Phosphorus Supply Increases Internode Length and Leaf Characteristics, and Increases Dry Matter Accumulation and Seed Yield in Soybean under Water Deficit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Leaf Photosynthetic Rate and the Leaf P Concentration Measurements
2.2. Leaf Area and Its Distribution on the Main Stem and Branches
2.3. Plant Height and Internode Number
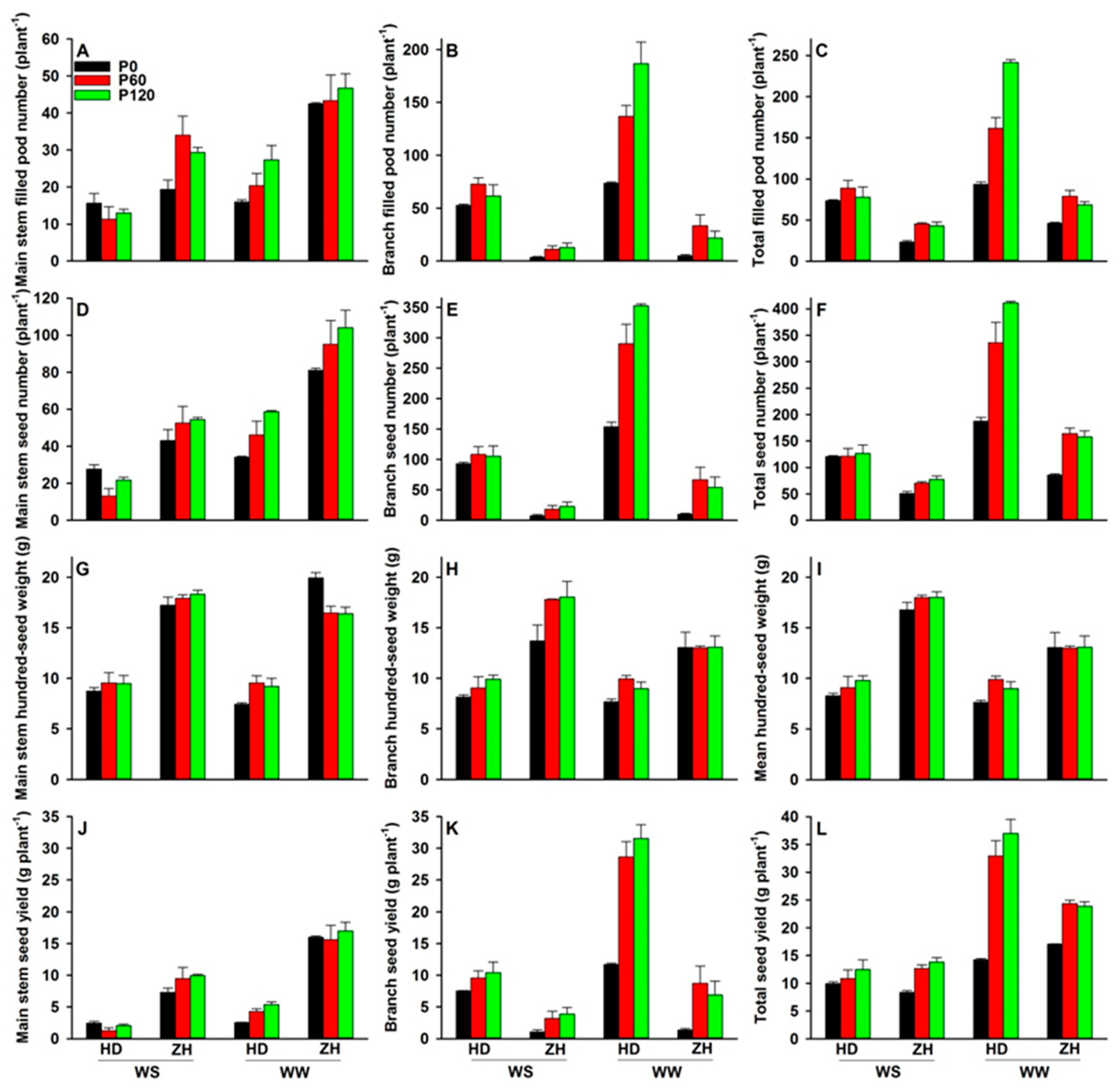
2.4. Shoot and Root Dry Weights, Seed Yield and Yield Components
2.5. Statistics
3. Results
3.1. Genotypic Variation
3.2. Response of Leaf Traits to Water and P Supply
3.3. Changes in Plant Height, Internode Length and Internode Number with Water and P Supply
3.4. Dry Matter Accumulation and Yield Performance under Water and P Deficits
4. Discussion
4.1. Interaction of Water Treatment and P Level on Leaf Traits
4.2. Leaf Traits and Dry Matter Accumulation
4.3. Dry Matter Accumulation and Yield Components
4.4. Shoots and Roots
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frederick, J.R.; Camp, C.R.; Bauer, P.J. Drought-stress effects on branch and mainstem seed yield and yield components of determinate soybean. Crop Sci. 2001, 41, 759–763. [Google Scholar] [CrossRef]
- He, J.; Du, Y.-L.; Wang, T.; Turner, N.C.; Xi, Y.; Li, F.-M. Old and new cultivars of soya bean (Glycine max L.) subjected to soil drying differ in abscisic acid accumulation, water relations characteristics and yield. J. Agron. Crop Sci. 2015, 202, 372–383. [Google Scholar] [CrossRef]
- He, J.; Du, Y.-L.; Wang, T.; Turner, N.C.; Yang, R.-P.; Jin, Y.; Xi, Y.; Zhang, C.; Cui, T.; Fang, X.-W.; et al. Conserved water use improves the yield performance of soybean (Glycine max (L.) Merr.) under drought. Agric. Water Manag. 2017, 179, 236–245. [Google Scholar] [CrossRef]
- Wang, X.; Yan, X.; Liao, H. Genetic improvement for phosphorus efficiency in soybean: A radical approach. Ann. Bot. 2010, 106, 215–222. [Google Scholar] [CrossRef]
- He, J.; Jin, Y.; Du, Y.-L.; Wang, T.; Turner, N.C.; Yang, R.-P.; Siddique, K.H.M.; Li, F.-M. Genotypic variation in yield, yield components, root orphology and architecture, in soybean in relation to water and phosphorus supply. Front. Plant Sci. 2017, 8, 1499. [Google Scholar] [CrossRef] [Green Version]
- Lynch, J.P.; Brown, K.M. Topsoil foraging—An architectural adaptation of plants to low phosphorus availability. Plant Soil 2001, 237, 225–237. [Google Scholar] [CrossRef]
- He, J.; Jin, Y.; Turner, N.C.; Chen, Z.; Liu, H.-Y.; Wang, X.-L.; Siddique, K.H.; Li, F.-M. Phosphorus application increases root growth, improves daily water use during the reproductive stage, and increases grain yield in soybean subjected to water shortage. Environ. Exp. Bot. 2019, 166, 103816. [Google Scholar] [CrossRef]
- Miller, C.R.; Ochoa, I.; Nielsen, K.L.; Beck, D.; Lynch, J.P. Genetic variation for adventitious rooting in response to low phosphorus availability: Potential utility for phosphorus acquisition from stratified soils. Funct. Plant Biol. 2003, 30, 973–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postma, J.A.; Dathe, A.; Lynch, J.P. The optimal Lateral root branching density for maize depends on nitrogen and phosphorus availability. Plant Physiol. 2014, 166, 590–602. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Liu, P.; Lynch, J.P. Greater lateral root branching density in maize (Zea mays L.) improves phosphorus acquisition from low phosphorus soil. J. Exp. Bot. 2018, 69, 4961–4970. [Google Scholar] [CrossRef] [Green Version]
- Rangarajan, H.; Postma, J.; Lynch, J.P. Co-optimization of axial root phenotypes for nitrogen and phosphorus acquisition in common bean. Ann. Bot. 2018, 122, 485–499. [Google Scholar] [CrossRef]
- Sun, B.; Gao, Y.; Lynch, J.P. Large crown root number improves topsoil foraging and phosphorus acquisition. Plant Physiol. 2018, 177, 90–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, A.; Fukuda, T.; Vejchasarn, P.; Nestler, J.; Pariasca-Tanaka, J.; Wissuwa, M. The role of root size versus root efficiency in phosphorus acquisition in rice. J. Exp. Bot. 2016, 67, 1179–1189. [Google Scholar] [CrossRef]
- Rodríguez, D.; Goudriaan, J.; Oyarzábal, M.; Pomar, M.C. Phosphorus nutrition and water stress tolerance in wheat plants. J. Plant Nutr. 1996, 19, 29–39. [Google Scholar] [CrossRef]
- Jones, C.; Jacobsen, J.; Wraith, J. The effects of P fertilization on drought tolerance of malt barley. In Proceedings of the Western Nutrient Management Conference, Salt Lake City, UT, USA, 6–7 March 2003; Volume 5, pp. 88–93. [Google Scholar]
- Jin, J.; Wang, G.; Liu, X.; Pan, X.; Herbert, S.J.; Tang, C. Interaction between phosphorus nutrition and drought on grain yield, and assimilation of phosphorus and nitrogen in two soybean cultivars differing in protein concentration in grains. J. Plant Nutr. 2006, 29, 1433–1449. [Google Scholar] [CrossRef]
- Namugwanya, M.; Tenywa, J.S.; Otabbong, E.; Mubiru, D.N.; Masamba, T.A. Development of common bean (Phaseolus vulgaris L.) production under low soil phosphorus and drought in Sub-Saharan Africa: A Review. J. Sustain. Dev. 2014, 7, 128. [Google Scholar] [CrossRef]
- Jemo, M.; Sulieman, S.; Bekkaoui, F.; Olomide, O.A.K.; Hashem, A.; Allah, E.F.A.; Alqarawi, A.A.; Tran, L.-S.P. Comparative analysis of the combined effects of different water and phosphate levels on growth and biological nitrogen fixation of nine cowpea varieties. Front. Plant Sci. 2017, 8, 2111. [Google Scholar] [CrossRef] [Green Version]
- Roucou, A.; Violle, C.; Fort, F.; Roumet, P.; Ecarnot, M.; Vile, D. Shifts in plant functional strategies over the course of wheat domestication. J. Appl. Ecol. 2017, 55, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Wang, B.; Liu, B.Q. Heredity of soybean nodes of main stem and internode length also its correlation with plant height. Soybean Sci. 2010, 29, 942–947. [Google Scholar]
- Allen, L.H.; Zhang, L.; Boote, K.J.; Hauser, B.A. Elevated temperature intensity, timing, and duration of exposure affect soybean internode elongation, mainstem node number, and pod number per plant. Crops J. 2018, 6, 148–161. [Google Scholar] [CrossRef]
- Wang, X.M.; Zhang, Z.; Shi, G.R. Interaction of phosphorus concentration and soil moisture on photosynthetic traits in soybean plants. Agric. Res. Arid Areas 2010, 28, 143–148. [Google Scholar]
- Turner, N.C. Imposing and maintaining soil water deficits in drought studies in pots. Plant Soil 2018, 439, 45–55. [Google Scholar] [CrossRef]
- Fehr, W.R.; Caviness, C.E.; Burmood, D.T.; Pennington, J.S. Stage of development descriptions for soybeans, Glycine max (L.) Merrill. Crops Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Chen, L.; Nan, H.; Kong, L.; Yue, L.; Yang, H.; Zhao, Q.; Fang, C.; Li, H.; Cheng, Q.; Lu, S.; et al. Soybean AP1 homologs control flowering time and plant height. J. Integr. Plant Biol. 2020, 62, 1868–1879. [Google Scholar] [CrossRef]
- Yang, Q.; Lin, G.; Lv, H.; Wang, C.; Yang, Y.; Liao, H. Environmental and genetic regulation of plant height in soybean. BMC Plant Biol. 2021, 21, 1–15. [Google Scholar] [CrossRef]
- Mahamood, J.; Abayomi, Y.A.; Aduloju, M.O. Comparative growth and grain yield responses of soybean genotypes to phosphorus fertilizer application. Afr. J. Biotechnol. 2009, 8, 1030–1036. [Google Scholar]
- Singh, S.K.; Reddy, V.R.; Fleisher, D.H.; Timlin, D.J. Phosphorus nutrition affects temperature response of soybean growth and canopy photosynthesis. Front. Plant Sci. 2018, 9, 9. [Google Scholar] [CrossRef]
- Gutierrez-Boem, F.H.; Thomas, G.W. Phosphorus nutrition and water deficits in field-grown soybeans. Plant Soil 1998, 207, 87–96. [Google Scholar] [CrossRef]
- Jaidee, R.; Polthanee, A.; Saenjan, P. Growth and yield of soybean cultivars as affected by ground water levels and phosphorus rates grown under greenhouse and field conditions. Aust. J. Crop Sci. 2012, 6, 81–92. [Google Scholar]
- Zhong, P.; Wu, J.J.; Liu, L.J.; Lin, W.G.; Dong, D.J.; Wang, J.L. Effects of phosphorus deficiency and drought stress on photosynthetic characters in different genotypic soybeans. Soybean Sci. 2009, 28, 806–881. [Google Scholar]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Sinebo, W. Trade off between yield increase and yield stability in three decades of barley breeding in a tropical highland environment. Field Crops Res. 2005, 92, 35–52. [Google Scholar] [CrossRef]
- Pennacchi, J.P.; Carmo-Silva, E.; Andralojc, P.J.; Lawson, T.; Allen, A.M.; Raines, C.A.; Parry, M.A.J. Stability of wheat grain yields over three field seasons in the UK. Food Energy Secur. 2019, 8, e00147. [Google Scholar] [CrossRef] [Green Version]
- Del Moral, L.F.G.; Del Moral, M.B.G.; Molina-Cano, J.L.; Slafer, G.A. Yield stability and development in two- and six-rowed winter barleys under Mediterranean conditions. Field Crops Res. 2003, 81, 109–119. [Google Scholar] [CrossRef]
- Zhang, X.C.; Chen, H.; Huang, S.L.; Yin, X.W.; Du, C.Z.; Zhang, J.J. Optimal combination of nitrogen fertilizer and spring soybean varieties in Chongqing. Soybean Sci. 2012, 31, 255–258. [Google Scholar]





| Source of Variability | G | W | P | G*W | G*P | W*P | G*W*P |
|---|---|---|---|---|---|---|---|
| Plant height at 65 DAS (cm) | *** (1.8) | *** (1.8) | *** (2.2) | ** (2.5) | ** (3.1) | *** (3.1) | * (4.3) |
| Plant height at maturity (cm) | *** (6.2) | *** (6.2) | ** (7.6) | *** (8.7) | n.s | n.s | n.s |
| Average internode length at 65 DAS (cm) | *** (0.2) | *** (0.2) | *** (0.2) | n.s | * (0.3) | ** (0.3) | n.s |
| Average internode length at maturity (cm) | *** (0.3) | *** (0.3) | * (0.3) | *** (0.4) | n.s | * (0.5) | n.s |
| Internode number at 65 DAS (plant−1) | n.s | *** (0.3) | * (0.4) | n.s | n.s | n.s | n.s |
| Internode number at maturity (plant−1) | *** (0.6) | *** (0.6) | *** (0.8) | n.s | n.s | n.s | n.s |
| Branch numbers at 65 DAS (plant−1) | *** (0.8) | n.s | *** (0.9) | n.s | n.s | n.s | n.s |
| Branch numbers at maturity (plant−1) | *** (0.5) | *** (0.5) | *** (0.6) | n.s | n.s | n.s | n.s |
| Leaf area on main stem at 65 DAS (cm2) | * (115) | *** (115) | *** (141) | n.s | n.s | ** (199) | n.s |
| Leaf area on main stem at maturity (cm2) | ** (159) | *** (159) | *** (195) | n.s | * (276) | n.s | n.s |
| Leaf area on branches at 65 DAS (cm2) | *** (255) | *** (255) | *** (312) | *** (360) | *** (441) | ** (441) | ** (624) |
| Leaf area on branches at maturity (cm2) | *** (365) | *** (365) | *** (447) | *** (517) | * (633) | * (633) | n.s |
| LMA on main stem at 65 DAS (g cm−2) | *** (3.1) | * (3.1) | n.s | n.s | n.s | n.s | n.s |
| LMA on main stem at maturity (g cm−2) | *** (1.8) | *** (1.8) | *** (2.2) | ** (2.5) | ** (3.1) | * (3.1) | n.s |
| LMA on branches at 65 DAS (g cm−2) | * (8.0) | n.s | n.s | n.s | n.s | n.s | n.s |
| LMA on branches at maturity (g cm−2) | ** (3.2) | *** (3.2) | * (3.9) | n.s | n.s | n.s | n.s |
| Pn at 50 DAS (μmol CO2 m−2 s−1) | n.s | * (1.0) | ** (1.2) | n.s | n.s | n.s | n.s |
| Leaf P concentration at 65 DAS (mg g−1) | ** (0.5) | n.s | *** (0.6) | ** (0.7) | n.s | n.s | n.s |
| Shoot dry weight at 65 DAS (g plant−1) | *** (1.7) | *** (1.7) | *** (2.1) | n.s | n.s | ** (3.0) | n.s |
| Shoot dry weight at maturity (g plant−1) | *** (5.1) | *** (5.1) | *** (6.3) | *** (7.8) | ** (8.8) | *** (8.8) | * (12.5) |
| Root dry weight at 65 DAS (g plant−1) | *** (0.4) | *** (0.4) | *** (0.4) | n.s | * (0.6) | n.s | n.s |
| Root dry weight at maturity (g plant−1) | *** (2.7) | *** (2.7) | *** (3.3) | *** (3.8) | n.s | * (4.7) | n.s |
| Filled pod number on main stem (plant−1) | *** (4.1) | *** (4.1) | n.s | n.s | n.s | n.s | * (10.2) |
| Seed number on main stem (plant−1) | *** (7.4) | *** (7.4) | * (9.0) | * (10.4) | n.s | n.s | n.s |
| Hundred-seed weight on main stem (g) | *** (0.8) | n.s | n.s | n.s | ** (1.3) | n.s | ** (1.9) |
| Seed yield on main stem (g plant−1) | *** (0.9) | *** (0.9) | * (1.1) | *** (1.3) | n.s | n.s | * (2.2) |
| Filled pod number on branches (plant−1) | *** (11) | *** (11) | *** (14) | *** (16) | ** (19) | *** (19) | ** (27) |
| Seed number on branches (plant−1) | *** (16) | *** (16) | *** (19) | *** (22) | ** (28) | *** (28) | *** (39) |
| Hundred-seed weight on branches (g) | *** (1.2) | * (1.2) | n.s | n.s | n.s | * (2.0) | * (2.9) |
| Seed yield on branches (g plant−1) | *** (1.7) | *** (1.7) | *** (2.1) | *** (2.4) | ** (3.0) | *** (3.0) | ** (4.2) |
| Total filled pod number (plant−1) | *** (10) | *** (10) | *** (12) | *** (14) | *** (18) | *** (18) | *** (25) |
| Total seed number (plant−1) | *** (11) | *** (11) | *** (13) | *** (15) | *** (19) | *** (19) | *** (27) |
| Mean hundred-seed weight (g) | *** (0.3) | *** (0.3) | n.s. | * (0.4) | *** (0.5) | *** (0.5) | *** (0.7) |
| Total seed yield (g plant−1) | *** (1.4) | *** (1.4) | *** (1.7) | *** (2.0) | ** (2.4) | *** (2.4) | *** (3.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.-Y.; He, J.; Turner, N.C.; Siddique, K.H.M.; Li, F.-M. Phosphorus Supply Increases Internode Length and Leaf Characteristics, and Increases Dry Matter Accumulation and Seed Yield in Soybean under Water Deficit. Agronomy 2021, 11, 930. https://doi.org/10.3390/agronomy11050930
Feng Y-Y, He J, Turner NC, Siddique KHM, Li F-M. Phosphorus Supply Increases Internode Length and Leaf Characteristics, and Increases Dry Matter Accumulation and Seed Yield in Soybean under Water Deficit. Agronomy. 2021; 11(5):930. https://doi.org/10.3390/agronomy11050930
Chicago/Turabian StyleFeng, Yun-Yin, Jin He, Neil C. Turner, Kadambot H. M. Siddique, and Feng-Min Li. 2021. "Phosphorus Supply Increases Internode Length and Leaf Characteristics, and Increases Dry Matter Accumulation and Seed Yield in Soybean under Water Deficit" Agronomy 11, no. 5: 930. https://doi.org/10.3390/agronomy11050930
APA StyleFeng, Y.-Y., He, J., Turner, N. C., Siddique, K. H. M., & Li, F.-M. (2021). Phosphorus Supply Increases Internode Length and Leaf Characteristics, and Increases Dry Matter Accumulation and Seed Yield in Soybean under Water Deficit. Agronomy, 11(5), 930. https://doi.org/10.3390/agronomy11050930








