Common Bean Yield and Zinc Use Efficiency in Association with Diazotrophic Bacteria Co-Inoculations
Abstract
:1. Introduction
2. Materials and Methods
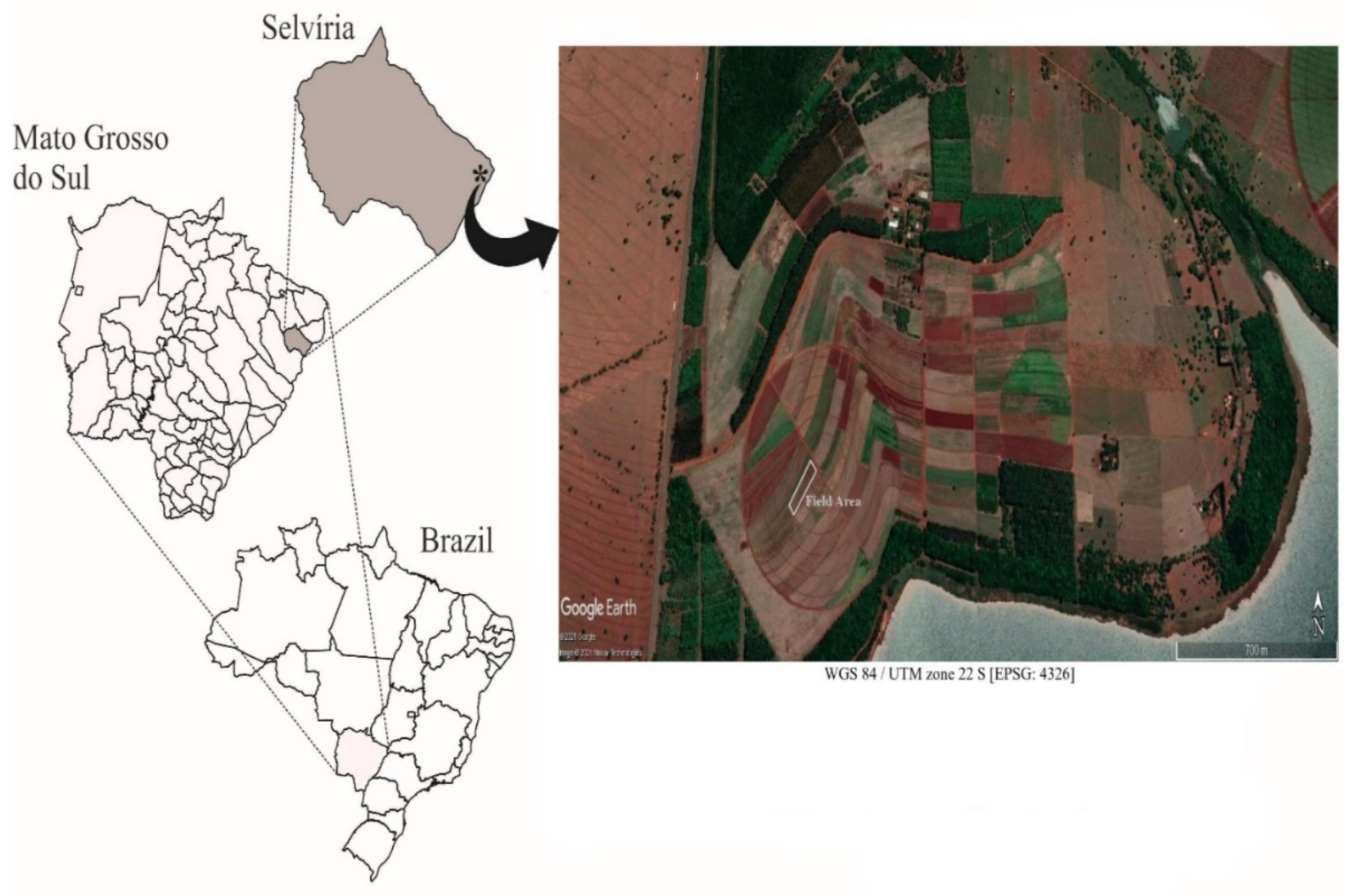
2.1. Experimental Area and Location
2.2. Soil Analysis
2.3. Experimental Design and Treatments
2.4. Plant Materials
2.5. Evaluations and Analysis
2.5.1. Zinc Soil and Plant Nutritional Analysis
2.5.2. Shoot Dry Matter and Yield
2.5.3. Zn Plant Accumulation
2.5.4. Zinc Partitioning and Intake
2.5.5. Zinc Use Efficiency
2.6. Statistical Analysis
3. Results
3.1. Zinc Nutrition in Soil, Plants and Grains
3.2. Zinc Accumulation in Plant and Grains, and Partitioning Index (ZPI)
3.3. Plant Height, Dry Matter, Grain Yield and Zn Intake
3.4. Zinc Efficiencies
3.5. Pearson’s Linear Correlation among Zn Content in Tissues and Soil, Zn Efficiencies and Common Bean Grain Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Long, J.; Zhang, J.; Zhang, X.; Wu, J.; Chen, H.; Wang, P.; Wang, Q.; Du, C. Genetic Diversity of Common Bean (Phaseolus vulgaris L.) Germplasm Resources in Chongqing, Evidenced by Morphological Characterization. Front. Genet. 2020, 11, 697. [Google Scholar] [CrossRef]
- Hayat, I.; Ahmad, A.; Masud, T.; Ahmed, A.; Bashir, S. Nutritional and Health Perspectives of Beans (Phaseolus vulgaris L.): An Overview. Crit. Rev. Food Sci. Nutr. 2014, 54, 580–592. [Google Scholar] [CrossRef]
- Rivera, A.; Plans, M.; Sabaté, J.; Casañas, F.; Casals, J.; Rull, A.; Simó, J. The Spanish Core Collection of Common Beans (Phaseolus vulgaris L.): An Important Source of Variability for Breeding Chemical Composition. Front. Plant Sci. 2018, 9, 1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Agriculture Organization of the United Nations. FAOSTAT. Food and Agriculture Data; FAOSTAT: Rome, Italy, 2018. [Google Scholar]
- Conab Follow-Up of Brazilian Grain Safra, National Supply Company. Bean Harvest-Safra 2018/19, n.7-Seventh Survey. Available online: https://www.conab.gov.br/info-agro/safras/graos (accessed on 16 February 2021).
- Soratto, R.P.; Fernandes, A.M.; Dos Santos, L.A.; Job, A.L.G. Nutrient extraction and exportation by common bean cultivars under different fertilization levels: I-macronutrients. Rev. Bras. Ciência Solo 2013, 37, 1027–1042. [Google Scholar] [CrossRef] [Green Version]
- Estrada-Domínguez, V.; Sánchez-Chávez, E.; de-la-Cruz-Lázaro, E.; Márquez-Quiroz, C.; Osorio-Osorio, R. Effect of zinc chelate and sulfate on mineral content, antioxidant activity and grain yield of Vigna unguiculata L. Philipp. Agric. Sci. 2020, 103, 47–54. [Google Scholar]
- Costa, H.; Saliba, E.; Galvani, D.; Bomfim, M.; Lana, Â.M.; Borges, A.L.; Landim, A.; Faciola, A. Effects of Zinc Sulfate or Propylene Glycol on Intake, Digestibility, and Forage Selection by Grazing Sheep in a Semi-Arid Region During the Rainy Season. Animals 2019, 9, 867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaseen, M.K.; Hussain, S. Zinc-biofortified wheat required only a medium rate of soil zinc application to attain the targets of zinc biofortification. Arch. Agron. Soil Sci. 2021, 67, 551–562. [Google Scholar] [CrossRef]
- Melo, F.D.B.; Bastos, E.A.; Cardoso, M.J.; Ribeiro, V.Q. Cowpea response to phosphorus and zinc. Rev. Caatinga 2018, 31, 240–245. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, R.; Hossain, A.; Sharma, P. Zinc biofortification as an innovative technology to alleviate the zinc deficiency in human health: A review. Open Agric. 2020, 5, 176–187. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Perkowska, K.; Rzymski, P. Food Fortification. Vitam. Miner. Biofortification Edible Plants 2020, 27–44. [Google Scholar] [CrossRef]
- Maxfield, L.; Crane, J.S. Zinc Deficiency. In StatPearls [Internet]: StatPearls Publishing 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493231/ (accessed on 13 March 2021).
- World Health Organization (WHO). Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; WHO Technical Report Series; WHO: Geneva, Switzerland, 2007; Available online: https://www.who.int/nutrition/publications/nutrientrequirements/WHO_TRS_935/en (accessed on 16 November 2020).
- Bollinedi, H.; Yadav, A.K.; Vinod, K.K.; Krishnan, S.G.; Bhowmick, P.K.; Nagarajan, M.; Neeraja, C.N.; Ellur, R.K.; Singh, A.K. Genome-Wide Association Study Reveals Novel Marker-Trait Associations (MTAs) Governing the Localization of Fe and Zn in the Rice Grain. Front. Genet. 2020, 11, 213. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Kishore, A.; Alvi, M.F.; Singh, V. Designing better input support programs: Lessons from zinc subsidies in Andhra Pradesh, India. PLoS ONE 2020, 15, e0242161. [Google Scholar] [CrossRef]
- Gammoh, N.Z.; Rink, L. Zinc in Infection and Inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uwitonze, A.M.; Ojeh, N.; Murererehe, J.; Atfi, A.; Razzaque, M.S. Zinc Adequacy Is Essential for the Maintenance of Optimal Oral Health. Nutrients 2020, 12, 949. [Google Scholar] [CrossRef] [Green Version]
- Skalny, A.V.; Rink, L.; Ajsuvakova, O.P.; Aschner, M.; Gritsenko, V.A.; Alekseenko, S.I.; Svistunov, A.A.; Petrakis, D.; Spandidos, D.A.; Aaseth, J.; et al. Zinc and respiratory tract infections: Perspectives for COVID-19. Int. J. Mol. Med. 2020, 46, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kryvoruchko, I. Zn-use efficiency for optimization of symbiotic nitrogen fixation in chickpea (Cicer arietinum L.). Turk. J. Bot. 2017, 41, 423–441. Available online: https://journals.tubitak.gov.tr/botany/abstract.htm?id=21254 (accessed on 13 March 2021).
- Doolette, C.L.; Read, T.L.; Li, C.; Scheckel, K.G.; Donner, E.; Kopittke, P.M.; Schjoerring, J.K.; Lombi, E. Foliar application of zinc sulphate and zinc EDTA to wheat leaves: Differences in mobility, distribution, and speciation. J. Exp. Bot. 2018, 69, 4469–4481. [Google Scholar] [CrossRef]
- Rehman, A.; Farooq, M.; Asif, M.; Ozturk, L. Supra-optimal growth temperature exacerbates adverse effects of low Zn supply in wheat. J. Plant Nutr. Soil Sci. 2019, 182, 656–666. [Google Scholar] [CrossRef]
- Ullah, A.; Farooq, M.; Nadeem, F.; Rehman, A.; Hussain, M.; Nawaz, A.; Naveed, M. Zinc Application in Combination with Zinc Solubilizing Enterobacter sp. MN17 Improved Productivity, Profitability, Zinc Efficiency, and Quality of Desi Chickpea. J. Soil Sci. Plant Nutr. 2020, 20, 2133–2144. [Google Scholar] [CrossRef]
- Farooq, M.; Ullah, A.; Rehman, A.; Nawaz, A.; Nadeem, A.; Wakeel, A.; Nadeem, F.; Siddique, K.H. Application of zinc improves the productivity and biofortification of fine grain aromatic rice grown in dry seeded and puddled transplanted production systems. Field Crop. Res. 2018, 216, 53–62. [Google Scholar] [CrossRef]
- Montanha, G.S.; Rodrigues, E.S.; Romeu, S.L.; De Almeida, E.; Reis, A.R.; Lavres, J.; De Carvalho, H.W.P. Zinc uptake from ZnSO4 (aq) and Zn-EDTA (aq) and its root-to-shoot transport in soybean plants (Glycine max) probed by time-resolved in vivo X-ray spectroscopy. Plant Sci. 2020, 292, 110370. [Google Scholar] [CrossRef]
- Haider, M.U.; Hussain, M.; Farooq, M.; Nawaz, A. Soil application of zinc improves the growth, yield and grain zinc biofortification of mungbean). J. Plant Nutr. 2018, 37, 123–128. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, N.; Fu, H.; Chen, T.; Liu, S.; Zheng, S.; Zhang, J. Effect of zinc oxide nanoparticles on nitrogen removal, microbial activity and microbial community of CANON process in a membrane bioreactor. Bioresour. Technol. 2017, 243, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Hakim, S.; Naqqash, T.; Nawaz, M.S.; Laraib, I.; Siddique, M.J.; Zia, R.; Mirza, M.S.; Imran, A. Rhizosphere Engineering with Plant Growth-Promoting Microorganisms for Agriculture and Ecological Sustainability. Front. Sustain. Food Syst. 2021, 5, 16. [Google Scholar] [CrossRef]
- Khoshru, B.; Mitra, D.; Khoshmanzar, E.; Myo, E.M.; Uniyal, N.; Mahakur, B.; Mohapatra, P.K.; Panneerselvam, P.; Boutaj, H.; Alizadeh, M.; et al. Current scenario and future prospects of plant growth-promoting rhizobacteria: An economic valuable resource for the agriculture revival under stressful conditions. J. Plant Nutr. 2020, 43, 3062–3092. [Google Scholar] [CrossRef]
- Sedlakova-Kadukova, J.; Kopcakova, A.; Gresakova, L.; Godany, A.; Pristas, P. Bioaccumulation and biosorption of zinc by a novel Streptomyces K11 strain isolated from highly alkaline aluminium brown mud disposal site. Ecotoxicol. Environ. Saf. 2019, 167, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Yahaghi, Z.; Shirvani, M.; Nourbakhsh, F.; Pueyo, J. Uptake and effects of lead and zinc on alfalfa (Medicago sativa L.) seed germination and seedling growth: Role of plant growth promoting bacteria. S. Afr. J. Bot. 2019, 124, 573–582. [Google Scholar] [CrossRef]
- Lambrese, Y.; Guiñez, M.; Calvente, V.; Sansone, G.; Cerutti, S.; Raba, J.; Sanz, M.I. Production of siderophores by the bacterium Kosakonia radicincitans and its application to control of phytopathogenic fungi. Bioresour. Technol. Rep. 2018, 3, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Khoshru, B.; Mitra, D.; Mahakur, B.; Sarikhani, M.R.; Mondal, R.; Verma., D.; Pant, K. Role of soil rhizobacteria in utilization of an indispensable micronutrient zinc for plant growth promotion. J. Nat. Remedies 2020, 21, 4644–4654. [Google Scholar]
- Mumtaz, M.Z.; Ahmad, M.; Jamil, M.; Hussain, T. Zinc solubilizing Bacillus spp. potential candidates for biofortification in maize. Microbiol. Res. 2017, 202, 51–60. [Google Scholar] [CrossRef]
- Kushwaha, P.; Srivastava, R.; Pandiyan, K.; Singh, A.; Chakdar, H.; Kashyap, P.L.; Bhardwaj, A.K.; Murugan, K.; Karthikeyan, N.; Bagul, S.Y.; et al. Enhancement in Plant Growth and Zinc Biofortification of Chickpea (Cicer arietinum L.) by Bacillus altitudinis. J. Soil Sci. Plant Nutr. 2021, 1–14. [Google Scholar] [CrossRef]
- USDA Foreign Agriculture Services; Brazil Grain and Feed Annual. 2019. Available online: https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Grain%20and%20Feed%20Annual_Brasilia_Brazil_4-8-2019.pdf (accessed on 15 February 2021).
- Calegari, A.; Tiecher, T.; Hargrove, W.L.; Ralisch, R.; Tessier, D.; de Tourdonnet, S.; Guimarães, M.D.F.; dos Santos, D.R. Long-term effect of different soil management systems and winter crops on soil acidity and vertical distribution of nutrients in a Brazilian Oxisol. Soil Tillage Res. 2013, 133, 32–39. [Google Scholar] [CrossRef]
- Raij, B.; van Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Chemical Analysis for Fertility Evaluation of Tropical Soils; IAC: Campinas, Brazil, 2001; p. 285. (In Portuguese) [Google Scholar]
- Fageria, N.K.; Baligar, V.C.; Clark, R.B. Micronutrients in crop production. Advan. Agron. 2002, 77, 185–268. [Google Scholar]
- Fageria, N.K.; Stone, L.F. Bean productivity in no-tillage system with application of lime and zinc. Pesqui. Agropecu. 2004, 39, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Evaluation of the Nutritional Status of Plants: Principles and Applications, 2nd ed.; Potafos: Piracicaba, Brazil, 1997; p. 319. (In Portuguese) [Google Scholar]
- Lessa, J.H.D.L.; Araujo, A.M.; Ferreira, L.A.; Júnior, E.C.D.S.; De Oliveira, C.; Corguinha, A.P.B.; Martins, F.A.D.; De Carvalho, H.W.P.; Guilherme, L.R.G.; Lopes, G. Agronomic biofortification of rice (Oryza sativa L.) with selenium and its effect on element distributions in biofortified grains. Plant Soil 2019, 444, 331–342. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciência Agrotecnologia 2011, 35, 1039–1042. [Google Scholar] [CrossRef] [Green Version]
- Fageria, N.K.; Dos Santos, A.B.; Cobucci, T. Zinc Nutrition of Lowland Rice. Commun. Soil Sci. Plant Anal. 2011, 42, 1719–1727. [Google Scholar] [CrossRef]
- IBGE. Directorate of Research: Coordination of Work and Income [Family Budget Survey 2017–2018: First Results]; IBGE: Rio de Janeiro, Brazil. Available online: https://agenciadenoticias.ibge.gov.br/agencia-sala-de-imprensa/2013-agencia-de-noticias/releases/28646-pof-2017-2018-brasileiro-ainda-mantem-dieta-a-base-de-arroz-e-feijao-mas-consumo-de-frutas-e-legumes-e-abaixo-do-esperado (accessed on 24 March 2021).
- Van Raij, B.; Cantarella, H.; Quaggio, J.A.; Furlani, A.M.C. Liming and Fertilization Recommendations for the State of São Paulo; Instituto Agronômico de Campinas: Campinas, Brazil, 1997; p. 285. (In Portuguese) [Google Scholar]
- Rehman, A.; Farooq, M.; Naveed, M.; Ozturk, L.; Nawaz, A. Pseudomonas-aided zinc application improves the productivity and biofortification of bread wheat. Crop. Pasture Sci. 2018, 69, 659. [Google Scholar] [CrossRef]
- Hussain, A.; Zahir, Z.A.; Asghar, H.N.; Imran, M.; Ahmad, M.; Hussain, S. Integrating the potential of Bacillus sp. Az6 and organic waste for zinc oxide bio-activation to improve growth, yield and zinc content of maize grains. Pak. J. Agric. Sci. 2020, 57, 123–130. [Google Scholar] [CrossRef]
- Imran, M.; Arshad, A.; Khalid, S.; Kanwal, S.; Crowley, D.E. Perspectives of rhizospheremicroflora for improving Zn bioavailability and acquisition by higher plants. Int. J. Agric. Biol 2014, 16, 653–662. [Google Scholar]
- Ullah, A.; Romdhane, L.; Rehman, A.; Farooq, M. Adequate zinc nutrition improves the tolerance against drought and heat stresses in chickpea. Plant Physiol. Biochem. 2019, 143, 11–18. [Google Scholar] [CrossRef]
- Saini, P.; Nagpal, S.; Saini, P.; Kumar, A.; Gani, M. Microbial Mediated Zinc Solubilization in Legumes for Sustainable Agriculture. Phytomicrobiome 2021, 254–276. [Google Scholar] [CrossRef]
- Pawar, A.; Ismail, S.; Mundhe, S.; Patil, V.D. Solubilization of insoluble zinc compounds by different microbial isolates in vitro condition. Int. J. Trop. Agric. 2015, 33, 865–869. [Google Scholar]
- Saravanan, V.; Madhaiyan, M.; Thangaraju, M. Solubilization of zinc compounds by the diazotrophic, plant growth promoting bacterium Gluconacetobacter diazotrophicus. Chemosphere 2007, 66, 1794–1798. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.C.; Sharma, S.K.; Ramesh, A.; Sharma, K.; Sharma, P.K.; Varma, A. Contribution of Zinc-Solubilizing and-Mobilizing Microorganisms (ZSMM) to enhance zinc bioavailability for better soil, plant, and human health. In Rhizosphere Microbes; Springer: Singapore, 2020; pp. 357–386. [Google Scholar] [CrossRef]
- Khande, R.; Sharma, S.K.; Ramesh, A.; Sharma, M.P. Zinc solubilizing Bacillus strains that modulate growth, yield and zinc biofortification of soybean and wheat. Rhizosphere 2017, 4, 126–138. [Google Scholar] [CrossRef]
- Sindhu, S.S.; Sharma, R.; Sindhu, S.; Phour, M. Plant nutrient management through inoculation of zinc-solubilizing bacteria for sustainable agriculture. In Biofertilizers for Sustainable Agriculture and Environment; Giri, B., Prasad, R., Wu, Q.S., Varma, A., Eds.; Soil Biology; Springer: Cham, Switzerland, 2019; Volume 55, pp. 173–201. [Google Scholar] [CrossRef]
- Kumar, A.; Dewangan, S.; Lawate, P.; Bahadur, I.; Prajapati, S. Zinc-Solubilizing Bacteria: A Boon for Sustainable Agriculture; Springer: Singapore, 2019; pp. 139–155. [Google Scholar] [CrossRef]
- Ramesh, A.; Sharma, S.K.; Sharma, M.P.; Yadav, N.; Joshi, O.P. Inoculation of zinc solubilizing Bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in Vertisols of central India. Appl. Soil Ecol. 2014, 73, 87–96. [Google Scholar] [CrossRef]
- Rana, A.; Saharan, B.; Nain, L.; Prasanna, R.; Shivay, Y.S. Enhancing micronutrient uptake and yield of wheat through bacterial PGPR consortia. Soil Sci. Plant Nutr. 2012, 58, 573–582. [Google Scholar] [CrossRef]
- Singh, B.; Natesan, S.K.A.; Singh, B.K.; Usha, K. Improving zinc efficiency of cereals under zinc deficiency. Curr. Sci. 2005, 36–44. Available online: https://www.jstor.org/stable/24110091 (accessed on 21 March 2021).
- Sharma, S.K.; Sharma, M.P.; Ramesh, A.; Joshi, O.P. Characterization of zinc-solubilizing Bacillus isolates and their potential to influence zinc assimilation in soybean seeds. J. Microbiol. Biotechnol. 2012, 22, 352–359. [Google Scholar] [CrossRef] [Green Version]
- Goteti, P.K.; Emmanuel, L.D.A.; Desai, S.; Shaik, M.H.A. Prospective Zinc Solubilising Bacteria for Enhanced Nutrient Uptake and Growth Promotion in Maize (Zea mays L.). Int. J. Microbiol. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Rajawat, M.V.S.; Kaushik, R.; Prasanna, R.; Saxena, A.K. Beneficial role of endophytes in biofortification of Zn in wheat genotypes varying in nutrient use efficiency grown in soils sufficient and deficient in Zn. Plant Soil 2017, 416, 107–116. [Google Scholar] [CrossRef]
- Gontia-Mishra, I.; Sapre, S.; Tiwari, S. Zinc solubilizing bacteria from the rhizosphere of rice as prospective modulator of zinc biofortification in rice. Rhizosphere 2017, 3, 185–190. [Google Scholar] [CrossRef]
- Khanghahi, M.Y.; Ricciuti, P.; Allegretta, I.; Terzano, R.; Crecchio, C. Solubilization of insoluble zinc compounds by zinc solubilizing bacteria (ZSB) and optimization of their growth conditions. Environ. Sci. Pollut. Res. 2018, 25, 25862–25868. [Google Scholar] [CrossRef] [PubMed]
- Sunithakumari, K.; Devi, S.P.; Vasandha, S. Zinc solubilizing bacterial isolates from the agricultural fields of Coimbatore, Tamil Nadu, India. Curr. Sci. 2016, 25, 196–205. Available online: https://www.jstor.org/stable/24906745 (accessed on 26 March 2021). [CrossRef]
- Jalal, A.; Shah, S.; Teixeira Filho, M.C.M.; Khan, A.; Shah, T.; Hussain, Z.; Younis, M.; Ilyas, M. Yield and phenological indices of wheat as affected by exogenous fertilization of Zinc and Iron. Braz. J. Agric. Sci./Rev. Bras. Ciências Agrárias 2020, 15. [Google Scholar] [CrossRef] [Green Version]
- Jalal, A.; Shah, S.; Filho, M.C.M.T.; Khan, A.; Shah, T.; Ilyas, M.; Rosa, P.A.L. Agro-Biofortification of Zinc and Iron in Wheat Grains. Gesunde Pflanz. 2020, 72, 227–236. [Google Scholar] [CrossRef]
- Boleta, E.H.; Shintate Galindo, F.; Jalal, A.; Santini, J.M.; Rodrigues, W.L.; Lima, B.H.; Arf, O.; Silva, M.R.; Buzetti, S.; Teixeira Filho, M.C.M. Inoculation with Growth-Promoting Bacteria Azospirillum brasilense and Its Effects on Productivity and Nutritional Accumulation of Wheat Cultivars. Front. Sustain. Food Syst. 2020, 4, 265. [Google Scholar] [CrossRef]
- Ramesh, A.; Sharma, S.K.; Joshi, O.P.; Khan, I.R. Phytase, Phosphatase Activity and P-Nutrition of Soybean as Influenced by Inoculation of Bacillus. Indian J. Microbiol. 2011, 51, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Vaid, S.; Kumar, B.; Sharma, A.; Shukla, A.; Srivastava, P. Effect of Zn solubilizing bacteria on growth promotion and Zn nutrition of rice. J. Soil Sci. Plant Nutr. 2014, 14, 889–910. [Google Scholar] [CrossRef] [Green Version]
- Cambraia, T.L.L.; Fontes, R.L.F.; Vergütz, L.; Vieira, R.F.; Neves, J.C.L.; Corrêa Netto, P.S.; Dias, R.F.N. Agronomic biofortification of common bean grain with zinc. Pesqu. Agropecu. 2019, 54, 54. [Google Scholar] [CrossRef]




| Properties | Units | Values |
|---|---|---|
| Clay | g kg−1 | 433 |
| Sand | g kg−1 | 471 |
| Silt | g kg−1 | 90 |
| pH (CaCl2) | ---- | 5.2 |
| Organic matter | mg dm−3 | 18 |
| P (resin) | mg dm−3 | 38 |
| K | mmolc dm−3 | 1.7 |
| Ca | mmolc dm−3 | 21 |
| Mg | mmolc dm−3 | 15 |
| B (hot water) | mg dm−3 | 0.14 |
| Cu (DTPA) | mg dm−3 | 3.4 |
| Fe (DTPA) | mg dm−3 | 25 |
| Zn (DTPA) | mg dm−3 | 0.9 |
| Mn (DTPA) | mg dm−3 | 38.1 |
| S-SO4 | mg dm−3 | 4.0 |
| H + Al | mmolc dm−3 | 34 |
| CEC (pH 7.0) | mmolc dm−3 | 75.7 |
| V | % | 50 |
| Treatments | Zn-Soil Concentration | Zn-Leaf Concentration | Zn-Shoot Concentration | Zn-Grains Concentration | ||||
|---|---|---|---|---|---|---|---|---|
| mg dm−3 | mg kg−1 | |||||||
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| Zinc (Zn) application (kg ha−1) | ||||||||
| 0 | 2.8 b | 4.6 b | 35.9 b | 42.7 b | 30.7 b | 41.1 b | 47.4 b | 54.0 b |
| 8 | 3.7 a | 6.5 a | 38.2 a | 46.4 a | 38.6 a | 46.6 a | 48.6 a | 56.5 a |
| Diazotrophic bacterial inoculations (I) | ||||||||
| Without | 2.7 c | 4.8 c | 33.3 b | 40.6 b | 27.8 c | 41.2 d | 43.4 e | 52.5 d |
| R. tropici | 2.7 c | 5.6 b | 36.7 b | 42.6 b | 29.8 c | 43.4 c | 47.0 c | 54.5 c |
| R. tropici + A. brasilense | 3.5 b | 5.7 b | 40.4 a | 56.7 a | 33.7 b | 45.5 b | 49.3 b | 57.0 b |
| R. tropici + B. subtilis | 4.6 a | 7.8 a | 42.8 a | 45.5 b | 39.6 a | 48.5 a | 54.5 a | 60.7 a |
| R. tropici + P. fluorescens | 3.5 b | 5.5 b | 36.6 b | 43.5 b | 42.0 a | 43.7 c | 48.8 b | 53.7 d |
| R. tropici + A. brasilense + B. subtilis | 3.5 b | 4.8 c | 35.2 c | 43.3 b | 39.8 a | 42.8 c | 47.1 c | 55.2 c |
| R. tropici + A. brasilense + P. fluorescens | 2.7 c | 4.2 c | 34.5 b | 39.8 b | 30.0 c | 41.6 d | 45.8 d | 53.1 d |
| F-values | ||||||||
| Zn | 86.2 ** | 112 ** | 6.7 * | 9.1 * | 85.4 ** | 239 ** | 5.3 * | 67.3 ** |
| I | 25.3 ** | 23 ** | 9.2 ** | 12.4 ** | 25.9 ** | 28.4 ** | 24.1 ** | 48.1 ** |
| Zn x I | 7.8 ** | 20 ** | 0.9 ns | 0.4 ns | 5.8 * | 1.2 ns | 1.8 ns | 1.6 ns |
| CV (%) | 10.7 | 12.3 | 8.6 | 10.2 | 9.2 | 3.0 | 4.35 | 2.09 |
| Treatments | Zn-Shoot Accumulation | Zn Grain Accumulation | Zn-Plant Accumulation | Zn Partitioning Index | ||||
|---|---|---|---|---|---|---|---|---|
| g ha−1 | % | |||||||
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| Zinc (Zn) application (kg ha−1) | ||||||||
| 0 | 116 b | 161 b | 159 b | 233 b | 276 b | 394 b | 122 b | 145 b |
| 8 | 166 a | 189 a | 187 a | 277 a | 340 a | 466 a | 133 a | 152 a |
| Diazotrophic bacterial inoculations (I) | ||||||||
| Without | 89 d | 162 c | 140 e | 223 e | 244 f | 386 d | 126 b | 142 e |
| R. tropici | 124 c | 176 b | 173 b | 250 c | 295 d | 426 c | 126 b | 146 d |
| R. tropici + A. brasilense | 133 c | 191 a | 170 b | 271 b | 300 d | 461 b | 123 b | 149 c |
| R. tropici + B. subtilis | 202 a | 188 a | 228 a | 307 a | 386 a | 496 a | 137 a | 158 a |
| R. tropici + P. fluorescens | 153 b | 174 b | 155 d | 246 c | 318 c | 420 c | 125 b | 152 b |
| R. tropici + A. brasilense + B. subtilis | 170 b | 173 b | 180 b | 252 c | 338 b | 425 c | 129 b | 149 c |
| R. tropici + A. brasilense + P. fluorescens | 119 c | 163 c | 162 c | 234 d | 277 e | 397 d | 129 b | 144 d |
| F-values | ||||||||
| Zn | 122 ** | 196 ** | 97.1 ** | 341 ** | 208 ** | 507 ** | 54.3 ** | 138 ** |
| I | 38 ** | 17 ** | 55.6 ** | 75.9 ** | 60.5 ** | 79 ** | 5.0 * | 39 ** |
| Zn x I | 7.3 ** | 1.5 ns | 6.5 * | 3.7 * | 13.6 ** | 1.56 ns | 1.42 ns | 2.7 * |
| CV (%) | 12.01 | 4.24 | 6.16 | 3.50 | 5.35 | 2.78 | 4.38 | 1.57 |
| Treatments | Plant Height | Shoot Dry Matter | Grain Yield | Zn Intake | ||||
|---|---|---|---|---|---|---|---|---|
| cm | kg ha−1 | g Person−1 Day−1 | ||||||
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| Zinc (Zn) application (kg ha−1) | ||||||||
| 0 | 95 b | 92 a | 3934 b | 3930 b | 3758 b | 4303 b | 6.73 b | 7.68 b |
| 8 | 102 a | 97 a | 4040 a | 4075 a | 4269 a | 4888 a | 6.92 a | 8.04 a |
| Diazotrophic bacterial inoculations (I) | ||||||||
| Without (Control) | 92.1 b | 99.8 a | 3843 d | 3818 d | 3268 e | 4252 e | 6.16 e | 7.46 d |
| R. tropici | 98.6 b | 94.1 a | 3952 c | 3989 c | 4121 b | 4590 c | 6.68 c | 7.75 c |
| R. tropici + A. brasilense | 103.7 a | 95.4 a | 4060 b | 4063 b | 3899 c | 4748 b | 7.01 b | 8.11 b |
| R. tropici + B. subtilis | 104.1 a | 93.0 a | 4207 a | 4164 a | 5069 a | 5052 a | 7.75 a | 8.64 a |
| R. tropici + P. fluorescens | 92.2 b | 90.1 a | 3978 c | 4018 b | 3542 d | 4564 c | 6.95 b | 7.64 d |
| R. tropici + A. brasilense + B. subtilis | 103.7 a | 94.1 a | 3962 c | 4012 b | 4234 b | 4558 c | 6.70 c | 7.85 c |
| R. tropici + A. brasilense + P. fluorescens | 96.0 b | 98.1 a | 3905 d | 3955 c | 3962 c | 4405 d | 6.52 d | 7.55 d |
| F-values | ||||||||
| Zn | 24.1 ** | 3.4 ns | 23.6 ** | 74.9 ** | 161 ** | 306 ** | 5.3 * | 67.2 ** |
| I | 7.2 ** | 0.85 ns | 16.4 ** | 22.7 ** | 115 ** | 33.1 ** | 22.1 * | 48.2 ** |
| Zn x I | 4.8 * | 0.84 ns | 0.13 ns | 2.68 * | 10.2 ** | 5.1 ** | 1.8 ns | 1.7 ns |
| CV (%) | 5.71 | 10.44 | 2.06 | 1.56 | 3.76 | 2.72 | 4.35 | 2.09 |
| Treatments | ZnUE | APE | UE | AZnR | ||||
|---|---|---|---|---|---|---|---|---|
| kg kg−1 | % | |||||||
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| Diazotrophic bacterial inoculations (I) | ||||||||
| Without (Control) | 24 d | 64 d | 6.95 a | 5.15 c | 41 d | 133 c | 3 e | 12 d |
| R. tropici | 168 b | 104 c | 9.88 a | 6.36 b | 209 b | 183 b | 17 c | 16 c |
| R. tropici + A. brasilense | 140 b | 123 b | 7.68 a | 5.90 b | 228 b | 208 b | 18 c | 21 b |
| R. tropici + B. subtilis | 250 a | 174 a | 7.17 a | 6.56 b | 447 a | 273 a | 35 a | 26 a |
| R. tropici + P. fluorescens | 106 c | 132 b | 4.96 a | 7.66 a | 212 b | 204 b | 21 b | 17 c |
| R. tropici + A. brasilense + B. subtilis | 167 b | 121 b | 7.68 a | 7.02 a | 238 b | 198 b | 22 b | 17 c |
| R. tropici + A. brasilense + P. fluorescens | 106 c | 104 c | 10.20 a | 7.48 a | 148 c | 175 b | 10 d | 14 d |
| F-values | ||||||||
| I | 51 * | 16 * | 2.96 | 9.10 * | 44.2 * | 13.4 * | 62.2 * | 32.4 * |
| CV (%) | 14.2 | 13.9 | 26.7 | 8.9 | 16.8 | 11.8 | 13.6 | 9.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalal, A.; Galindo, F.S.; Boleta, E.H.M.; Oliveira, C.E.d.S.; Reis, A.R.d.; Nogueira, T.A.R.; Moretti Neto, M.J.; Mortinho, E.S.; Fernandes, G.C.; Teixeira Filho, M.C.M. Common Bean Yield and Zinc Use Efficiency in Association with Diazotrophic Bacteria Co-Inoculations. Agronomy 2021, 11, 959. https://doi.org/10.3390/agronomy11050959
Jalal A, Galindo FS, Boleta EHM, Oliveira CEdS, Reis ARd, Nogueira TAR, Moretti Neto MJ, Mortinho ES, Fernandes GC, Teixeira Filho MCM. Common Bean Yield and Zinc Use Efficiency in Association with Diazotrophic Bacteria Co-Inoculations. Agronomy. 2021; 11(5):959. https://doi.org/10.3390/agronomy11050959
Chicago/Turabian StyleJalal, Arshad, Fernando Shintate Galindo, Eduardo Henrique Marcandalli Boleta, Carlos Eduardo da Silva Oliveira, André Rodrigues dos Reis, Thiago Assis Rodrigues Nogueira, Mário João Moretti Neto, Emariane Satin Mortinho, Guilherme Carlos Fernandes, and Marcelo Carvalho Minhoto Teixeira Filho. 2021. "Common Bean Yield and Zinc Use Efficiency in Association with Diazotrophic Bacteria Co-Inoculations" Agronomy 11, no. 5: 959. https://doi.org/10.3390/agronomy11050959
APA StyleJalal, A., Galindo, F. S., Boleta, E. H. M., Oliveira, C. E. d. S., Reis, A. R. d., Nogueira, T. A. R., Moretti Neto, M. J., Mortinho, E. S., Fernandes, G. C., & Teixeira Filho, M. C. M. (2021). Common Bean Yield and Zinc Use Efficiency in Association with Diazotrophic Bacteria Co-Inoculations. Agronomy, 11(5), 959. https://doi.org/10.3390/agronomy11050959










