for the Field Phenotyping of Shoot Biomass, Leaf Area Index and Height of West African Sorghum Varieties under Two Contrasted Water Conditions. Agronomy, 11(5), 850. https://doi.org/10.3390/agronomy11050850








