The Participation of Microbiota in the Transformation of Nitrogen Compounds in the Soil—A Review
Abstract
:1. Introduction
2. Biological Changes of Nitrogen
2.1. Binding of Atmospheric Nitrogen
2.1.1. Non-Symbiotic Nitrogen Fixation
2.1.2. Symbiotic Nitrogen Fixation
2.2. Decomposition of Organic Nitrogen Compounds
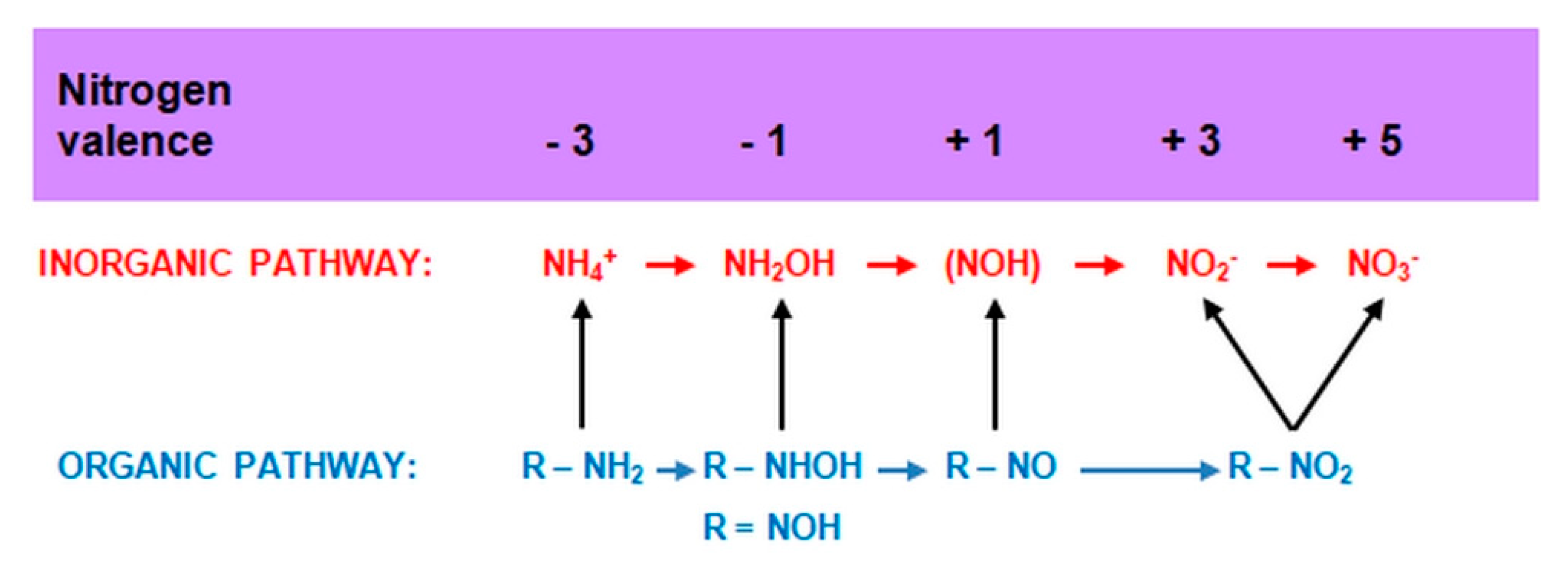
2.3. Nitrification
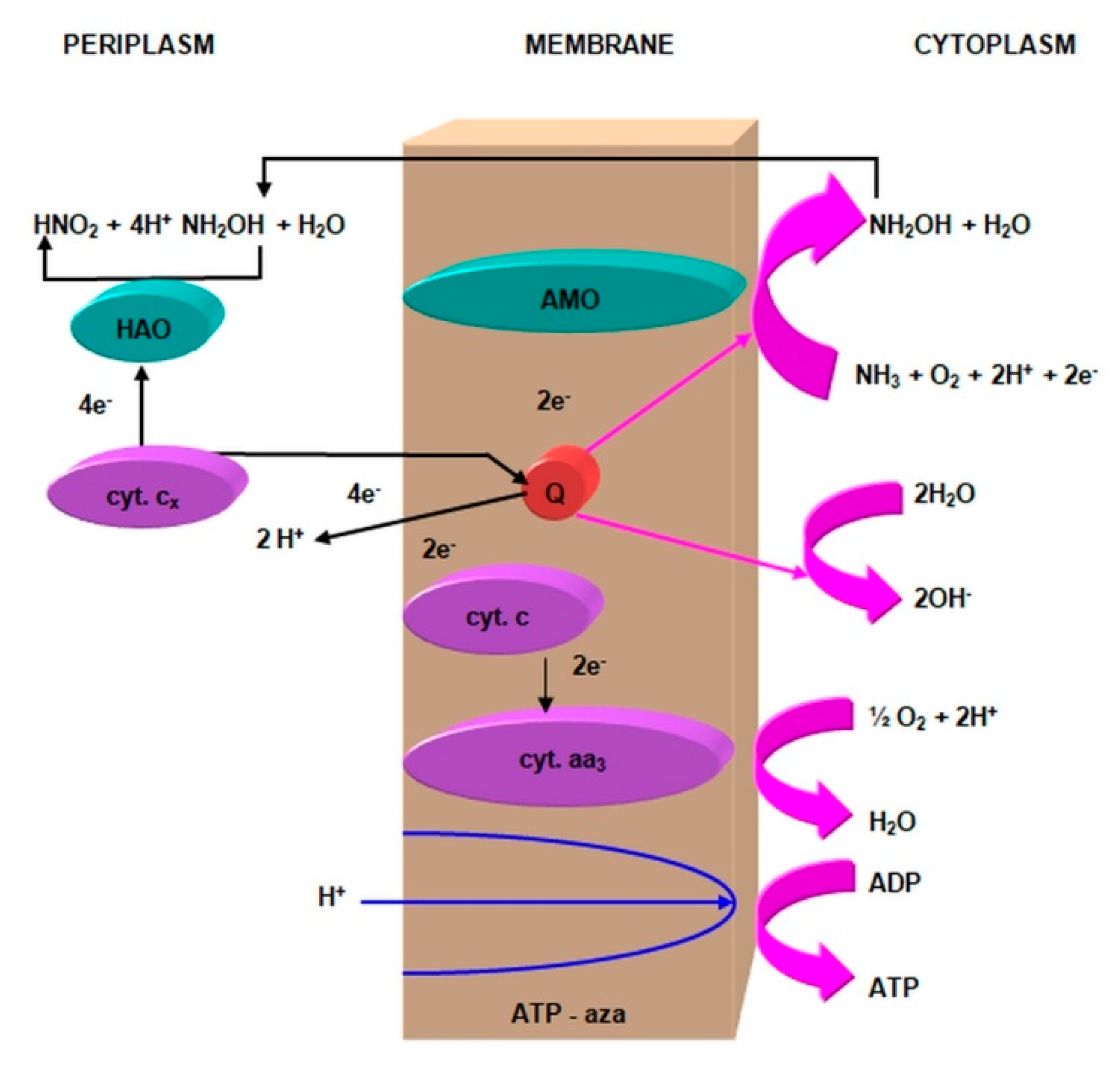
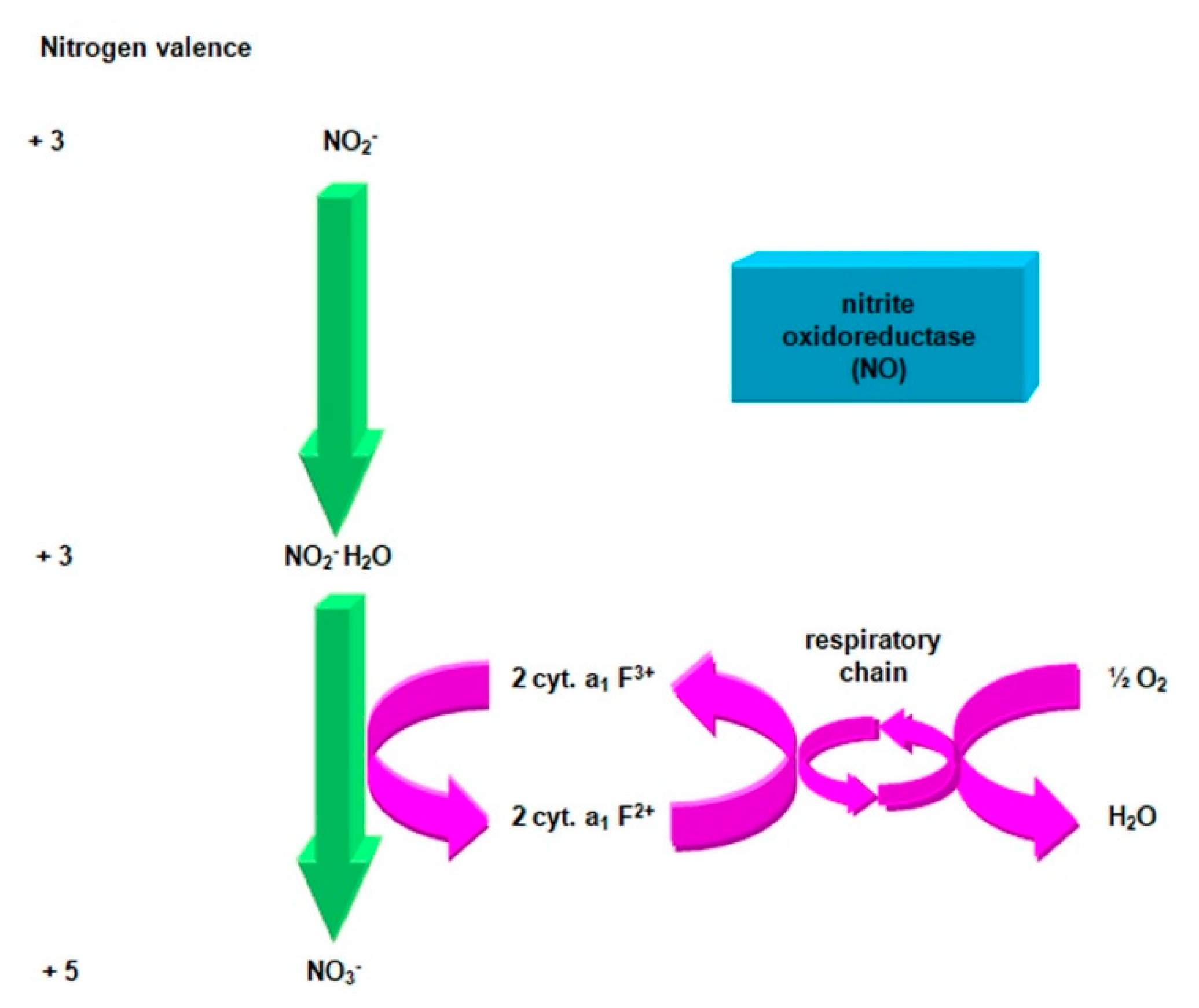
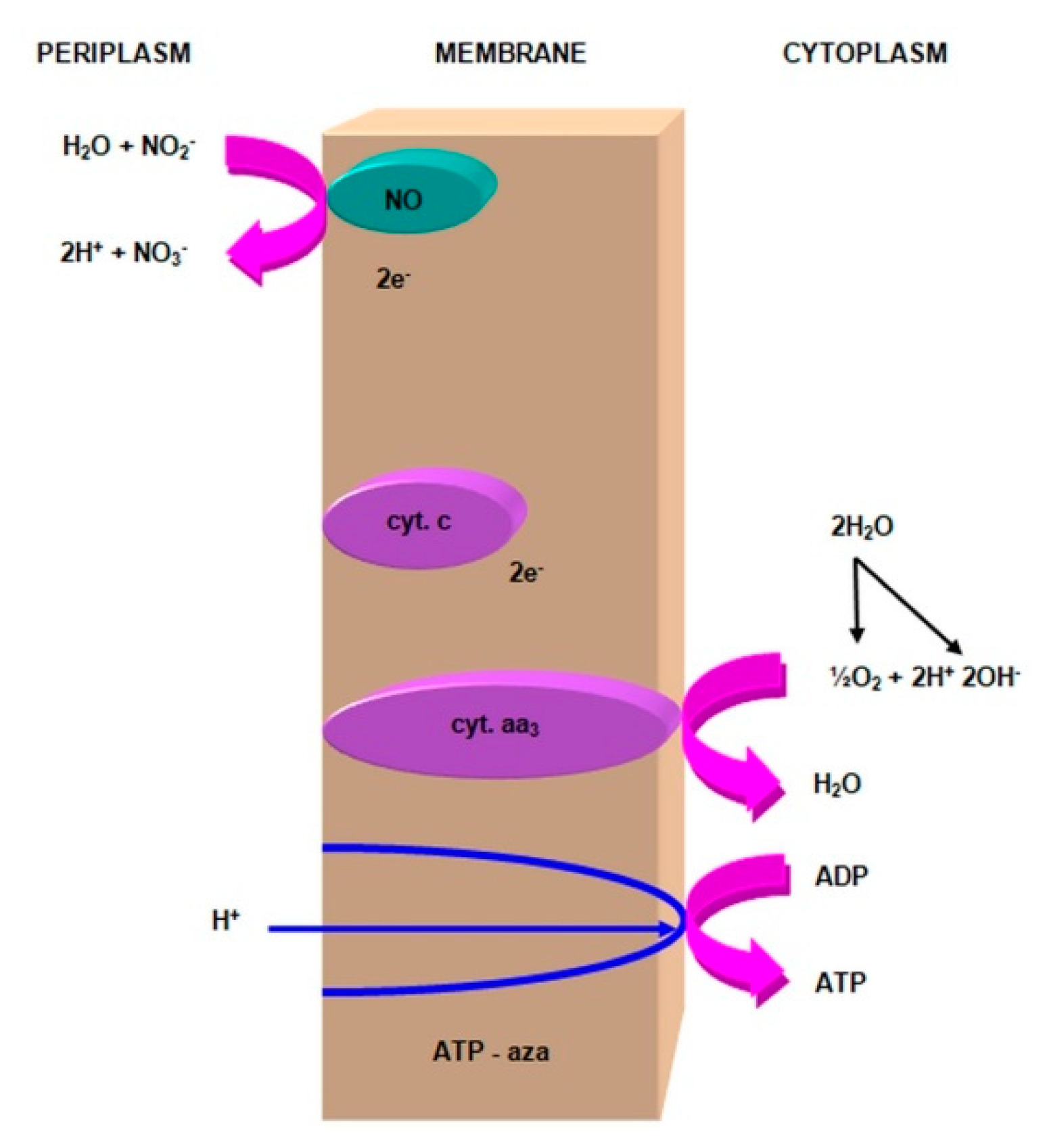
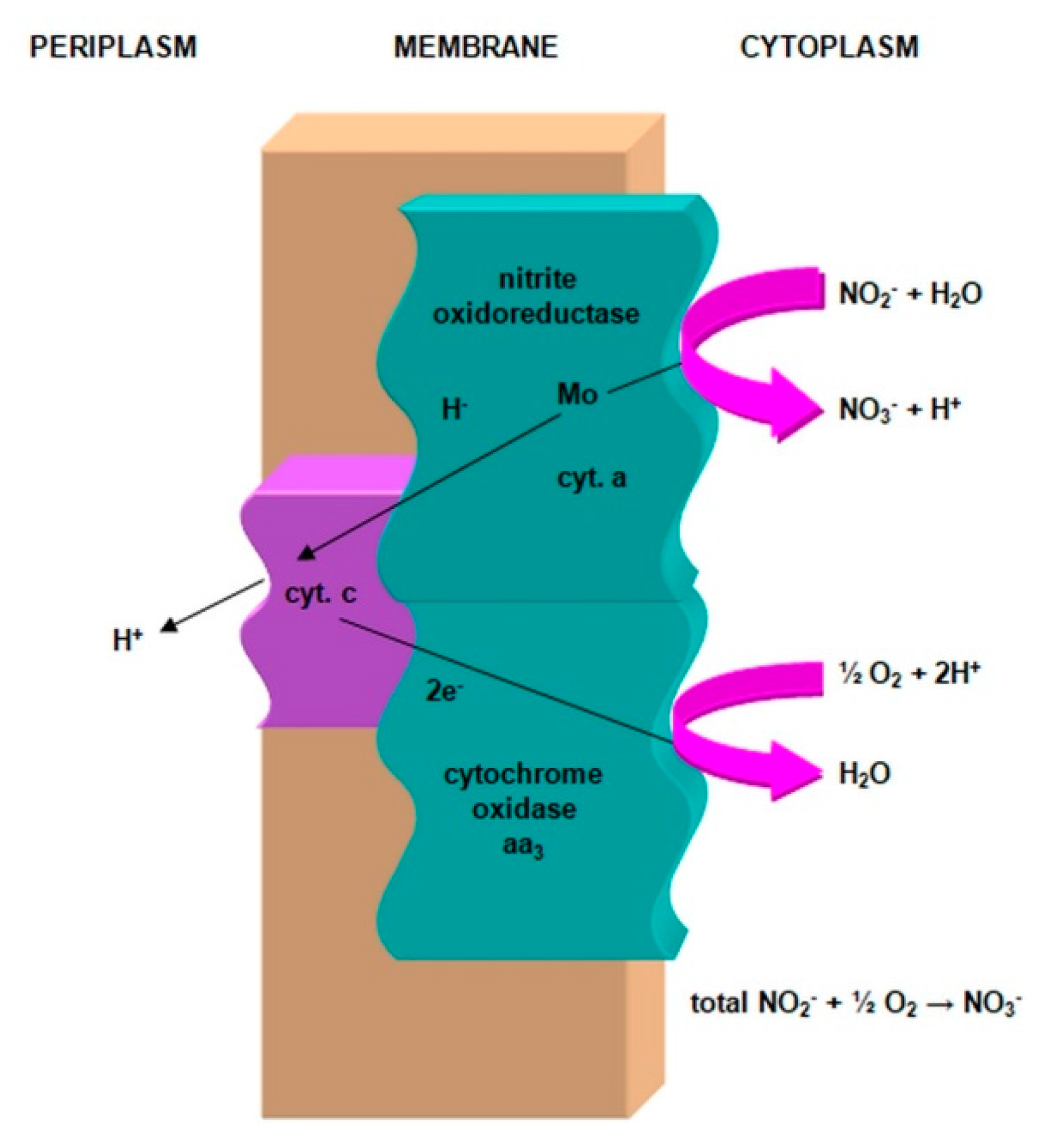
2.3.1. The Morphology and Ecology of Nitrifying Bacteria and the Biochemistry of the Nitrification Process
- catalyzes the inclusion of one oxygen atom in the substrate (NH3);
- highly unstable nitrile (NOH) radical;
- nitrohydroxylamine (NO2NHOH).
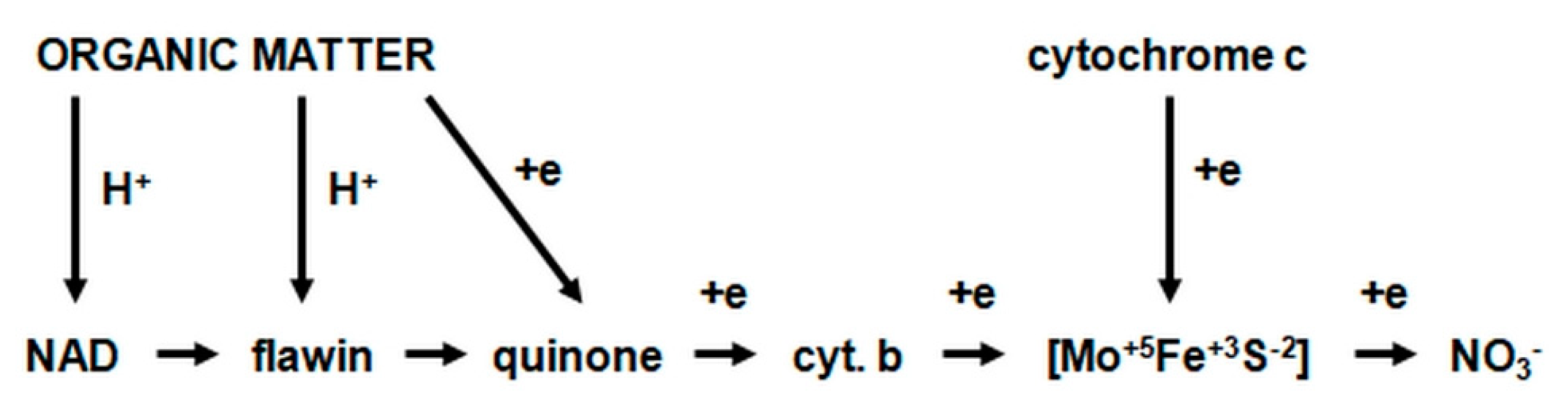
- nitrite oxidase (NO), located in the cell membrane, which contains hemi c and a, in the form of cytochromes c1 and a1 and three proteins, with different molar masses, forming the molecule transporting electrons;
- cytochrome c;
- unknown quinone;
- NADH dehydrogenase;
- molybdenum center;
- nonhemous iron and sulfur proteins [54].
- is an electron donor for oxidative phosphorylation;
- is an electron donor for the synthesis of NADH [41].
2.3.2. Heterotrophic Nitrification
2.4. Denitrification
2.4.1. Biochemistry of Nitrate Reduction
- Alcaligenes (A. eutrophus, A. faecalis),
- Arthrobacter (A. citreus, A. simolex, A. terregens),
- Bacillus (B. licheniformis, B. stearothermophilus),
- Chromobacterium (C. lividum, C. violaceum),
- Clostridium (C. aurantobutyricum, C. limosum, C. oroticum, C. rectum),
- Corynebacterium (C. mycetoides),
- Cytophaga (C. johansone),
- Dactylosporangium (D. aurantiacum),
- Eubacterium (E. nitritogenes),
- Hyphomicrobium (H. vulgare),
- Micromonospora (M. chalacea),
- Mycobacterium (M. intercellulare),
- Moraxella (M. kingie),
- Nocardia (N. otididis-caviarum),
- Paracoccus (P. denitrificans, P. halodenitrificans),
- Propionibacterium (P. acidi-propionici),
- Pseudomonas (P. aeruginosa, P. aureofaciens, P. fluorescens, P. stutzeri),
- Rhizobium (R. leguminosarum, R. japonicum),
- Rhodopseudomonas (R. capsulata, R. palustris, R. sheroides),
- Spirillum (S. lipoferum),
- Streptosporangium (S. pseudovulgare),
- Thiobacillus (T. denitrificans),
- Vibrio (V. succinogenes).
- assimilatory reduction of nitrate (NO3−);
- dissimilatory reduction of nitrate (NO3−), also known as denitrification.
2.4.2. Assimilatory Reduction of Nitrate (NO3−)
- AnaR—assimilatory nitrate reductase, which occurs in the cytoplasm and works under aerobic conditions, initiating a chain of biochemical transformations of assimilated nitrate (NO3−).
- AniR—assimilatory nitrite reductase, which catalyzes the reduction of nitrite (NO2−). The cofactor of this enzyme is a reduced nicotinamide adenic dinucleotide (NADH + H+) as a hydrogen donor. The enzyme also interacts with cytochrome c.
- ANH2OHR—assimilatory hydroxylamine reductase, which catalyzes the reduction of hydroxylamine to ammonia in the presence of manganese (IV).
2.4.3. Dissimilatory Reduction of Nitrate (NO3−)
- DnaR—denitrifying nitrate reductase, which is associated with the cytoplasmic membrane of the cell and initiates chain biochemical transformations of nitrate (NO3−) during denitrification. This enzyme belongs to flavoproteids. In addition to the flavin fragment (FAD), it contains molybdenum and iron. It is active in the presence of cofactors NADH + H+ and FMN and interacts with cytochromes b and c, which transfer electrons to nitrate (NO3−). The activity of denitrifying nitrate reductase is inhibited by molecular oxygen.
- 2.
- DniR—denitrifying nitrite reductase. This enzyme catalyzes the reduction of nitrite (NO2−) to nitric oxide. The denitrifying nitrite reductase consists of iron, FAD and a protein called azurine, containing copper. This enzyme interacts with cytochromes c and d in the presence of the cofactor NADH + H+. The optimum activity of this reductase is at 30 °C and pH = 7.0.
- 3.
- DNOR—denitrifying nitrogenous oxide reductase. This enzyme catalyses the reduction of nitric oxide to nitrous oxide. It contains FAD and works with cytochromes b and c in the presence of cofactor NADH + H+.
- 4.
- DN2OR—denitrifying nitrous oxide reductase. This enzyme catalyzes the reduction of nitrous oxide to atmospheric nitrogen. Nitrous oxide is the penultimate stage of denitrification in many bacteria, but in some may be the final product of this process. It interacts with cytochromes b and c and probably contains copper. Denitrifying nitrous oxide reductase is extremely sensitive and deactivates after just one hour at room temperature [78].
- 5.
- DNH2OHR—denitrifying hydroxylamine reductase catalyzes the reduction of hydroxylamine to ammonia. This enzyme is activated by manganese, but under anaerobic conditions, in the presence of reduced forms of NADH + H+, pyocyanin and methylene blue, as hydrogen donors, the demand for this metal is much lower.
3. Future Perspectives
4. Conclusions
- establishing different types of symbiosis with soil microorganisms;
- stimulating the activity of microorganisms near the roots to increase nitrogen availability;
- reduction of nitrogen losses in the soil by limiting microbial processes such as nitrifiction and denitrification, directly by releasing inhibitors from the plant roots.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, X.; Zhang, C. Nitrogen cycling and environmental impacts in upland agricultural soils in North China: A review. J. Integr. Agric. 2017, 16, 2848–2862. [Google Scholar] [CrossRef]
- Martinez-Espinosa, R.M.; Cole, J.A.; Richardson, D.J.; Wartmough, N.J. Enzymology and ecology of the nitrogen cycle. Biochem. Soc. Trans. 2011, 39, 175–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purwanto, P.; Yuwariah, Y.; Sumadi, S.; Simarmata, T. Nitrogenase Activity and IAA Production of Indigenous Diazotroph and Its Effect on Rice Seedling Growth. AGRIVITA J. Agric. Sci. 2017, 39, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Rütting, T.; Aronsson, H.; Delin, S. Efficient use of nitrogen in agriculture. Nutr. Cycling Agroecosyst. 2018, 110, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Gholami, A.; Biyari, A.; Gholipoor, M.; Rahmani, H.A. Growth Promotion of Maize (Zea mays L.) by Plant-Growth-Promoting Rhizobacteria under Field Conditions. Commun. Soil. Sci. Plant Anal. 2012, 43, 1263–1272. [Google Scholar] [CrossRef]
- Ibáñez, F.; Tonelli, M.L.; Muñoz, V.; Figueredo, M.S.; Fabra, A. Bacterial Endophytes of Plants: Diversity, Invasion Mechanisms and Effects on the Host. In Endophytes: Biology and Biotechnology. Sustainable Development and Biodiversity; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 15. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, Y.; Zhang, W.; Zhou, H.; Chen, X.; Li, Y.; Wei, D.; Wei, Z. Roles of bacterial community in the transformation of organic nitrogen toward enhanced bioavailability during composting with different waste. Bioresour. Technol. 2019, 285, 121326. [Google Scholar] [CrossRef]
- Garrido-Oter, R.; Nakano, R.T.; Dombrowski, N.; Ma, K.W.; McHardy, A.C.; Schulze-Lefert, P. Modular Traits of the Rhizobiales Root Microbiota and Their Evolutionary Relationship with Symbiotic Rhizobia. Cell Host Microbe 2018, 24, 155–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolyzed-based products and humic substances in plant metabolizm. Sci. Agric. 2016, 73, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; El-Sawah, A.M.; Ali, D.F.I.; Alhaj Hamoud, Y.; Shaghaleh, H.; Sheteiwy, M.S. The Integration of Bio and Organic Fertilizers Improve Plant Growth, Grain Yield, Quality and Metabolism of Hybrid Maize. Agronomy 2020, 10, 319. [Google Scholar] [CrossRef] [Green Version]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Senatore, M.; Giordano, M.; El-Nakhel, C.; Sacco, A.; Rouphael, Y.; Colla, G.; Mori, M. Plant-Based Biostimulants Influence the Agronomical, Physiological, and Qualitative Responses of Baby Rocket Leaves under Diverse Nitrogen Conditions. Plants 2019, 8, 522. [Google Scholar] [CrossRef] [Green Version]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.K.; Subler, S.; Edwards, C.A. Effects of agricultural biostimulants on soil microbial activity and nitrogen dynamics. Appl. Soil Ecol. 2002, 19, 249–259. [Google Scholar] [CrossRef]
- Sangiorgio, D.; Cellini, A.; Donati, I.; Pastore, C.; Onofrietti, C.; Spinelli, F. Facing Climate Change: Application of Microbial Biostimulants to Mitigate Stress in Horticultural Crops. Agronomy 2020, 10, 794. [Google Scholar] [CrossRef]
- De Luca, V.; De Barreda, D.G.; Lidón, A.; Lull, C. Effect of Nitrogen-fixing Microorganisms and Amino Acid-based Biostimulants on Perennial Ryegrass. ASHS 2020, 30, 280–291. [Google Scholar] [CrossRef] [Green Version]
- Berg, S.; Dennis, P.G.; Paungfoo-Lonhienne, C.; Anderson, J.; Robinson, N.; Brackin, R.; Royle, A.; DiBella, L.; Schmidt, S. Effects of commercial microbial biostimulants on soil and root microbial communities and sugarcane yield. Biol. Fertil. Soils 2020, 56, 565–580. [Google Scholar] [CrossRef]
- Chen, S.K.; Edwards, C.A.; Subler, S. The influence of two agricultural biostimulants on nitrogen transformations, microbial activity, and plant growth in soil microcosms. Soil Biol. Biochem. 2003, 35, 9–19. [Google Scholar] [CrossRef]
- Pylak, M.; Oszust, K.; Frąc, M. Review report on the role of bioproducts, biopreparations, biostimulants and microbial inoculants in organic production of fruit. Rev. Environ. Sci. Biotechnol. 2019, 18, 597–616. [Google Scholar] [CrossRef] [Green Version]
- Puri, A.; Padda, K.P.; Chanway, C.P. Nitrogen-fixation by endophytic bacteria in agricultural crops: Recent advances. In Nitrogen in Agriculture—Updates; Khan, A., Fahad, S., Eds.; InTech: Rijeka, Croatia, 2018; pp. 73–94. Available online: https://www.intechopen.com/books/nitrogen-in-agriculture-updates/nitrogen-fixation-by-endophytic-bacteria-in-agricultural-crops-recent-advances (accessed on 11 March 2021).
- Boliyevich, M.S.; Asrorovna, S.G.; Ugli, B.D.K. Biological Nitrogen. AJMR 2020, 9, 66–68. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Shen, C.; Yang, F.; Wang, J.; Ge, Y. Significant dose effects of fertilizers on soil diazotrophic diversity, community composition, and assembly processes ina long-term paddy field fertilization experiment. Land Degrad. Dev. 2020, 1–10. [Google Scholar] [CrossRef]
- Vu HT, L.; Yukphan, P.; Charoenyingcharoen, P.; Malimas, S.; Nguyen, L.K.; Muramatsu, Y.; Yamada, Y. Acetobacter sacchari sp. nov., for a plant growth-promoting acetic acid bacterium isolated in Vietnam. Ann. Microbiol. 2019, 69, 1155–1163. [Google Scholar] [CrossRef]
- Papik, J.; Folkmanova, M.; Polivkova-Majorova, M.; Suman, J.; Uhlik, O. The invisible life inside plants: Deciphering the riddles of endophytic bacterial diversity. Biotechnol. Adv. 2020, 44, 107614. [Google Scholar] [CrossRef] [PubMed]
- Latef, A.A.H.A.; Alhmad, M.F.A.; Kordrostami, M.; Abo–Baker, A.B.A.E.; Zakir, A. Inoculation with Azospirillum lipoferum or Azotobacter chroococcum Reinforces Maize Growth by Improving Physiological Activities under Saline Conditions. J. Plant Growth Regul. 2020, 39, 1293–1306. [Google Scholar] [CrossRef]
- Batista, M.B.; Dixon, R. Manipulating nitrogen regulation in diazotrophic bacteria for agronomic benefit. Biochem. Soc. Trans. 2019, 47, 603–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kargapolova, K.Y.; Burygin, G.L.; Tkachenko, O.V.; Evseeva, N.V.; Pukhalskiy, Y.V.; Belimov, A.A. Effectiveness of inoculation of in vitro-grown potato microplants with rhizosphere bacteria of the genus Azospirillum. Plant Cell Tissue Organ Cult. 2020, 141, 351–359. [Google Scholar] [CrossRef]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting Biological Nitrogen Fixation: A Route Towards a Sustainable Agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef]
- Aasfar, A.; Bargaz, A.; Yaakoubi, K.; Hilali, A.; Bennis, I.; Zeroual, Y.; Meftah Kadmiri, I.M. Nitrogen Fixing Azotobacter Species as Potential Soil Biological Enhancers for Crop Nutrition and Yield Stability. Front. Microbiol. 2021, 12, 628379. [Google Scholar] [CrossRef]
- Baldani, J.I.; Baldani, V.D.L. History on the biological nitrogen fixation research in graminaceous plants: Special emphasis on the Brazilian experience. An. Acad. Bras. Ciênc. 2005, 77, 549–579. [Google Scholar] [CrossRef] [Green Version]
- Torres-Cruz, T.J.; Howell, A.J.; Reibold, R.H.; McHugh, T.A.; Eickhoff, M.A.; Reed, S.C. Species-specific nitrogenase activity in lichen-dominated biological soil crusts from the Colorado Plateau, USA. Plant Soil 2018, 429, 113–125. [Google Scholar] [CrossRef]
- Wolińska, A.; Kuźniar, A.; Zielenkiewicz, U.; Banach, A.; Izak, D.; Stępniewska, Z.; Błaszczyk, M. Metagenomic Analysis of Some Potential Nitrogen-Fixing Bacteria in Arable Soils at Different Formation Processes. Microb. Ecol. 2017, 73, 162–176. [Google Scholar] [CrossRef] [Green Version]
- Czaban, J.; Wróblewska, B. The Effect of Bentonite on the Survival of Azotobacter chroococcum in Sandy Soil in a Long-Term Plot Experiment. Pol. J. Environ. Stud. 2017, 26, 1–8. [Google Scholar] [CrossRef]
- Zheng, Y.; Liang, J.; Zhao, D.L.; Meng, C.; Xu, Z.C.; Xie, Z.H.; Zhang, C.S. The Root Nodule Microbiome of Cultivated and Wild Halophytic Legumes Showed Similar Diversity but Distinct Community Structure in Yellow River Delta Saline Soils. Microorganisms 2020, 8, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, J.; Wei, K.; Condron, L.M.; Chen, Z.; Xu, Z.; Feng, J.; Chen, L. Effects of elevated nitrogen and precipitation on soil organic nitrogen fractions and nitrogen-mineralizing enzymes in semi-arid steppe and abandoned cropland. Plant Soil 2017, 417, 217–229. [Google Scholar] [CrossRef]
- Fujii, K.; Yamada, T.; Hayakawa, C.; Nakanishi, A.; Funakawa, S. Decoupling of protein depolymerization and ammonification in nitrogen mineralization of acidic forest soils. Appl. Soil Ecol. 2020, 153, 103572. [Google Scholar] [CrossRef]
- Wang, C.; Wang, N.; Zhu, J.; Liu, Y.; Xu, X.; Niu, S.; He, N. Soil Gross N ammonification and nitrification from tropi cal to tempera te forests in ekstern China. Funct. Ecol. 2018, 32, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Mariano, E.; Jones, D.L.; Hill, P.W.; Trivelina, P.C.O. Mineralisation and sorption of dissolved organic nitrogen compounds in litter and soil from sugarcane fields. Soil Biol. Biochem. 2016, 103, 522–532. [Google Scholar] [CrossRef]
- Ye, J.; An, N.; Chen, H.; Ying, Z.; Zhang, S.; Zhao, J. Performance and mechanism of carbon dioxide fixation by a newly isolated chemoautotrophic strain Paracoccus denitrificans PJ-1. Chemosphere 2020, 252, 126473. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chapman, S.J.; Nicol, G.W.; Yao, H. Nitrification and nitrifiers in acidic soils. Soil Biol. Biochem. 2018, 116, 290–301. [Google Scholar] [CrossRef]
- Meinhardt, K.A.; Stopnisek, N.; Pannu, M.W.; Strand, S.E.; Fransen, S.C.; Casciotti, K.L.; Stahl, D.A. Ammonia-oxidizing bacteria are the primary N2O producers in an ammonia-oxidizing archaea dominated alkaline agricultural soil. Environ. Microbiol. 2018, 20, 2195–2206. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Ren, B.; Wang, X.C.; Jin, X.; Shi, X. Mechanism of microbial metabolic responses and ecological system conversion under different nitrogen conditions in sewers. Water Res. 2020, 186, 116320. [Google Scholar] [CrossRef]
- Liu, W.; Nasry, A.A.N.B.; Zhao, J.; Laoyongxay, H.; Dai, W.; Zhao, Q. Start-up of the Simultaneous Nitrification, Anammox, and Denitrification (SNAD) Reactor and Efficacy of a Small Amount of Organic Carbon. Water Air Soil Pollut. 2019, 230, 256. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Lv, W.; Xia, S.; Han, J.; Wang, Z.; Yu, X. Enhancement of nitrogen removal by supplementing fluidized-carriers into the aerobic tank in a full-scale A2/O system. Sci. Total Environ. 2019, 660, 817–825. [Google Scholar] [CrossRef]
- Wang, H.; He, X.; Nakhla, G.; Zhu, J.; Su, Y.K. Performance and bacterial community structure of a novel inverse fluidized bed bioreactor (IFBBR) treating synthetic municipal wastewater. Sci. Total Environ. 2020, 718, 137288. [Google Scholar] [CrossRef]
- Williams, S.T.; Sharpe, M.E.; Holt, J.G. (Eds.) Bergey’s Manual of Determinative Bacteriology, 8th ed.; Williams & Wilkins Company: Baltimore, ML, USA, 1989; pp. 1808–1834. [Google Scholar]
- Bergey, D.H.; Holt, J.G. (Eds.) Bergey’s Manual of Determinative Bacteriology, 9th ed.; Williams & Wilkins Company: Baltimore, ML, USA, 1994; pp. 447–455. [Google Scholar]
- Chen, R.; Takemura, Y.; Liu, Y.; Ji, J. Using Partial Nitrification and Anammox To Remove Nitrogen from Low-Strength Wastewater by Co-immobilizing Biofilm inside a Moving Bed Bioreactor. ACS Sustain. Chem. Eng. 2019, 7, 1353–1361. [Google Scholar] [CrossRef]
- Purkhold, U.; Pommerening-Röser, A.; Juretschko, S.; Schmid, M.C.; Koops, H.P.; Wagner, M. Phylogeny of all recognized species of ammonia oxidizers based on comparative 16S rRNA and amaA sequence analysis: Implications for molecular diversity surveys. Appl. Environ. Microbiol. 2000, 66, 5368–5382. [Google Scholar] [CrossRef] [Green Version]
- Cai, M.; Ng, S.K.; Lim, C.K.; Lu, H.; Jia, Y. Physiological and Metagenomic Characterizations of the Synergistic Relationships between Ammonia- and Nitrite-Oxidizing Bacteria in Freshwater Nitrification. Front. Microbiol. 2018, 9, 280. [Google Scholar] [CrossRef] [PubMed]
- Ginawi, A.; Yan, Y. The Function of Ammonia Oxidizers Community in the Environment. PSM Microbiol. 2019, 4, 20–36. Available online: https://www.journals.psmpublishers.org/index.php/microbiol/article/view/311 (accessed on 26 March 2021).
- Duddleston, K.N.; Bottomley, P.J.; Porter, A.J.; Arp, D.J. New Insights into Methyl Bromide Cooxidation by Nitrosomonas europaea Obtained by Experimenting with Moderately Low Density Cell Suspensions. Appl. Environ. Microbiol. 2000, 66, 2726–2731. [Google Scholar] [CrossRef] [Green Version]
- Barker, L.K.; Giska, J.R.; Radniecki, T.S.; Semprini, L. Effects of short- and long-term exposure of silver nanoparticles and silver ions to Nitrosomonas europaea biofilms and planktonic cells. Chemosphere 2018, 206, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Soman, C.; Li, D.; Wander, M.M.; Kent, A.D. Long-term fertilizer and crop-rotation treatments differentially affect soil bacterial community structure. Plant Soil 2017, 413, 145–159. [Google Scholar] [CrossRef]
- Jeong, D.; Cho, K.; Lee, C.H.; Lee, S.; Bae, H. Effects of salinity on nitrification efficiency and bacterial community structure in a nitrifying osmotic membrane bioreaktor. Process Biochem. 2018, 73, 132–141. [Google Scholar] [CrossRef]
- Bennett, K.; Sadler, N.C.; Wright, A.T.; Hyman, M.R. Activity-Based Protein Profiling of Ammonia Monooxygenase in Nitrosomonas europaea. Appl. Environ. Microbiol. 2016, 82, 2270–2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, C.L.; Schatteman, A.; Crombie, A.T.; Murrell, J.C.; Lehtovirta-Morley, L.E. Inhibition of Ammonia Monooxygenase from Ammonia-Oxidizing Archaea by Linear and Aromatic Alkynes. Appl. Environ. Microbiol. 2020, 86, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ginawi, A.; Yan, Y. Molecular Techniques Applied to Investigations of Abundance of the Ammonia Oxidizing Bacteria and Ammonia Oxidizing Archaea Microorganisms in the Environment. IOSR J. Environ. Sci. Toxicol. Food Technol. 2019, 13, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Ngo, H.H.; Guo, W.; Wang, D.; Peng, L.; Ni, B.J. New perspectives on microbial communities and biological nitrogen removal processes in wastewater treatment systems. Bioresour. Technol. 2020, 297, 122491. [Google Scholar] [CrossRef]
- Cole, J.A. Biodegradation of inorganic nitrogen compounds. In Biochemistry of Microbial Degradation; Ratledge, C., Ed.; Springer: Dordrecht, Germany, 1994; pp. 487–512. [Google Scholar] [CrossRef]
- Liu, H.; Ding, Y.; Zhang, Q.; Liu, X.; Xu, J.; Li, Y.; Di, H. Heterotrophic nitrification and denitrification are the main sources of nitrous oxide in two paddy soils. Plant Soil 2019, 445, 39–53. [Google Scholar] [CrossRef]
- Duan, P.; Shen, H.; Jiang, X.; Yan, X.; Xiong, Z. The contributions of hydroxylamine and nitrite to NO and N2O production in alkaline and acidic vegetable soils. J. Soils Sediments 2020, 20, 2903–2911. [Google Scholar] [CrossRef]
- Ushiki, N.; Jinno, M.; Fujitani, H.; Suenaga, T.; Terada, A.; Tsuneda, S. Nitrite oxidation kinetics of two Nitrospira strains: The quest for competition and ecological niche differentiation. J. Biosci. Bioeng. 2017, 123, 581–589. [Google Scholar] [CrossRef]
- Giguere, A.T.; Taylor, A.E.; Myrold, D.D. Nitrite-oxidizing activity responds to nitrite accumulation in soil. FEMS Microbiol. Ecol. 2018, 94, 1–9. [Google Scholar] [CrossRef]
- Daims, H.; Lücker, S.; Wagner, M. A New Perspective on Microbes Formerly Known as Nitrite-Oxidizing Bacteria. Trends Microbiol. 2016, 24, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhao, J.; Su, J.; Jia, Z.; Shi, X.; Wright, A.L. Neutrophilic bacteria are responsible for autotrophic ammonia oxidation in an acidic forest soil. Soil Biol. Biochem. 2018, 119, 83–89. [Google Scholar] [CrossRef]
- Ho, A.; Di Lonardo, D.P.; Bodelier, P.L.E. Revisiting life strategy concepts in environmental microbial ecology. FEMS Microbiol. Ecol. 2017, 93, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.; Wang, T.; Ye, J.; Zhao, J.; Yang, L.; Wu, P. Effects of carbon sources and operation modes on the performances of aerobic denitrification process and its microbial community shifts. J. Environ. Manag. 2019, 239, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Forsmark, B.; Nordin, A.; Maaroufi, N.I.; Lundmark, T. Low and High Nitrogen Deposition Rates in Northern Coniferous Forests Have Different Impacts on Aboveground Litter Production, Soil Respiration, and Soil Carbon Stocks. Ecosystems 2020. [Google Scholar] [CrossRef] [Green Version]
- Gayon, U.; Dupetit, G. Sur la fermentation des nitrates en nitrites. C. R. Acad. Sci. 1882, 95, 664–666. [Google Scholar]
- Guo, B.; Zheng, X.; Yu, J.; Ding, H.; Pan, B.; Lu, S. Dissolved organic carbon enhances both soil N2O production and uptake. Glob. Ecol. Conserv. 2020, 24. [Google Scholar] [CrossRef]
- Liu, C.W.; Sung, Y.; Chen, B.C.; Lai, H.Y. Effects of Nitrogen Fertilizers on the Growth and Nitrate Content of Lettuce (Lactuca sativa L.). Int. J. Environ. Res. Public Health 2014, 11, 4427–4440. [Google Scholar] [CrossRef] [Green Version]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition-Current Knowledge and Future Directions. Front. Plant Sci. 2017, 19. [Google Scholar] [CrossRef] [Green Version]
- Dey, S.; Awata, T.; Mitsushita, J.; Zhang, D.; Kasai, T.; Matsuura, N.; Katayama, A. Promotion of biological nitrogen fixation activity of an anaerobic consortium using humin as an extracellular electron mediator. Sci. Rep. 2021, 11. [Google Scholar] [CrossRef]
- Tiso, M.; Schechter, A.N. Nitrate Reduction to Nitrite, Nitric Oxide and Ammonia by Gut Bacteria under Physiological Conditions. PLoS ONE 2015. [Google Scholar] [CrossRef] [Green Version]
- Maintinguer, S.I.; Sakamoto, I.K.; Adorno, M.A.T.; Varesche, M.B.A. Evaluation of the microbial diversity of denitrifying bacteria in batch reactor. Braz. J. Chem. Eng. 2013, 30. [Google Scholar] [CrossRef]
- Beristain-Cardoso, R.; Sierra-Alvarez, R.; Rowlette, P.; Razo-Flores, E. Sulfide oxidation under chemolithoautotrophic denitrifying conditions. Biotechnol. Bioeng. 2006, 95, 11481157. [Google Scholar] [CrossRef]
- Sparacino-Watkins, C.; Stolz, J.F.; Basu, P. Nitrate and periplasmic nitrate reductases. Chem. Soc. Rev. 2014, 43, 676–706. [Google Scholar] [CrossRef] [PubMed]
- Ona-Nguema, G.; Guerbois, D.; Pallud, C.; Brest, J.; Abdelmoula, M.; Morin, G. Biogenic Fe(II-III) Hydroxycarbonate Green Rust Enhances Nitrate Removal and Decreases Ammonium Selectivity during Heterotrophic Denitrification. Minerals 2020, 10, 818. [Google Scholar] [CrossRef]
- Olaya-Abril, A.; Hidalgo-Carrillo, J.; Luque-Almagro, V.M.; Fuentes-Almagro, C.; Urbano, F.J.; Moreno-Vivián, C.; Richardson, D.J.; Roldán, M.D. Exploring the Denitrification Proteome of Paracoccus denitrificans PD1222. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Natywa, M.; Selwet, M.; Maciejewski, T. Effect of some agrotechnical factors on the number and activity soil microorganisms. Fragm. Agron. 2014, 31, 56–63. [Google Scholar]
- Buckley, S.; Brackin, R.; Jämtgård, S.; Näsholm, T.; Schmidt, S. Microdialysis in soil environments: Current practice and future perspectives. Soil Biol. Biochem. 2020, 143. [Google Scholar] [CrossRef]
- Inselsbacher, E.; Öhlund, J.; Jämtgård, S.; Huss-Danell, K.; Näsholm, T. The potential of microdialysis to monitor organic and inorganic nitrogen compounds in soil. Soil Biol. Biochem. 2011, 43, 1321–1332. [Google Scholar] [CrossRef]
- Homyak, P.M.; Slessarev, E.W.; Hagerty, S.; Greene, A.C.; Marchus, K.; Dowdy, K.; Iverson, S.; Schimel, J.P. Amino acids dominate diffusive nitrogen fluxes across soil depths in acidic tussock tundra. New Phytol. 2021. [Google Scholar] [CrossRef]
- Heinzle, J.; Wanek, W.; Tian, Y.; Kwatcho Kengdo, S.; Borken, W.; Schindlbacher, A.; Inselsbacher, E. No effect of long-term soil warming on diffusive soil inorganic and organic nitrogen fluxes in a temperate forest soil. Soil Biol. Biochem. 2021, 158. [Google Scholar] [CrossRef]
- Gao, S.; DeLuca, T.H. Influence of fire retardant and pyrogenic carbon on microscale changes in soil nitrogen and phosphorus. Biogeochemistry 2021, 152, 117–126. [Google Scholar] [CrossRef]
- Buckley, S.; Brackin, R.; Näsholm, T.; Schmidt, S.; Jämtgård, S. Improving in situ recovery of soil nitrogen using the microdialysis technique. Soil Biol. Biochem. 2017, 114, 93–101. [Google Scholar] [CrossRef]
- Buckley, S.; Allen, D.; Brackin, R.; Jämtgård, S.; Näsholm, T.; Schmidt, S. Microdialysis as an in situ technique for sampling soil enzymes. Soil Biol. Biochem. 2019, 135, 20–27. [Google Scholar] [CrossRef]
- Scarlett, K.; Denman, S.; Clark, D.R.; Forster, J.; Vanguelova, E.; Brown, N.; Whitby, C. Relationships between nitrogen cycling microbial community abundance and composition reveal the indirect effect of soil pH on oak decline. ISME J. 2021, 15, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Hartman, K.; Tringe, S.G. Interactions between plants and soil shaping the root microbiome under abiotic stress. Biochem. J. 2019, 476, 2705–2724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreau, D.; Bardgett, R.D.; Finlay, R.D.; Jones, D.L.; Philippot, L. A plant perspective on nitrogen cycling in the rhizosphere. Funct. Ecol. 2019, 33, 540–552. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Liu, J.; Zhu, H. Genetic and Molecular Mechanisms Underlying Symbiotic Specificity in Legume-Rhizobium Interactions. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Zarama-Alvarado, S. The Challenges of Dealing with Nitrogen Pollutants in Groundwater. Rev. Cient. 2018, 33, 230–242. [Google Scholar] [CrossRef]
- Veraart, A.J.; de Bruijne, W.J.; de Klein, J.J.; Peeters, E.T.; Scheffer, M. Effects of aquatic vegetation type on denitrification. Biogeochemistry 2011, 104, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Lata, J.C.; Degrange, V.; Raynaud, X.; Maron, P.A.; Lensi, R.; Abbadie, L. Grass populations control nitrification in savanna soils. Funct. Ecol. 2004, 18, 605–611. [Google Scholar] [CrossRef]
- Escuer-Gatius, J.; Shanskiy, M.; Mander, Ü.; Kauer, K.; Astover, A.; Vahter, H.; Soosaar, K. Intensive Rain Hampers the Effectiveness of Nitrification Inhibition in Controlling N2O Emissions from Dairy Slurry-Fertilized Soils. Agriculture 2020, 10, 497. [Google Scholar] [CrossRef]
- Tao, K.; Kelly, S.; Radutoiu, S. Microbial associations enabling nitrogen acquisition in plants. Curr. Opin. Microbiol. 2019, 49, 83–89. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, A.; Singh, M.; Maurya, S.K.; Pandey, K.D. Microbial Diversity in the Rhizosphere of Momordica charantia L. (Bitter Gourd). Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Kozieł, M.; Gałązka, A. Characteristics of selected molecular methods used in identification and assessment of genetic diversity of bacteria belonging to the genus Azotobacter. PJA 2019, 38, 37–45. [Google Scholar] [CrossRef]
- Jiménez, D.J.; Montaña, J.S.; Martínez, M.M. Characterization of free nitrogen fixing bacteria of the genus Azotobacter in organic vegetable—grown colombian soils. Braz. J. Microbiol. 2011, 42, 846–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juárez, B.; Martínez, M.M.; González, J. Growth of Azotobacter chroococcum in chemically defined media containing p—hydroxybenzoic acid and protocatechuic acid. Chemosphere 2004, 59, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Gradova, N.B.; Gornova, I.B.; Eddaudi, R.; Salina, N.R. Use of Bacteria of the Genus Azotobacter for Bioremediation of Oil-Contaminated Soils. Appl. Biochem. Microbiol. 2003, 39, 279–281. [Google Scholar] [CrossRef]
- Gurikar, C.; Naik, M.K.; Sreenivasa, M.Y. Azotobacter: PGPR Activities with Special Reference to Effect of Pesticides and Biodegradation. In Microbial Inoculants in Sustainable Agricultural Productivity; Singh, D., Singh, H., Prabha, R., Eds.; Springer: New Delhi, India, 2016; pp. 229–244. [Google Scholar] [CrossRef]



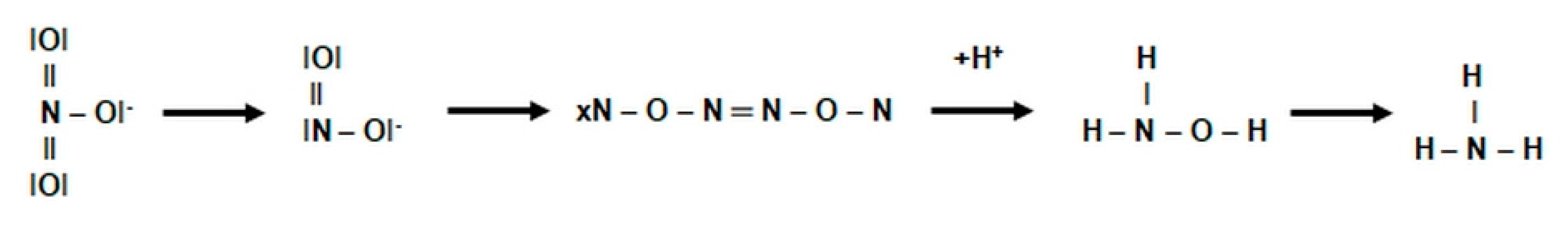




| Bacterial Species | Plant |
|---|---|
| Azospirillum brasilense | Millet, sorghum, wheat, corn |
| Azospirillum lipoferum | Millet, wheat, corn |
| Azospirillum amazonense | Wheat |
| Azospirillum doeberainerae | Miscanthus |
| Herbaspirillum seropedicae | Wheat, sugar cane, rice |
| Herbaspirillum rubrisubalbicans | Sugar cane, rice |
| Herbaspirillum frisingense | Miscanthus |
| Acetobacter diazotrophicus | Sugar cane, sweet potatoes, wheat |
| Bacterial Species | Plant |
|---|---|
| Rhizobium leguminosarum biowar viviae | Pea |
| Rhizobium leguminosarum biowar trifolii | Clover |
| Rhizobium leguminosarum browar phaseoli | Bean |
| Mesorhizobium lori | Pigweed |
| Sinorhizobium melilori | Lucerne, melilot |
| Azorhizobium caulinodans | Sesbania rostrata |
| Bradyrhizobium sp. | Lupine |
| Bradyrhizobium japonicum | Soya |
| Bacterial Species | Habitat |
|---|---|
| I. Bacteria Oxidizing NH3 To NO2− | |
| Nitrosomonas europaea | Soil, sea water, sweet water, sewage |
| Nitrosomonas cryotolerans | Marine environment |
| Nitrosococcus nitrosus | Soil, sea water, industrial sewage |
| Nitrosococcus oceanus | Sea water |
| Nitrosococcus mobilis | Brackish water |
| Nitrosococcus halophilus | Salty lagoons, salt lakes |
| Nitrosospira briensis | Soil, fresh wate |
| Nitrosolobus multiformis | Soil |
| Nitrosovibrio tenuis | Soil |
| II. Bacteria Oxidizing NO2− To NO3− | |
| Nitrobacter winogradskyi | Soil, sea water, fresh water, sewage |
| Nitrobacter hamburgensis | Soil |
| Nitrospina gracilis | Sea water |
| Nitrococcus mobilis | Sea water |
| Nitrospira marina | Soil, sea water, sediments |
| Bacteria | Fungi |
|---|---|
| Achromobacter fisheri Agrobacterium tumefaciens Alcaligenes eutrophus Azospirillum brasilense Bacillus licheniformis Micrococcus denitryficans Nitrosomonas eutropha Pseudomonas aeruginosa Thiobacillus denitryficans | Aspergillus nidulans Fusarium oxysporum Penicillum sp. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paśmionka, I.B.; Bulski, K.; Boligłowa, E. The Participation of Microbiota in the Transformation of Nitrogen Compounds in the Soil—A Review. Agronomy 2021, 11, 977. https://doi.org/10.3390/agronomy11050977
Paśmionka IB, Bulski K, Boligłowa E. The Participation of Microbiota in the Transformation of Nitrogen Compounds in the Soil—A Review. Agronomy. 2021; 11(5):977. https://doi.org/10.3390/agronomy11050977
Chicago/Turabian StylePaśmionka, Iwona Beata, Karol Bulski, and Elżbieta Boligłowa. 2021. "The Participation of Microbiota in the Transformation of Nitrogen Compounds in the Soil—A Review" Agronomy 11, no. 5: 977. https://doi.org/10.3390/agronomy11050977
APA StylePaśmionka, I. B., Bulski, K., & Boligłowa, E. (2021). The Participation of Microbiota in the Transformation of Nitrogen Compounds in the Soil—A Review. Agronomy, 11(5), 977. https://doi.org/10.3390/agronomy11050977






