Bacillus Co-Inoculation Alleviated Salt Stress in Seedlings Cucumber
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Screening of Bacillus spp.
2.2. Screening of Salt-Tolerant Strains
2.3. Screening for Growth-Promoting Strains
2.4. Identification and Phylogenetic Analysis
2.5. Pot Experiments with Salt-Tolerant and Growth-Promoting Compound Bacteria
2.6. Analysis Methods
2.7. Statistical Analysis
3. Results
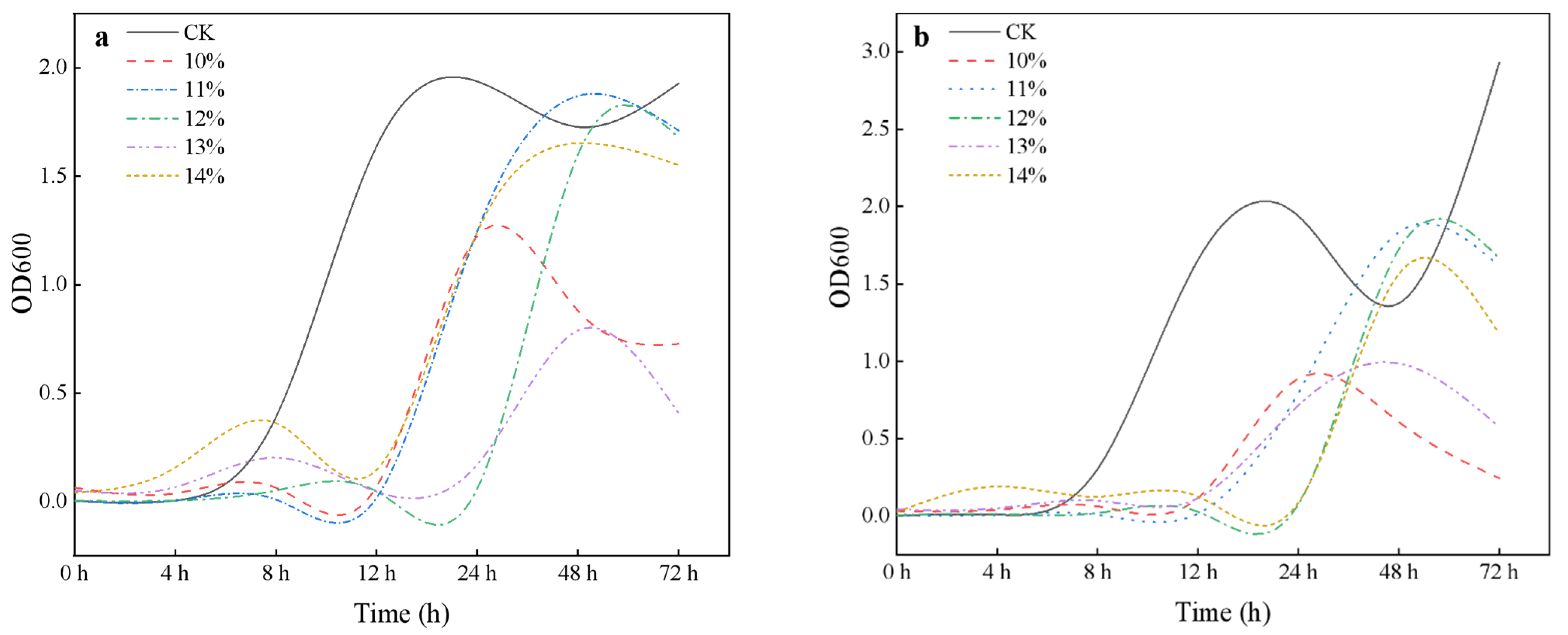
3.1. Isolation of Salt Tolerance and Growth Promotion
3.2. Identification of Isolated Strains
3.3. Seedling Growth
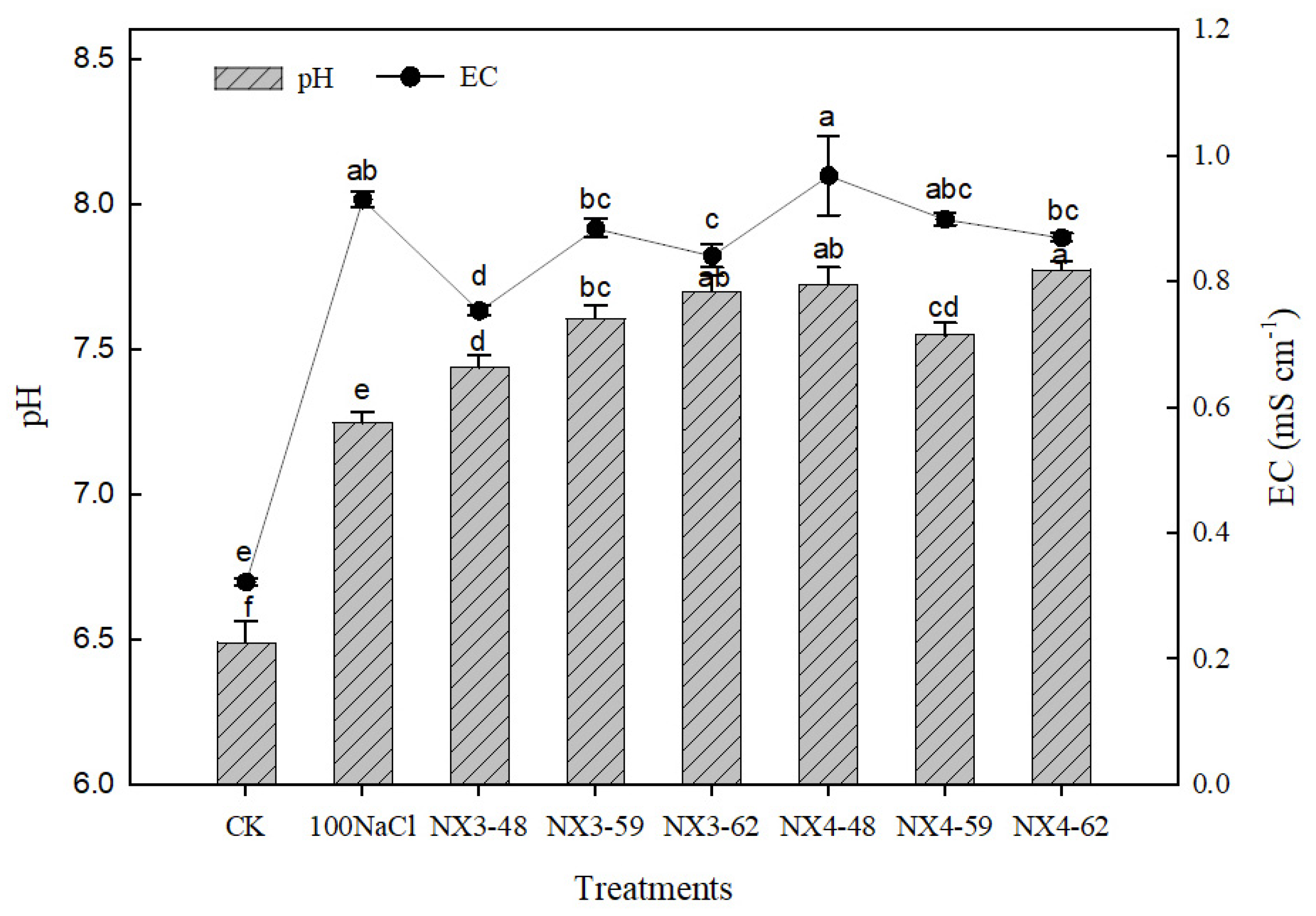
3.4. Substrate Nutrients
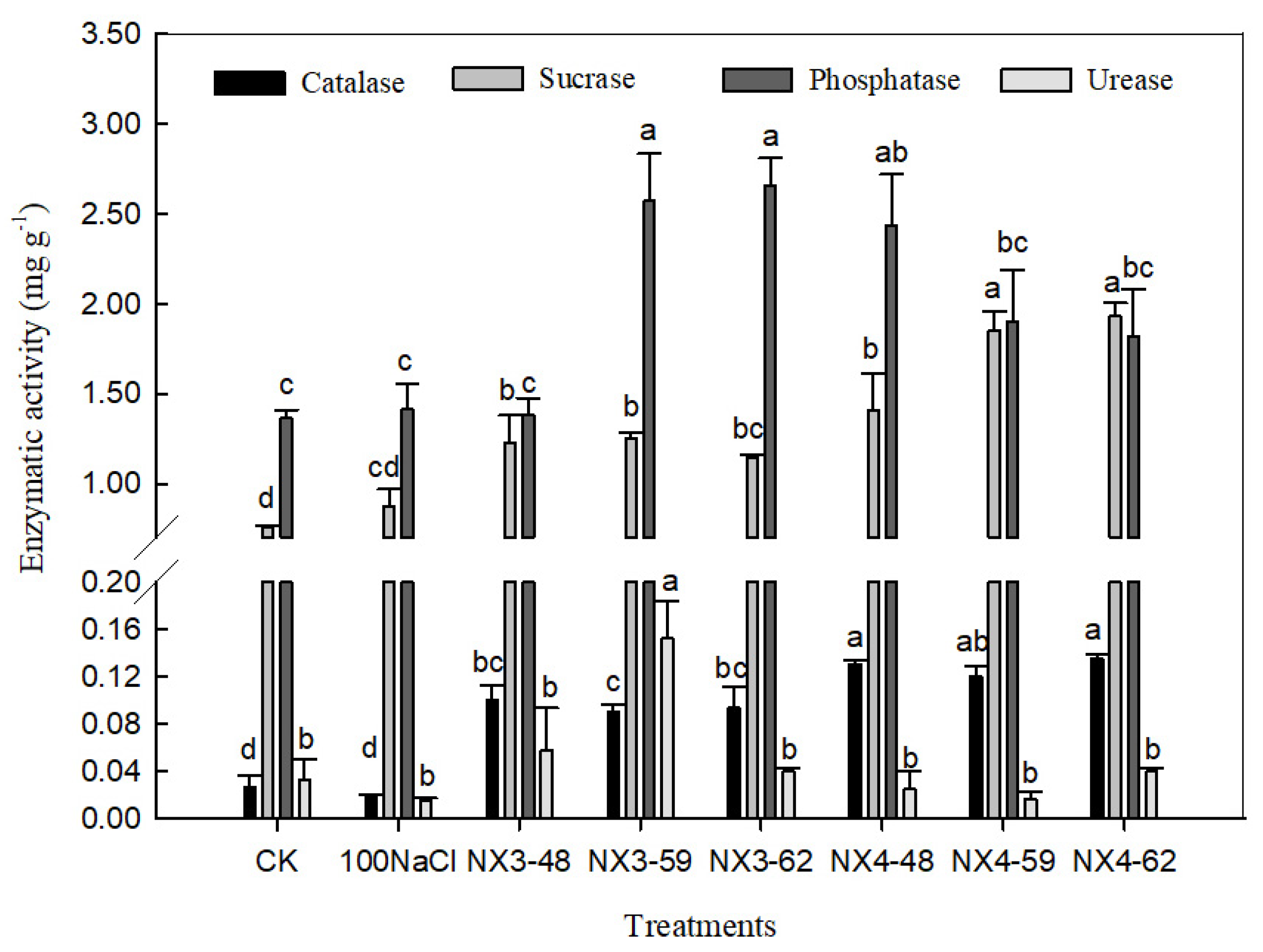
3.5. Substrate Enzyme Activity
3.6. Correlation Analysis
3.7. PCA Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singam, K.; Juntawong, N.; Cha-Um, S.; Kirdmanee, C. Salt stress induced ion accumulation, ion homeostasis, membrane injury and sugar contents in salt-sensitive rice (Oryza sativa L. spp. indica) roots under isoosmotic conditions. Afr. J. Biotechnol. 2011, 10, 1340–1346. [Google Scholar] [CrossRef]
- Abbasi, G.H.; Akhtar, J.; Ahmad, R.; Jamil, M.; Anwar-ul-Haq, M.; Ali, S.; Ijaz, M. Potassium application mitigates salt stress differentially at different growth stages in tolerant and sensitive maize hybrids. Plant Growth Regul. 2015, 76, 111–125. [Google Scholar] [CrossRef]
- Bodner, G.; Nakhforoosh, A.; Kaul, H.P. Management of crop water under drought: A review. Agron Sustain. Dev. 2015, 35, 401–442. [Google Scholar] [CrossRef]
- Silva, R.; Filgueiras, L.; Santos, B.; Coelho, M.; Silva, M.; Estrada-Bonilla, G.; Vidal, M.; Baldani, J.; Meneses, C. Gluconacetobacter diazotrophicus changes the molecular mechanisms of root development in Oryza sativa L. growing under water stress. Int. J. Mol. Sci. 2020, 21, 333. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, V.K.; Tripathi, V.; Singh, P.P.; Singh, A.K. Plant Growth-Promoting Rhizobacteria (PGPR): Perspective in agriculture under biotic and abiotic stress. New Future Dev. Microb. Biotechnol. Bioeng. 2018, 1, 333–342. [Google Scholar] [CrossRef]
- Rahnama, A.; James, R.A.; Poustini, K.; Munns, R. Stomatal conductance as a screen for osmotic stress tolerance in durum wheat growing in saline soil. Funct. Plant Biol. 2010, 37, 255–263. [Google Scholar] [CrossRef]
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 2012, 32, 181–200. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, K.; Wang, H.; Shang, Y.; Yang, X. Characteristics of phenol degradation in saline conditions of a halophilic strain JS3 isolated from industrial activated sludge. Mar. Pollut. Bull. 2015, 99, 230–234. [Google Scholar] [CrossRef]
- Yasar, E.; Sezai, E.; Ayhan, H.; Ramazan, C. Effects of plant growth promoting rhizobacteria (PGPR) on rooting and root growth of kiwifruit (Actinidia deliciosa) stem cuttings. Biol. Res. 2010, 43, 91–98. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Shaharoona, B.; Arshad, M.; Crowley, D.E. Population density and functional diversity of plant growth promoting rhizobacteria associated with avocado trees in saline soils. Appl. Soil. Ecol. 2012, 62, 147–154. [Google Scholar] [CrossRef]
- Wang, C.J.; Wei, Y.; Chao, W.; Gu, C.; Niu, D.D.; Liu, H.X.; Wang, Y.P.; Guo, J.H. Induction of Drought Tolerance in Cucumber Plants by a Consortium of Three Plant Growth-Promoting Rhizobacterium Strains. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Jha, Y.; Subramanian, R.B.; Patel, S. Combination of endophytic and rhizospheric plant growth promoting rhizobacteria in Oryza sativa shows higher accumulation of osmoprotectant against saline stress. Acta Physiol. Plant. 2011, 33, 797–802. [Google Scholar] [CrossRef]
- Abeer, H.; Abd_Allah, E.F.; Alqarawi, A.A.; Al-Huqail, A.A.; Stephan, W.; Dilfuza, E. The Interaction between Arbuscular Mycorrhizal Fungi and Endophytic Bacteria Enhances Plant Growth of Acacia gerrardii under Salt Stress. Front. Microbiol. 2016, 7, 1089. [Google Scholar] [CrossRef]
- Abbas, H.; Patel, R.M. Culturable endophytic bacteria from halotolerant Salicornia brachata L.: Isolation and plant growth promoting traits. Int. J. Microbiol. 2018, 10, 1074. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Li, L.; Lindstrom, K.; Rasanen, L.A. A synergistic interaction between salt-tolerant Pseudomonas and Mesorhizobium strains improves growth and symbiotic performance of liquorice (Glycyrrhiza uralensis Fish.) under salt stress. Appl. Microbiol. Biot. 2016, 100, 2829–2841. [Google Scholar] [CrossRef]
- Zhang, J.; Aziz, M.; Qiao, Y.; Han, Q.; Li, J.; Wang, Y.; Shen, X.; Wang, S.; Pare, P. Soil microbe Bacillus subtilis (GB03) induces biomass accumulation and salt tolerance with lower sodium accumulation in wheat. Crop Pastureence. 2014, 65, 423–427. [Google Scholar] [CrossRef]
- Abd_Allah, E.F.; Alqarawi, A.A.; Hashem, A.; Radhakrishnan, R.; Al-Huqail, A.A.; Al-Otibi, F.O.N.; Malik, J.A.; Alharbi, R.I.; Egamberdieva, D. Endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by regulating the plant defense mechanisms. J. Plant Interact. 2018, 13, 37–44. [Google Scholar] [CrossRef]
- Pinyapach, S.; Andi, K.; Tzu-Chuan, K.; Huey-Wen, C. A multifaceted rhizobacterium Bacillus licheniformis functions as a fungal antagonist and a promoter of plant growth and abiotic stress tolerance. Environ. Exp. Bot. 2018, 155, 541–551. [Google Scholar] [CrossRef]
- Chookietwattana, K.; Maneewan, K. Selection of efficient salt-tolerant bacteria containing ACC deaminase for promotion of tomato growth under salinity stress. Plant Soil Environ. 2012, 31, 30–36. [Google Scholar]
- He, Y.; Pantigoso, H.A.; Wu, Z.; Vivanco, J.M. Co-inoculation of Bacillus spp. and Pseudomonas putida at different development stages acts as a biostimulant to promote growth, yield and nutrient uptake of tomato. J. Appl. Microbiol. 2019, 127, 196–207. [Google Scholar] [CrossRef]
- Pandey, C.; Bajpai, V.K.; Negi, Y.K.; Rather, I.A.; Maheshwari, D.K. Effect of plant growth promoting Bacillus spp. on nutritional properties of Amaranthus hypochondriacus grains. Saudi. J. Biol. Sci. 2018, 25, 1066–1071. [Google Scholar] [CrossRef]
- Prakash, V.J.; Kumar, J.D.; Ram, K.; Satya, P.; Janardan, Y.; Vijai, S. Characterization and Screening of Thermophilic Bacillus Strains for Developing Plant Growth Promoting Consortium From Hot Spring of Leh and Ladakh Region of India. Front. Microbiol. 2018, 9, 1293. [Google Scholar] [CrossRef]
- Walker, R.; Powell, A.A.; Seddon, B. Bacillus isolates from the spermosphere of peas and dwarf French beans with antifungal activity against Botrytis cinerea and Pythium species. J. Appl. Microbiol. 2010, 84, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Vashisth, A.; Nagarajan, S. Effect on germination and early growth characteristics in sunflower (Helianthus annuus) seeds exposed to static magnetic field. J. Plant Physiol. 2010, 167, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 1950, 1, 121–160. [Google Scholar] [CrossRef]
- Wang, R.; Liu, J.Q.; Zhou, H.X.; Lan, Z.Q.; Han, Z.Y.; Zhang, X.Y. Effects of Bio-organic Fertilizer and Water-retaining Agent on Cucumber Growth and Soil Fertility under Continuous Cropping in Greenhouse. J. Henan Agric. Sci. 2018, 47, 45–53. [Google Scholar] [CrossRef]
- Nkonge, C.; Ballance, G.M. A sensitive colorimetric procedure for nitrogen determination in micro-Kjeldahl digests. J. Agric. Food Chem. 1982, 30, 416–420. [Google Scholar] [CrossRef]
- Kalembasa, S.J.; Jenkinson, D.S. A Comparative Study of Titrimetric and Gravimetric Methods for the Determination of Organic Carbon in Soil. J. Sci. Food Agric. 1973, 24, 1085–1090. [Google Scholar] [CrossRef]
- Houba, V.; Temminghoff, E.; Gaikhorst, G.A.; Vark, W.V. Soil analysis procedures using 0.01M calcium chloride as extraction regent. Commun. Soil Sci. Plan. 2000, 31, 1299–1396. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A.; Watanabe, F.; Dean, L. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; United States Department of Agriculture: Washington, DC, USA, 1954; Volume 939, pp. 1–19. [Google Scholar]
- Mehta, A. Physico-Chemical Analysis of Agriculture Soil Effluents from Pharmaceutical Industry at Mandideep Bhopal in Central India. Int. J. New Innov. Sci. Technol. 2014, 2, 8–24. [Google Scholar]
- Nannipieri, P.; Ceccanti, B.; Cervelli, S.; Matarese, E. Extraction of Phosphatase, Urease, Proteases, Organic Carbon, and Nitrogen from Soil. Soil Sci. Soc. Am. J. 1980, 45, 1011–1016. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.W.; Bin, W.U. Comprehensive Evaluation of Salt Tolerance and Screening for Salt Tolerant Accessions of Naked Oat (Avena nuda L.) at Germination Stage. Sci. Agric. Sin. 2014, 47, 2038–2046. [Google Scholar] [CrossRef]
- Nowak, J. Benefits of in vitro “biotization” of plant tissue cultures with microbial inoculants. In Vitro Cell. Dev. Biol. Plant. 1998, 34, 122–130. [Google Scholar] [CrossRef]
- Khan, M.A.; Sahile, A.A.; Jan, R.; Asaf, S.; Hamayun, M.; Imran, M.; Adhikari, A.; Kang, S.M.; Kim, K.M.; Lee, I.J. Halotolerant bacteria mitigate the effects of salinity stress on soybean growth by regulating secondary metabolites and molecular responses. BMC Plant Biol. 2021, 21, 1–176. [Google Scholar] [CrossRef]
- Pellegrini, M.; Pagnani, G.; Bernardi, M.; Mattedi, A.; Spera, D.; Del Gallo, M. Cell-Free Supernatants of Plant Growth-Promoting Bacteria: A Review of Their Use as Biostimulant and Microbial Biocontrol Agents in Sustainable Agriculture. Sustainability 2020, 12, 9917. [Google Scholar] [CrossRef]
- Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.; Baba, Z.; Sheikh, T.; Reddy, M.; El Enshasy, H.; Gafur, A. Bacterial Plant Biostimulants: A Sustainable Way towards Improving Growth, Productivity, and Health of Crops. Sustainability 2021, 13, 2856. [Google Scholar] [CrossRef]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur. J. Plant Pathol. 2007, 119, 329–339. [Google Scholar] [CrossRef]
- Chandra, D.; Srivastava, R.; Gupta, V.; Franco, C.; Sharma, A. Evaluation of ACC-deaminase-producing rhizobacteria to alleviate water-stress impacts in wheat (Triticum aestivum L.) plants. Can. J. Microbiol. 2019, 65, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qiu, Y.; Yao, T.; Ma, Y.; Zhang, H.; Yang, X. Effects of PGPR microbial inoculants on the growth and soil properties of Avena sativa, Medicago sativa, and Cucumis sativus seedlings. Soil Till Res. 2020, 199, 104577. [Google Scholar] [CrossRef]
- Ejaz, S.; Batool, S.; Anjum, M.A.; Naz, S.; Qayyum, M.F.; Naqqash, T.; Shah, K.H.; Ali, S. Effects of inoculation of root-associative Azospirillum and Agrobacterium strains on growth, yield and quality of pea (Pisum sativum L.) grown under different nitrogen and phosphorus regimes. Sci. Hortic. 2020, 270, 109401. [Google Scholar] [CrossRef]
- Amp, N.T.; Saraf, M. Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants. J. Plant Interact. 2010, 5, 51–58. [Google Scholar] [CrossRef]
- Park, Y.G.; Mun, B.G.; Kang, S.M.; Hussain, A.; Yun, B.W. Bacillus aryabhattai SRB02 tolerates oxidative and nitrosative stress and promotes the growth of soybean by modulating the production of phytohormones. PLoS ONE 2017, 12, e0173203. [Google Scholar] [CrossRef]
- Cohen, A.C.; Bottini, R.; Pontin, M.; Berli, F.J.; Moreno, D.; Bo Cc Anlandro, H.; Travaglia, C.N.; Piccoli, P.N. Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol. Plantarum. 2015, 153, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhu, L.; Xie, Y.; Li, F.; Xin, X.; Ma, Z.; Wang, J. Bacillus licheniformis SA03 Confers Increased Saline–Alkaline Tolerance in Chrysanthemum Plants by Induction of Abscisic Acid Accumulation. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Esitken, A.; Yildiz, H.E.; Ercisli, S.; Donmez, M.F.; Gunes, A. Effects of plant growth promoting bacteria (PGPB) on yield, growth and nutrient contents of organically grown strawberry. Sci. Hortic. 2010, 124, 62–66. [Google Scholar] [CrossRef]
- Berg, G.; Alavi, M.; Schmidt, C.S.; Zachow, C.; Egamberdieva, D.; Kamilova, F.; Lugtenberg, B. Biocontrol and Osmoprotection for Plants under Salinated Conditions. Mol. Microb. Ecol. Rhizosphere 2013, 1, 561–573. [Google Scholar]
- Jamal, Q.; Yong, S.L.; Jeon, H.D.; Kim, K.Y. Effect of plant growth-promoting bacteria Bacillus amylliquefaciens Y1 on soil properties, pepper seedling growth, rhizosphere bacterial flora and soil enzymes. Plant Protect. Sci. 2018, 54, 129–137. [Google Scholar] [CrossRef]
- García-López, A.M.; Recena, R.; Avilés, M.; Delgado, A. Effect of Bacillus subtilis QST713 and Trichoderma asperellum T34 on P uptake by wheat and how it is modulated by soil properties. J. Soil Sediment. 2018, 18, 727–738. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Liu, N.; He, H.; Cao, X.; Lv, C.; Zhang, K.; Dai, J. Effects of different types of microbial inoculants on available nitrogen and phosphorus, soil microbial community, and wheat growth in high-P soil. Environ. Sci. Pollut. R. 2021. [Google Scholar] [CrossRef]
- Azri, M.H. Effects of Bacillus salmalaya Strain 139SI Inoculation on Yield and Nutrients Uptake of Oil Palm. Int. J. Agric. Biol. 2018, 20, 499–506. [Google Scholar] [CrossRef]
- Daraz, U.; Li, Y.; Sun, Q.; Zhang, M.; Ahmad, I. Inoculation of Bacillus spp. Modulate the soil bacterial communities and available nutrients in the rhizosphere of vetiver plant irrigated with acid mine drainage. Chemosphere 2021, 263, 128345. [Google Scholar] [CrossRef] [PubMed]
- Hassan, T.U.; Bano, A. The stimulatory effects of L-tryptophan and plant growth promoting rhizobacteria (PGPR) on soil health and physiology of wheat. J. Soil Sci. Plant. Nut. 2015, 15, 190–201. [Google Scholar] [CrossRef]
- Nakatani, A.S.; Fernandes, M.F.; Souza, R.A.D.; Silva, A.P.D.; Reis, F.B.D.; Mendes, I.C.; Hungria, M.E. Effects of the glyphosate-resistance gene and of herbicides applied to the soybean crop on soil microbial biomass and enzymes. Field Crop. Res. 2014, 162, 20–29. [Google Scholar] [CrossRef]
- Chen, S.Y.; Guo, L.Y.; Bai, J.G.; Zhang, Y.; Wang, X.J. Biodegradation of p-hydroxybenzoic acid in soil by Pseudomonas putida CSY-P1 isolated from cucumber rhizosphere soil. Plant Soil 2015, 389. [Google Scholar] [CrossRef]
- Xun, F.; Xie, B.; Liu, S.; Guo, C. Effect of plant growth-promoting bacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) inoculation on oats in saline-alkali soil contaminated by petroleum to enhance phytoremediation. Environ. Sci. Pollut. R. 2015, 22, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhou, J.J.; Wang, E.T.; Chen, Q.; Xu, J.; Sun, J.G. Multiphasic characterization of a plant growth promoting bacterial strain, Burkholderia sp. 7016 and its effect on tomato growth in the field. J. Integr. Agric. 2015, 14, 1855–1863. [Google Scholar] [CrossRef]
- Rosier, A.; Medeiros, F.; Bais, H.P. Defining plant growth promoting rhizobacteria molecular and biochemical networks in beneficial plant-microbe interactions. Plant Soil 2018, 428, 35–55. [Google Scholar] [CrossRef]




| Treatments | The Seeding Vigor Index (VI) | |||
|---|---|---|---|---|
| CK | NX-48 | NX-59 | NX-62 | |
| 0 mM·L−1 NaCl | 74.12 | 96.71 | 77.46 | 84.95 |
| 100 mM·L−1 NaCl | 14.49 | 17.39 | 16.83 | 22.78 |
| Treatment | Organic Matter (g·kg−1) | Total N (g·kg−1) | C/N | Available N (mg·kg−1) | Available P (mg·kg−1) | Available K (mg·kg−1) |
|---|---|---|---|---|---|---|
| CK | 11.456 ± 0.64ab | 0.826 ± 0.10ab | 3.88 ± 0.18c | 8.587 ± 1.53bc | 1.829 ± 0.16c | 15.158 ± 2.95a |
| 100NaCl | 13.042 ± 0.71a | 0.820 ± 0.08ab | 3.84 ± 0.17c | 7.747 ± 0.25bc | 1.203 ± 0.03c | 9.263 ± 2.95ab |
| NX3-48 | 13.923 ± 1.77a | 0.905 ± 0.14ab | 3.83 ± 0.31c | 6.900 ± 0.56c | 2.532 ± 0.11a | 10.737 ± 1.47ab |
| NX3-59 | 14.893 ± 1.16a | 0.907 ± 0.04ab | 3.81 ± 0.18c | 12.973 ± 2.49a | 2.509 ± 0.10a | 6.316 ± 1.47b |
| NX3-62 | 12.690 ± 0.15a | 0.744 ± 0.01b | 3.85 ± 0.04c | 7.140 ± 0.73bc | 2.339 ± 0.06ab | 4.842 ± 1.47b |
| NX4-48 | 8.318 ± 0.32b | 0.879 ± 0.03ab | 4.55 ± 0.10a | 9.987 ± 1.36ab | 2.470 ± 0.20ab | 6.316 ± 1.47b |
| NX4-59 | 8.989 ± 1.46b | 1.009 ± 0.01a | 4.36 ± 0.32ab | 9.893 ± 1.39ab | 2.084 ± 0.09bc | 4.842 ± 1.47b |
| NX4-62 | 14.011 ± 1.33a | 0.784 ± 0.04ab | 4.07 ± 0.23bc | 11.107 ± 0.89ab | 2.718 ± 0.136a | 6.316 ± 1.47b |
| Index | pH | EC | OM | TN | C/N | AN | AP | AK | CA | SA | PA | UA | RL | SL | SD | CC | DW | FW |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1.00 | |||||||||||||||||
| EC | 0.881 ** | 1.00 | ||||||||||||||||
| OM | −0.08 | −0.17 | 1.00 | |||||||||||||||
| TN | 0.06 | 0.17 | −0.31 | 1.00 | ||||||||||||||
| C/N | 0.35 | 0.37 | −0.896 ** | 0.43 | 1.00 | |||||||||||||
| AN | 0.26 | 0.24 | −0.08 | 0.19 | 0.30 | 1.00 | ||||||||||||
| AP | 0.60 | 0.19 | 0.01 | 0.05 | 0.21 | 0.26 | 1.00 | |||||||||||
| AK | −0.910 ** | −0.848 ** | 0.17 | −0.13 | −0.41 | −0.39 | −0.40 | 1.00 | ||||||||||
| CA | 0.764 * | 0.46 | −0.30 | 0.27 | 0.59 | 0.30 | 0.869 ** | −0.66 | 1.00 | |||||||||
| SA | 0.69 | 0.52 | −0.17 | 0.35 | 0.88 ** | 0.42 | 0.59 | −0.68 | 0.852 ** | 1.00 | ||||||||
| PA | 0.64 | 0.49 | −0.22 | −0.08 | 0.25 | 0.48 | 0.79 * | −0.745 * | 0.50 | 0.25 | 1.00 | |||||||
| UA | 0.18 | 0.05 | 0.50 | 0.14 | −0.43 | 0.77 * | 0.43 | −0.11 | 0.11 | −0.06 | 0.42 | 1.00 | ||||||
| RL | −0.792 * | −0.974 ** | 0.09 | −0.06 | −0.26 | −0.26 | −0.05 | 0.754 * | −0.29 | −0.39 | −0.41 | −0.06 | 1.00 | |||||
| SL | −0.23 | 0.05 | −0.54 | 0.67 | 0.53 | −0.19 | −0.36 | 0.24 | −0.07 | 0.09 | −0.55 | −0.48 | −0.02 | 1.00 | ||||
| SD | 0.855 ** | 0.55 | 0.06 | 0.02 | 0.14 | 0.14 | 0.871 ** | −0.69 | 0.827 * | 0.56 | 0.66 | 0.42 | −0.42 | −0.43 | 1.00 | |||
| CC | 0.725 * | 0.35 | 0.02 | 0.19 | 0.24 | 0.19 | 0.899 ** | −0.64 | 0.908 ** | 0.723 * | 0.55 | 0.32 | −0.17 | −0.34 | 0.911 ** | 1.00 | ||
| DW | 0.61 | 0.23 | −0.20 | 0.09 | 0.40 | 0.09 | 0.784 * | −0.60 | 0.877 ** | 0.70 | 0.53 | 0.00 | −0.03 | −0.29 | 0.764 * | 0.921 ** | 1.00 | |
| FW | 0.744 * | 0.50 | 0.07 | 0.18 | 0.16 | 0.32 | 0.870 ** | −0.50 | 0.766 * | 0.52 | 0.52 | 0.60 | −0.42 | −0.24 | 0.893 ** | 0.770 * | 0.52 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, R.; Lin, W.; Gong, K.; Han, Z.; Ma, H.; Zhang, M.; Zhang, Q.; Gao, Y.; Li, J.; Zhang, X. Bacillus Co-Inoculation Alleviated Salt Stress in Seedlings Cucumber. Agronomy 2021, 11, 966. https://doi.org/10.3390/agronomy11050966
Qi R, Lin W, Gong K, Han Z, Ma H, Zhang M, Zhang Q, Gao Y, Li J, Zhang X. Bacillus Co-Inoculation Alleviated Salt Stress in Seedlings Cucumber. Agronomy. 2021; 11(5):966. https://doi.org/10.3390/agronomy11050966
Chicago/Turabian StyleQi, Ruixue, Wei Lin, Kaixuan Gong, Zeyu Han, Hui Ma, Miao Zhang, Qiannan Zhang, Yanming Gao, Jianshe Li, and Xueyan Zhang. 2021. "Bacillus Co-Inoculation Alleviated Salt Stress in Seedlings Cucumber" Agronomy 11, no. 5: 966. https://doi.org/10.3390/agronomy11050966
APA StyleQi, R., Lin, W., Gong, K., Han, Z., Ma, H., Zhang, M., Zhang, Q., Gao, Y., Li, J., & Zhang, X. (2021). Bacillus Co-Inoculation Alleviated Salt Stress in Seedlings Cucumber. Agronomy, 11(5), 966. https://doi.org/10.3390/agronomy11050966






