RNA-Seq Transcriptome Analysis of Potato with Differential Tolerance to Bentazone Herbicide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Potato Seedling Resistance to Bentazone
2.3. Determination of Photosynthetic Rate in Bentazone-Treated Potato Seedlings
2.4. Determination of Bentazone Residues in Bentazone-Treated Potato Seedlings
2.5. Pretranscriptome Sample Preparation
2.6. Transcriptome Sequencing and Assembly
2.7. Differential Gene Expression
2.8. Validation of Expression Using Quantitative Real-Time Polymerase Chain Reaction
3. Results
3.1. Difference Analysis of Potato Seedling Resistance to Bentazone
3.2. Effects of Bentazone on the Photosynthetic Rate of Sensitive and Resistant Potato Seedlings
3.3. Bentazone Residues in Sensitive and Resistant Potato Seedlings After Treatment
3.4. Quality Identification of Potato Seedlings with Different Resistance Levels
3.5. Principal Component Analysis
3.6. mRNA Differential Expression Analysis
3.7. Pathway Enrichment Analysis
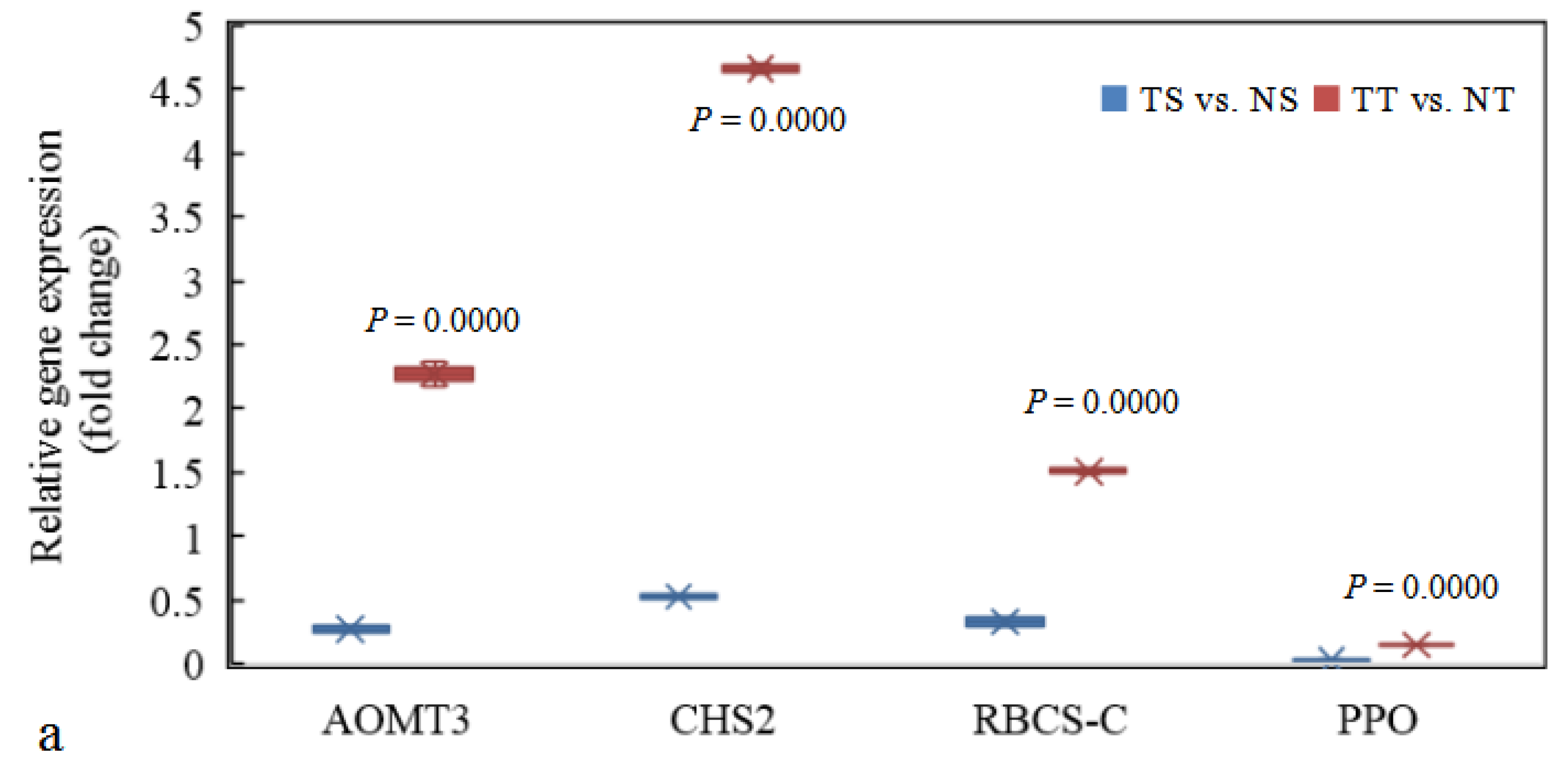
3.8. Identification and q-PCR Validation of DEGs
4. Discussion
4.1. Effects of Bentazone Application on Sensitive and Resistant Potato Varieties
4.2. DEGs in Bentazone-Resistant and -Susceptible Potato Seedlings
4.3. Screening of DEGs in Resistant Potato Plants
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, R.; Marshall, D.; Bryan, G.J.; Hornyik, C. Identification and characterization of miRNA transcriptome in potato by high-throughput sequencing. PLoS ONE 2013, 8, e57233. [Google Scholar] [CrossRef] [PubMed]
- Crookshanks, M.; Emmersen, J.; Welinder, K.G.; Nielsen, K.L. The potato tuber transcriptome: Analysis of 6077 expressed sequence tags. FEBS Lett. 2001, 506, 123–126. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Pan, S.; Cheng, S.; Zhang, B.; Mu, D.; Ni, P.; Zhang, G.; Yang, S.; Li, R.; Wang, J.; et al. Genome sequence and analysis of tuber crop potato. Nature 2011, 475, 189–195. [Google Scholar] [PubMed] [Green Version]
- Salmensuu, O. Macroeconomic trends and factors of production affecting potato producer prices in developing countries. J. Dev. Areas 2021, 55. [Google Scholar] [CrossRef]
- Tao, B.; Sun, S.; Zhang, L.; Guo, J.; Shao, B. Production and assessment of potato material resistant to the broadleaf herbicide bentazone. Potato Res. 2020. [Google Scholar] [CrossRef]
- Americanos, P.G. Post-Emergence Herbicides against Galium in Potatoes in Technical Bulletin; Press and Information Office: Nicosica, Cyprus, 1995; Volume 172. [Google Scholar]
- Huber, R.; Otto, S. Environmental behavior of bentazon herbicide. Rev. Environ. Contam. Toxicol. 1994, 137, 111–134. [Google Scholar] [CrossRef]
- Ponnam, V.; Katari, N.K.; Mandapati, R.N.; Nannapaneni, S.; Tondepu, S.; Jonnalagadda, S.B. Efficacy of biochar in removal of organic pesticide, bentazone from watershed systems. J. Environ. Sci. Health B 2020, 55, 396–405. [Google Scholar] [CrossRef]
- Lundegårdh, B. Differences in photosynthesis between a diuron-resistant, an atrazine-resistant, a bentazon-resistant and a susceptible biotype of the green alga Monoraphidium pusillum (Printz). In Progress in Photosynthesis Research; Springer: Dordrecht, The Netherlands, 1987; pp. 823–826. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Q.; Chen, H.; Pan, G.; Xiao, S.; Dai, Y.; Li, Q.; Zhang, J.; Wu, X.; Wu, J.; et al. Identification of a cytochrome P450 hydroxylase, CYP81A6, as the candidate for the bentazon and sulfonylurea herbicide resistance gene, Bel, in rice. Mol. Breed. 2006, 19, 59–68. [Google Scholar] [CrossRef]
- Liu, C.; Liu, S.; Wang, F.; Wang, Y.; Liu, K. Expression of a riceCYP81A6gene confers tolerance to bentazon and sulfonylurea herbicides in both Arabidopsis and tobacco. Plant Cell Tiss. Organ Cult. 2012, 109, 419–428. [Google Scholar] [CrossRef]
- Nicot, N.; Hausman, J.-F.; Hoffmann, L.; Evers, D. Housekeeping gene selection for real-time RT-PCR nor malization in potato during biotic and abiotic stress. J. Exp. Bot. 2005, 56, 2907–2914. [Google Scholar] [CrossRef]
- Salas-Perez, R.A.; Saski, C.A.; Noorai, R.E.; Srivastava, S.K.; Lawton-Rauh, A.L.; Nichols, R.L.; Roma-Burgos, N. RNA-Seq transcriptome analysis of Amaranthus palmeri with differential tolerance to glufosinate herbicide. PLoS ONE 2018, 13, e0195488. [Google Scholar] [CrossRef]
- Zeng, B.; Zhang, Y.; Zhang, A.; Qiao, D.; Ren, J.; Li, M.; Cai, K.; Zhang, J.; Huang, L. Transcriptome profiling of two Dactylis glomerata L. cultivars with different tolerance in response to submergence stress. Phytochemistry 2020, 175, 112378. [Google Scholar] [CrossRef] [PubMed]
- Etebari, K.; Lindsay, K.R.; Ward, A.L.; Furlong, M.J. Australian sugarcane soldier fly’s salivary gland transcriptome in response to starvation and feeding on sugarcane crops. Insect Sci. 2020, 27, 708–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.S.; Sun, M.N.; Lu, X.Z.; Gao, T.K.C.; Yang, J.B. HPLC determination of bentazon and its metabolites. Agrochemicals 2006, 45, 113–115. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [Green Version]
- DeLuca, D.S.; Levin, J.Z.; Sivachenko, A.; Fennell, T.; Nazaire, M.-D.; Williams, C.; Reich, M.; Winckler, W.; Getz, G. RNA-SeQC: RNA-seq metrics for quality control and process optimization. Bioinformatics 2012, 28, 1530–1532. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaines, T.A.; Lorentz, L.; Figge, A.; Herrmann, J.; Maiwald, F.; Ott, M.C.; Han, H.; Busi, R.; Yu, Q.; Powles, S.B.; et al. RNA-Seq transcriptome analysis to identify genes involved in metabolism-based diclofop resistance in Lolium rigidum. Plant J. 2014, 78, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Ma, Z.; Chen, H.; Liu, M. MYB gene family in potato (Solanum tuberosum L.): Genome-wide identification of hormone-responsive reveals their potential functions in growth and development. Int. J. Mol. Sci. 2019, 20, 4847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, Z.; Seleem, M.N.; Hussain, H.I.; Huang, L.; Hao, H.; Yuan, Z. Comparative virulence studies and transcriptome analysis of Staphylococcus aureus strains isolated from animals. Sci. Rep. 2016, 6, 35442. [Google Scholar] [CrossRef] [PubMed]
- Škavić, P.; Duraković, Z.; Nestić, M. Lethal bentazone intoxication-A report of two cases. J. Forensic Sci. 2018, 63, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Patzoldt, W.L.; Radwan, O.; Tranel, P.J.; Clough, S.J. Effects of photosystem-II-interfering herbicides atrazine and bentazon on the soybean transcriptome. Plant Genome 2009, 2, 191–205. [Google Scholar] [CrossRef] [Green Version]
- Bradshaw, L.D.; Barrett, M.; Poneleit, C.G. Physiological basis for differential bentazon susceptibility among corn (Zea mays) inbreds. Weed Sci. 1992, 40, 522–527. [Google Scholar] [CrossRef]
- Sterling, T.M.; Balke, N.E. Bentazon uptake and metabolism by cultured plant cells in the presence of monooxygenase inhibitors and cinnamic acid. Pestic. Biochem. Physiol. 1990, 38, 66–75. [Google Scholar] [CrossRef]
- Zuluaga, A.P.; Solé, M.; Lu, H.; Góngora-Castillo, E.; Vaillancourt, B.; Coll, N.S.; Buell, C.R.; Valls, M. Transcriptome responses to Ralstonia solanacearum infection in the roots of the wild potato Solanum commersonii. BMC Genomics. 2015, 16, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.-K. Cell signaling under salt, water and cold stresses. Curr. Opin. Plant Biol. 2001, 4, 401–406. [Google Scholar] [CrossRef]
- Délye, C. Unravelling the genetic bases of non-target-site-based resistance (NTSR) to herbicides: A major challenge for weed science in the forthcoming decade. Pest Manag. Sci. 2013, 69, 176–187. [Google Scholar] [CrossRef]
- Mayer, A.M. Polyphenol oxidases in plants and fungi: Going places? A review. Phytochemistry 2006, 67, 2318–2331. [Google Scholar] [CrossRef]
- Pialis, P.; Saville, B.A. Production of L-dopa from tyrosinase immobilized on nylon 6,6: Enzyme stability and scale-up. Enzyme Microb. Technol. 1998, 22, 261–268. [Google Scholar] [CrossRef]
- Trebst, A.; Department, K. B Polyphenol oxidase and photosynthesis research. Photosynth. Res. 1995, 46, 414–432. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, K.C.; Lax, A.R.; Duke, S.O. Polyphenol oxidase: The chloroplast oxidase with no established function. Physiol. Plant. 1988, 72, 659–665. [Google Scholar] [CrossRef]
- Qi, J.; Li, G.-Q.; Dong, Z.; Zhou, W. Transformation of tobacco plants by Yali PPO-GFP fusion gene and observation of subcellular localization. Am. J. Transl. Res. 2016, 8, 698–704. [Google Scholar] [PubMed]
- Ali, L.; Jo, H.; Song, J.T.; Lee, J.-D. The prospect of bentazone-tolerant soybean for conventional cultivation. Agronomy 2020, 10, 1650. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yang, L.Z.; Wang, T.; Wang, R.R.; Liu, J.; Shan, Y.; Zhang, Q.; Ding, S.H. Recent progress toward understanding the physiological function, purification, and enzymatic browning control of plant polyphenol oxidases. Food Sci. 2020, 41, 222–237. [Google Scholar]
- Cai, Z.; Liu, G.; Zhang, J.; Li, Y. Development of an activity-directed selection system enabled significant improvement of the carboxylation efficiency of RuBisCO. Protein Cell 2014, 5, 552–562. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, J.; Cao, K.; Xu, C.; Cao, W. An expressed sequence tags analysis for leaves of Chinese milk vetch (Astragalus sinicus). Legu. Rese. Inter. Jour. 2015, 38, 1–8. [Google Scholar] [CrossRef]
- Frukh, A.; Siddiqi, T.O.; Khan, M.I.R.; Ahmad, A. Modulation in growth, biochemical attributes and proteome profile of rice cultivars under salt stress. Plant Physiol. Biochem. 2020, 146, 55–70. [Google Scholar] [CrossRef]
- Henry-Kirk, R.A.; Plunkett, B.; Hall, M.; Mcghie, T.; Allan, A.C.; Wargent, J.J.; Espley, R.V. Solar UV light regulates flavonoid metabolism in apple(Malusxdomestica). Plant Cell Environ. 2018, 41, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Li, W.; Zhang, X.; Tang, Y.; Zhao, Z.; Gu, Y.; Qi, G.; Guo, S. Transcriptome sequencing and de novo assembly in red raspberry fruit development to elucidates the secondary metabolite pathways. J. Berry Res. 2020, 10, 497–511. [Google Scholar] [CrossRef]
- Silva-Navas, J.; Moreno-Risueno, M.A.; Manzano, C.; Téllez-Robledo, B.; Navarro-Neila, S.; Carrasco, V.; Pollmann, S.; Gallego, F.J.; Del Pozo, J.C. Flavonols mediate root phototropism and growth through regulation of proliferation-to-differentiation transition. Plant Cell 2016, 28, 1372–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, X.Q.; Du, Z.J.; Ding, X.M.; Jiang, W.B. Protective effects of dietary flavonoids against pesticide-induced toxicity: A review. Trends Food Sci. Tech. 2021, 109, 271–279. [Google Scholar] [CrossRef]
- Jhonsa, D.J.; Badgujar, L.B.; Sutariya, B.K.; Saraf, M.N. Neuroprotective effect of flavonoids against paraquat induced oxidative stress and neurotoxicity in Drosophila melanogaster. Curr. Top. Nutraceut. R. 2019, 14, 283–293. [Google Scholar]
- Yang, M.; Wang, L.; Belwal, T.; Zhang, X.; Lu, H.; Chen, C.; Li, L. Exogenous melatonin and abscisic acid expedite the flavonoids biosynthesis in grape berry of Vitis vinifera cv. Kyoho Mol. 2019, 25, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettinga, D.J.; Ou, J.; Patterson, E.L.; Jugulam, M.; Westra, P.; Gaines, T.A. Increased chalcone synthase (CHS) expression is associated with dicamba resistance in Kochia scoparia. Pest Manag. Sci. 2018, 74, 2306–2315. [Google Scholar] [CrossRef]







| Treatment Concentration (g a.i./ha) | Varieties | Survival Rate (%) |
|---|---|---|
| 1296 | 4–0 | 12 |
| 4–19 | 90 | |
| 648 | 4–0 | 62 |
| 4–19 | 96 | |
| 324 | 4–0 | 98 |
| 4–19 | 100 |
| Different Varieties | Treatment Concentration (g a.i./ha) | ||
|---|---|---|---|
| 1296 | 648 | 324 | |
| 4–0 | XXX | XX | − |
| 4–19 | X | − | − |
| Level of Gene Expression | Number of Differentially Expressed Genes | |||||||
|---|---|---|---|---|---|---|---|---|
| NT vs. NS | TS vs. NS | TT vs. NT | TT vs. TS | |||||
| Up | Down | Up | Down | Up | Down | Up | Down | |
| >1–2 | 1251 | 757 | 2700 | 2651 | 916 | 648 | 2594 | 2590 |
| >2–3 | 315 | 176 | 1184 | 1364 | 129 | 136 | 1490 | 1184 |
| >3–4 | 83 | 47 | 600 | 695 | 35 | 40 | 844 | 620 |
| >4–5 | 17 | 15 | 331 | 321 | 12 | 12 | 455 | 309 |
| >5 | 3 | 5 | 359 | 176 | 1 | 9 | 258 | 386 |
| ±inf | 27 | 7 | 155 | 125 | 4 | 21 | 125 | 169 |
| Total | 1696 | 1007 | 5329 | 5332 | 1097 | 866 | 5766 | 5258 |
| 2703 | 10,661 | 1963 | 11,024 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Song, X.; Sun, S.; Shao, B.; Tao, B.; Zhang, L. RNA-Seq Transcriptome Analysis of Potato with Differential Tolerance to Bentazone Herbicide. Agronomy 2021, 11, 897. https://doi.org/10.3390/agronomy11050897
Guo J, Song X, Sun S, Shao B, Tao B, Zhang L. RNA-Seq Transcriptome Analysis of Potato with Differential Tolerance to Bentazone Herbicide. Agronomy. 2021; 11(5):897. https://doi.org/10.3390/agronomy11050897
Chicago/Turabian StyleGuo, Jing, Xiuli Song, Shiqi Sun, Baihui Shao, Bo Tao, and Lili Zhang. 2021. "RNA-Seq Transcriptome Analysis of Potato with Differential Tolerance to Bentazone Herbicide" Agronomy 11, no. 5: 897. https://doi.org/10.3390/agronomy11050897





