Diversity in Phenological and Agronomic Traits of Miscanthus sinensis Collected in Korea and Eastern Asia
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of M. sinensis Accessions
2.2. Field Experiment
2.3. Assessment of Phenological and Agronomic Traits
2.4. Statistical Analysis
3. Results and Discussion
3.1. Diversity in Phenological and Agronomic Traits
3.2. Relationships between Latitude of Collection Site and Phenotypic Traits
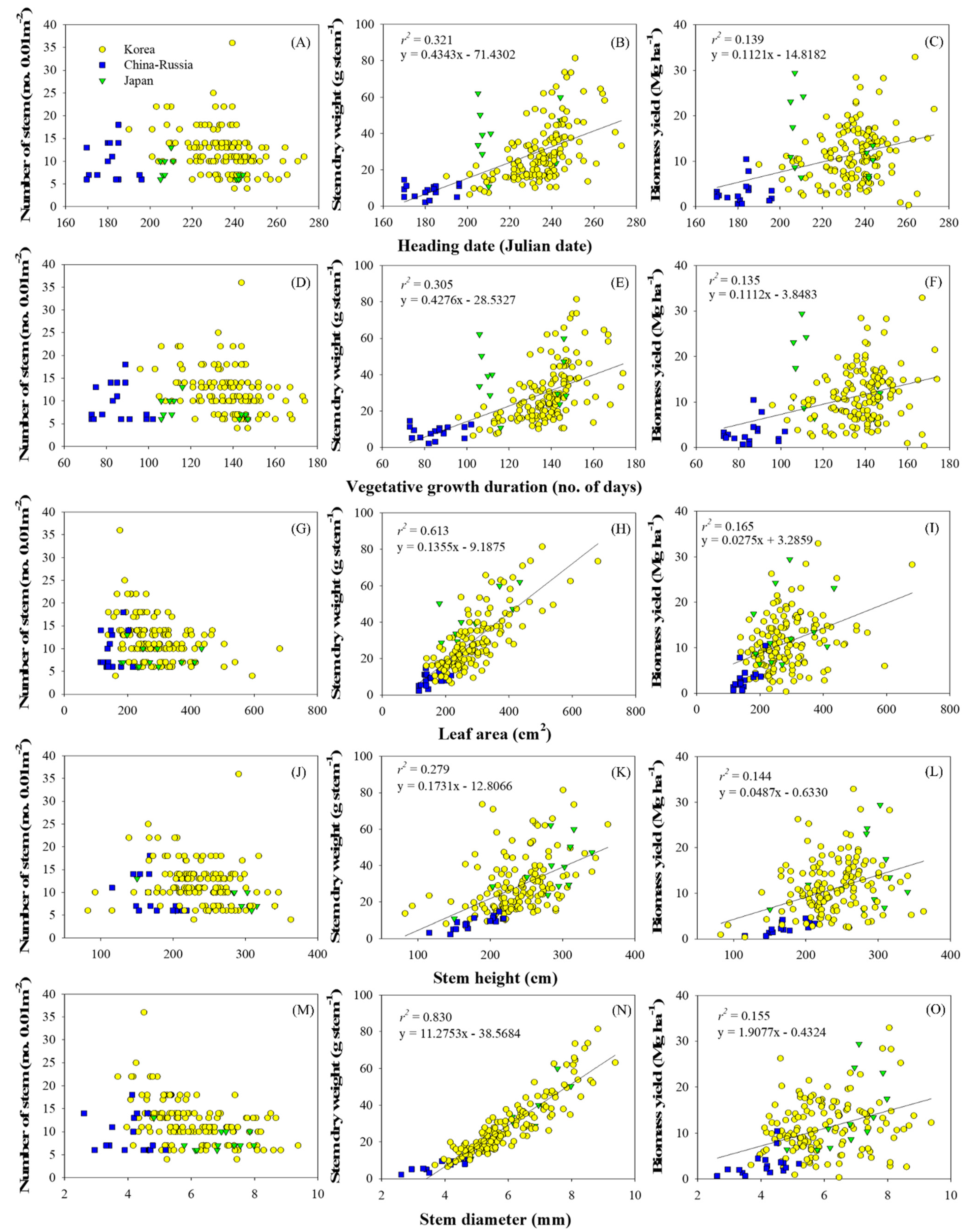
3.3. Relationships between Phenological and Agronomic Traits in Association with Biomass Yield
3.4. Early Traits Determining Biomass Yield Potential of Miscanthus
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dohleman, F.G.; Long, S.P. More Productive Than Maize in the Midwest: How Does Miscanthus Do It? Plant Physiol. 2009, 150, 2104–2115. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.-H.; Kim, D.-S. Miscanthus as a potential bioenergy crop in East Asia. J. Crop. Sci. Biotechnol. 2012, 15, 65–77. [Google Scholar] [CrossRef]
- Danalatos, N.G.; Archontoulis, S.V.; Mitsios, I.K. Potential growth and biomass productivity of Miscanthus × giganteus as affected by plant density and N-fertilization in central Greece. Biomass Bioenergy 2007, 31, 145–152. [Google Scholar] [CrossRef]
- Schwarz, H.; Liebhard, P.; Ehrendorfer, K.; Ruckenbauer, P. The effect of fertilization on yield and quality of Miscanthus sinensis ‘Giganteus’. Ind. Crop. Prod. 1994, 2, 153–159. [Google Scholar] [CrossRef]
- Hodkinson, T.R.; Chase, M.W.; Renvoize, S.A. Characterization of a Genetic Resource Collection for Miscanthus (Saccharinae, Andropogoneae, Poaceae) using AFLP and ISSR PCR. Ann. Bot. 2002, 89, 627–636. [Google Scholar] [CrossRef]
- Wühlisch, G.; Deuter, M.; Muhs, H.-J. Identifizierung verschiedener Miscanthus-Sorten mittels Isoenzymen. J. Agron. Crop. Sci. 1994, 172, 247–254. [Google Scholar] [CrossRef]
- Clifton-Brown, J.C.; Lewandowski, I.; Andersson, B.; Basch, G.; Christian, D.G.; Kjeldsen, J.B.; Jørgensen, U.; Mortensen, J.V.; Riche, A.B.; Schwarz, K.U.; et al. Performance of 15 Miscanthus Genotypes at Five Sites in Europe. Agron. J. 2001, 93, 1013–1019. [Google Scholar] [CrossRef]
- Ings, J.; Mur, L.A.J.; Robson, P.R.H.; Bosch, M. Physiological and growth responses to water deficit in the bioenergy crop Miscanthus × giganteus. Front. Plant. Sci. 2013, 4, 1–12. [Google Scholar] [CrossRef]
- Jahn, C.E.; McKay, J.K.; Mauleon, R.; Stephens, J.; McNally, K.L.; Bush, D.R.; Leung, H.; Leach, J.E. Genetic Variation in Biomass Traits among 20 Diverse Rice Varieties. Plant Physiol. 2011, 155, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Amini, F.; Saeidi, G.; Arzani, A. Study of genetic diversity in safflower genotypes using agro-morphological traits and RAPD markers. Euphytica 2007, 163, 21–30. [Google Scholar] [CrossRef]
- Kearsey, M.J.; Pooni, H.S. The Genetical Analysis of Quantitative Traits; Stanley Thornes (Publishers) Ltd.: Cheltenham, UK, 1998. [Google Scholar]
- Yook, M.J.; Lim, S.-H.; Song, J.-S.; Kim, J.-W.; Zhang, C.-J.; Lee, E.J.; Ibaragi, Y.; Lee, G.-J.; Nah, G.; Kim, D.-S. Assessment of genetic diversity of Korean Miscanthus using morphological traits and SSR markers. Biomass-Bioenergy 2014, 66, 81–92. [Google Scholar] [CrossRef]
- Nah, G.; Im, J.-H.; Lim, S.-H.; Kim, K.; Choi, A.Y.; Yook, M.J.; Kim, S.; Kim, C.; Kim, D.-S. Complete chloroplast genomes of two Miscanthus species. Mitochondrial DNA Part A 2015, 27, 4359–4360. [Google Scholar] [CrossRef]
- Chae, W.B.; Hong, S.J.; Gifford, J.M.; Rayburn, A.L.; Sacks, E.J.; Juvik, J.A. Plant morphology, genome size, and SSR markers differentiate five distinct taxonomic groups among accessions in the genus Miscanthus. GCB Bioenergy 2014, 6, 646–660. [Google Scholar] [CrossRef]
- Clark, L.V.; Brummer, J.E.; Głowacka, K.; Hall, M.C.; Heo, K.; Peng, J.; Yamada, T.; Yoo, J.H.; Yu, C.Y.; Zhao, H.; et al. A footprint of past climate change on the diversity and population structure of Miscanthus sinensis. Ann. Bot. 2014, 114, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Anzoua, K.G.; Suzuki, K.; Fujita, S.; Toma, Y.; Yamada, T. Evaluation of morphological traits, winter survival and biomass potential in wild Japanese Miscanthus sinensis Anderss. populations in northern Japan. Grassl. Sci. 2015, 61, 83–91. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, B.; He, J.; Yang, J.; Pan, L.; Sun, D.; Peng, J. Genetic Diversity and Population Structure of Miscanthus sinensis Germplasm in China. PLoS ONE 2013, 8, e75672. [Google Scholar] [CrossRef] [PubMed]
- Clifton-Brown, J.; Lewandowski, I. Screening Miscanthus genotypes in field trials to optimise biomass yield and quality in Southern Germany. Eur. J. Agron. 2002, 16, 97–110. [Google Scholar] [CrossRef]
- Jezowski, S.; Głowacka, K.; Kaczmarek, Z. Variation on biomass yield and morphological traits of energy grasses from the genus Miscanthus during the first years of crop establishment. Biomass- Bioenergy 2011, 35, 814–821. [Google Scholar] [CrossRef]
- Clifton-Brown, J.; Neilson, B.; Lewandowski, I.; Jones, M. The modelled productivity of Miscanthus × giganteus (GREEF et DEU) in Ireland. Ind. Crop. Prod. 2000, 12, 97–109. [Google Scholar] [CrossRef]
- Robson, P.R.H.; Jensen, E.F.; Hawkins, S.; White, S.R.; Kenobi, K.; Clifton-Brown, J.C.; Donnison, I.S.; Farrar, K. Accelerating the domestication of a bioenergy crop: Identifying and modelling morphological targets for sustainable yield increase in Miscanthus. J. Exp. Bot. 2013, 64, 4143–4155. [Google Scholar] [CrossRef]
- Robson, P.R.; Farrar, K.; Gay, A.P.; Jensen, E.F.; Clifton-Brown, J.C.; Donnison, I.S. Variation in canopy duration in the perennial biofuel crop Miscanthus reveals complex associations with yield. J. Exp. Bot. 2013, 64, 2373–2383. [Google Scholar] [CrossRef] [PubMed]
- Robson, P.R.H.; Mos, M.; Clifton-Brown, J.; Donnison, I.S. Phenotypic variation in senescence in Miscanthus: Towards optimising biomass quality and quantity. Bioenerg. Res. 2012, 5, 95–105. [Google Scholar] [CrossRef]
- Greef, J.; Deuter, M. Syntaxonomy of Miscanthus × giganteus GREEF et DEU. Angew. Bot. 1993, 67, 87–90. [Google Scholar]
- Bolaños, J.; Edmeades, G. Eight cycles of selection for drought tolerance in lowland tropical maize. II. Responses in reproductive behavior. Field Crop. Res. 1993, 31, 253–268. [Google Scholar] [CrossRef]
- Witcombe, J.; Joshi, A.; Goyal, S. Participatory plant breeding in maize: A case study from Gujarat, India. Euphytica 2003, 130, 413–422. [Google Scholar] [CrossRef]
- Murray, S.C.; Jessup, R.W. Breeding and Genetics of Perennial Maize. Perennial Crops for Food Security; Department of Soil and Crop Sciences: College Station, TX, USA, 2014; pp. 103–111. [Google Scholar]
- Casler, M.D. Switchgrass Breeding, Genetics, and Genomics. In Switchgrass: A Valuable Biomass Crop for Energy; Monti, A., Ed.; Springer: London, UK, 2012; pp. 29–53. [Google Scholar]
- Song, J.-S.; Lim, S.-H.; Lim, Y.; Nah, G.; Lee, D.K.; Kim, D.-S. Herbicide-based Weed Management in Miscanthus sacchariflorus. BioEnergy Res. 2016, 9, 326–334. [Google Scholar] [CrossRef]
- Lim, S.-H.; Yook, M.J.; Kim, J.-W.; Song, J.-S.; Zhang, C.-J.; Nah, G.; Kim, D.-S. Genetic diversity in agronomic traits associated with the biomass production of Miscanthus species collected in Northeast Asia. Plant Genet. Resour. 2014, 12, S137–S140. [Google Scholar] [CrossRef]
- Jensen, E.; Farrar, K.; Thomas-Jones, S.; Hastings, A.; Donnison, I.; Clifton-Brown, J. Characterization of flowering time diversity in Miscanthus species. Glob. Chang. Biol. Bioenergy 2011, 3, 387–400. [Google Scholar] [CrossRef]
- Jensen, E.; Robson, P.; Norris, J.; Cookson, A.; Farrar, K.; Donnison, I.; Clifton-Brown, J. Flowering induction in the bioenergy grass Miscanthus sacchariflorus is a quantitative short-day response, whilst delayed flowering under long days increases biomass accumulation. J. Exp. Bot. 2012, 64, 541–552. [Google Scholar] [CrossRef][Green Version]
- Casler, M.D.; Vogel, K.P.; Taliaferro, C.M.; Wynia, R.L. Latitudinal Adaptation of Switchgrass Populations. Crop. Sci. 2004, 44, 293–303. [Google Scholar] [CrossRef]
- Yan, J.; Chen, W.; Luo, F.; Ma, H.; Meng, A.; Li, X.; Zhu, M.; Li, S.; Zhou, H.; Zhu, W.; et al. Variability and adaptability of Miscanthus species evaluated for energy crop domestication. GCB Bioenergy 2011, 4, 49–60. [Google Scholar] [CrossRef]
- Zub, H.; Arnoult, S.; Brancourt-Hulmel, M. Key traits for biomass production identified in different Miscanthus species at two harvest dates. Biomass Bioenergy 2011, 35, 637–651. [Google Scholar] [CrossRef]
- Casler, M.D.; Vogel, K.P.; Taliaferro, C.M.; Ehlke, N.J.; Berdahl, J.D.; Brummer, E.C.; Kallenbach, R.L.; West, C.P.; Mitchell, R.B. Latitudinal and Longitudinal Adaptation of Switchgrass Populations. Crop. Sci. 2007, 47, 2249–2260. [Google Scholar] [CrossRef]
- Clark, L.V.; Dwiyanti, M.S.; Anzoua, K.G.; Brummer, J.E.; Ghimire, B.K.; Głowacka, K.; Hall, M.; Heo, K.; Jin, X.; Lipka, A.E.; et al. Biomass yield in a genetically diverse Miscanthus sinensis germplasm panel evaluated at five locations revealed individuals with exceptional potential. Glob. Chang. Biol. Bioenergy 2019, 11, 1125–1145. [Google Scholar] [CrossRef]
- Price, L.; Bullard, M.; Lyons, H.; Anthony, S.; Nixon, P. Identifying the yield potential of Miscanthus × giganteus: An assessment of the spatial and temporal variability of M. x giganteus biomass productivity across England and Wales. Biomass-Bioenergy 2004, 26, 3–13. [Google Scholar] [CrossRef]






| Source of Variation | Df | Estimates of Variance Components | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SE | HD | LA | PH | NS | SDW | SD | Yield | ||
| Accession | 172 | 12.24 *** | 389.15 *** | 3357.33 *** | 635.95 *** | 8.49 *** | 118.70 *** | 1.13 *** | 7.90 *** |
| Year | 3 | 19.55 *** | 22.68 *** | 6558.67 *** | 2098.49 *** | 6.96 *** | 37.29 *** | 1.64 *** | 19.72 *** |
| Accession × year | 516 | 37.69 | 26.27 | 2368.20 | 883.98 | 6.51 | 51.94 | 0.18 | 10.25 |
| Traits | Unit | Korea (n = 144) | Japan (n = 12) | China–Russia (n = 17) | M.× giganteus | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | Mean | Standard Error | Range | Mean | Standard Error | Range | Mean | Standard Error | |||
| Latitude | N° | 33.13–38.32 | 32.03–33.34 | 42.25–43.42 | 35.25 * | ||||||
| First shoot emergence date | Julian date | 92.0–100.0 | 96.7 | 0.13 | 94.0–99.0 | 97.5 | 0.54 | 94.0–99.0 | 96.4 | 0.28 | 97.3 |
| Heading date | Julian date | 184.0–273.0 | 234.2 | 1.18 | 205.0–244.0 | 222.0 | 5.28 | 170.0–196.0 | 181.7 | 2.11 | 235.8 |
| Leaf area ** | cm2 | 138.0–681.0 | 286.0 | 7.39 | 180.0–434.0 | 280.2 | 24.70 | 115.0–217.0 | 150.5 | 7.11 | 454.3 |
| Stem height | cm | 82.0–363.0 | 237.2 | 3.80 | 150.0–341.0 | 277.1 | 15.31 | 116.0–219.0 | 178.2 | 7.34 | 380.9 |
| Stem diameter | mm | 3.24–9.38 | 6.00 | 0.10 | 4.82–7.98 | 6.78 | 0.27 | 2.62–5.20 | 4.09 | 0.17 | 9.0 |
| No. of stem | Number 0.01 m−2 | 4.0–36.0 | 12.2 | 0.39 | 6.0–13.0 | 8.0 | 0.64 | 6.0–18.0 | 9.2 | 0.96 | 10.8 |
| Stem dry weight | g stem−1 | 6.50–81.49 | 29.10 | 1.29 | 10.85–62.13 | 37.81 | 4.36 | 2.25–14.68 | 8.46 | 0.82 | 76.1 |
| Biomass yield | Mg ha−1 | 0.37–32.92 | 11.42 | 0.49 | 6.32–29.43 | 14.10 | 2.24 | 0.67–10.47 | 3.24 | 0.61 | 18.5 |
| Traits in the 2nd Year after Planting | Traits in the 4th Year after Planting | |||||||
|---|---|---|---|---|---|---|---|---|
| Heading Date | Vegetative Growth Duration | Leaf Area | Stem Height | Stem Diameter | Number of Stem | Stem Dry Weight | Biomass Yield | |
| Heading date | 0.943 *** | 0.943 *** | 0.595 *** | 0.346 ** | 0.610 *** | −0.089 | 0.607 *** | 0.366 ** |
| Vegetative growth duration | 0.907 *** | 0.909 ** | 0.551 *** | 0.358 ** | 0.528 *** | 0.100 | 0.519 *** | 0.381 ** |
| Leaf area | 0.434 ** | 0.446 ** | 0.470 *** | 0.187 * | 0.406 ** | 0.291 ** | 0.368 ** | 0.404 ** |
| Stem height | 0.150 * | 0.160 * | 0.183 * | 0.270 ** | 0.131 * | 0.574 *** | 0.038 | 0.338 ** |
| Stem diameter | 0.600 *** | 0.586 *** | 0.735 *** | 0.559 *** | 0.869 *** | −0.267 ** | 0.808 *** | 0.360 ** |
| Number of stem | −0.037 | −0.020 | −0.202 ** | −0.171 * | −0.310 ** | 0.995 *** | −0.322 ** | 0.112 |
| Stem dry weight | 0.567 *** | 0.560 *** | 0.668 *** | 0.413 ** | 0.725 *** | −0.113 | 0.786 *** | 0.525 *** |
| Biomass yield | 0.375 ** | 0.372 ** | 0.304 ** | 0.338 ** | 0.281 ** | 0.259 ** | 0.337 ** | 0.785 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.-H.; Yook, M.-J.; Song, J.-S.; Kim, J.-W.; Zhang, C.-J.; Kim, D.-G.; Park, Y.-H.; Lee, D.; Kim, D.-S. Diversity in Phenological and Agronomic Traits of Miscanthus sinensis Collected in Korea and Eastern Asia. Agronomy 2021, 11, 900. https://doi.org/10.3390/agronomy11050900
Lim S-H, Yook M-J, Song J-S, Kim J-W, Zhang C-J, Kim D-G, Park Y-H, Lee D, Kim D-S. Diversity in Phenological and Agronomic Traits of Miscanthus sinensis Collected in Korea and Eastern Asia. Agronomy. 2021; 11(5):900. https://doi.org/10.3390/agronomy11050900
Chicago/Turabian StyleLim, Soo-Hyun, Min-Jung Yook, Jong-Seok Song, Jin-Won Kim, Chuan-Jie Zhang, Dong-Gil Kim, Yeon-Ho Park, DoKyoung Lee, and Do-Soon Kim. 2021. "Diversity in Phenological and Agronomic Traits of Miscanthus sinensis Collected in Korea and Eastern Asia" Agronomy 11, no. 5: 900. https://doi.org/10.3390/agronomy11050900
APA StyleLim, S.-H., Yook, M.-J., Song, J.-S., Kim, J.-W., Zhang, C.-J., Kim, D.-G., Park, Y.-H., Lee, D., & Kim, D.-S. (2021). Diversity in Phenological and Agronomic Traits of Miscanthus sinensis Collected in Korea and Eastern Asia. Agronomy, 11(5), 900. https://doi.org/10.3390/agronomy11050900






