Enhanced Nutritional Quality of Sweet Maize Kernel in Response to Cover Crops and Bio-Fertilizer
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material
- T1–common vetch (Vicia sativa L.);
- T2–field pea (Pisum sativum L.);
- T3–winter oats (Avena sativa L.);
- T4–fodder kale (Brassica oleracea (L.) convar. acephala);
- T5–common vetch + winter oats;
- T6–field pea + winter oats;
- T7–organic mulch;
- T8–control –fallow.
2.2. Field Experiment
2.3. Chemical Analysis
2.4. Statistical Analysis
2.5. Meteorological Conditions
3. Results
3.1. Cover Crops Biomass and Sweet Maize Kernel Yield
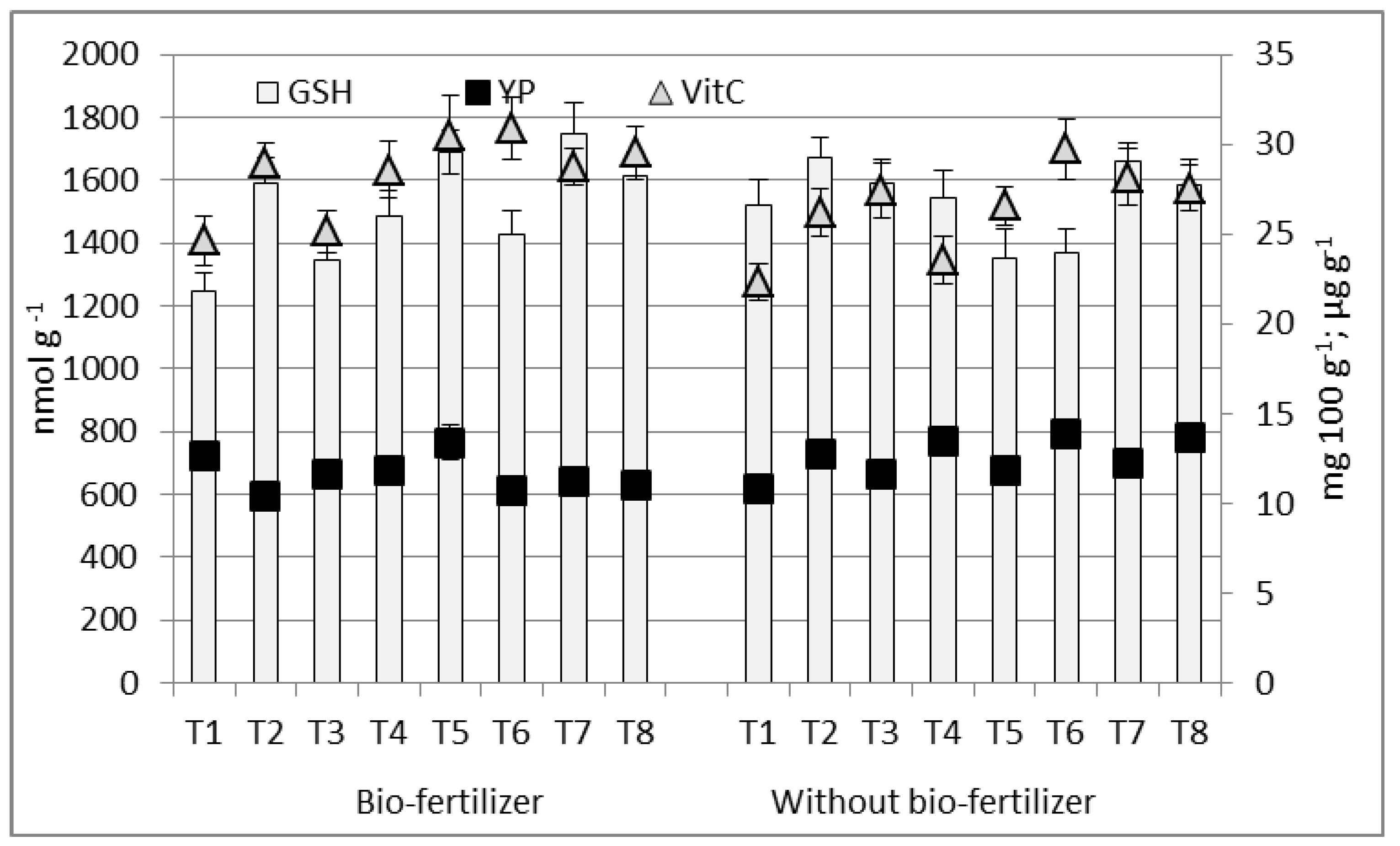
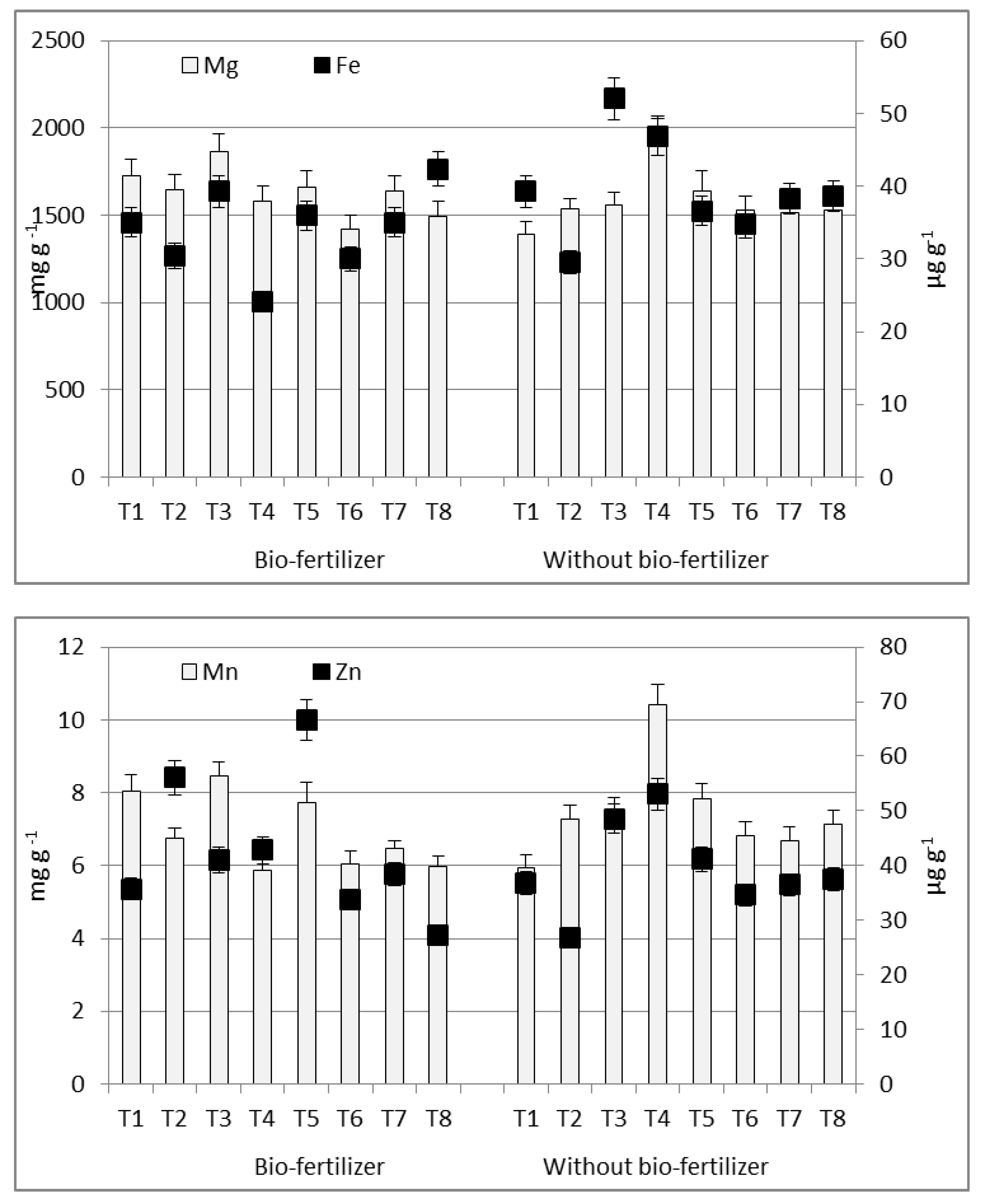
3.2. Variability in Chemical Composition of Sweet Maize Kernel
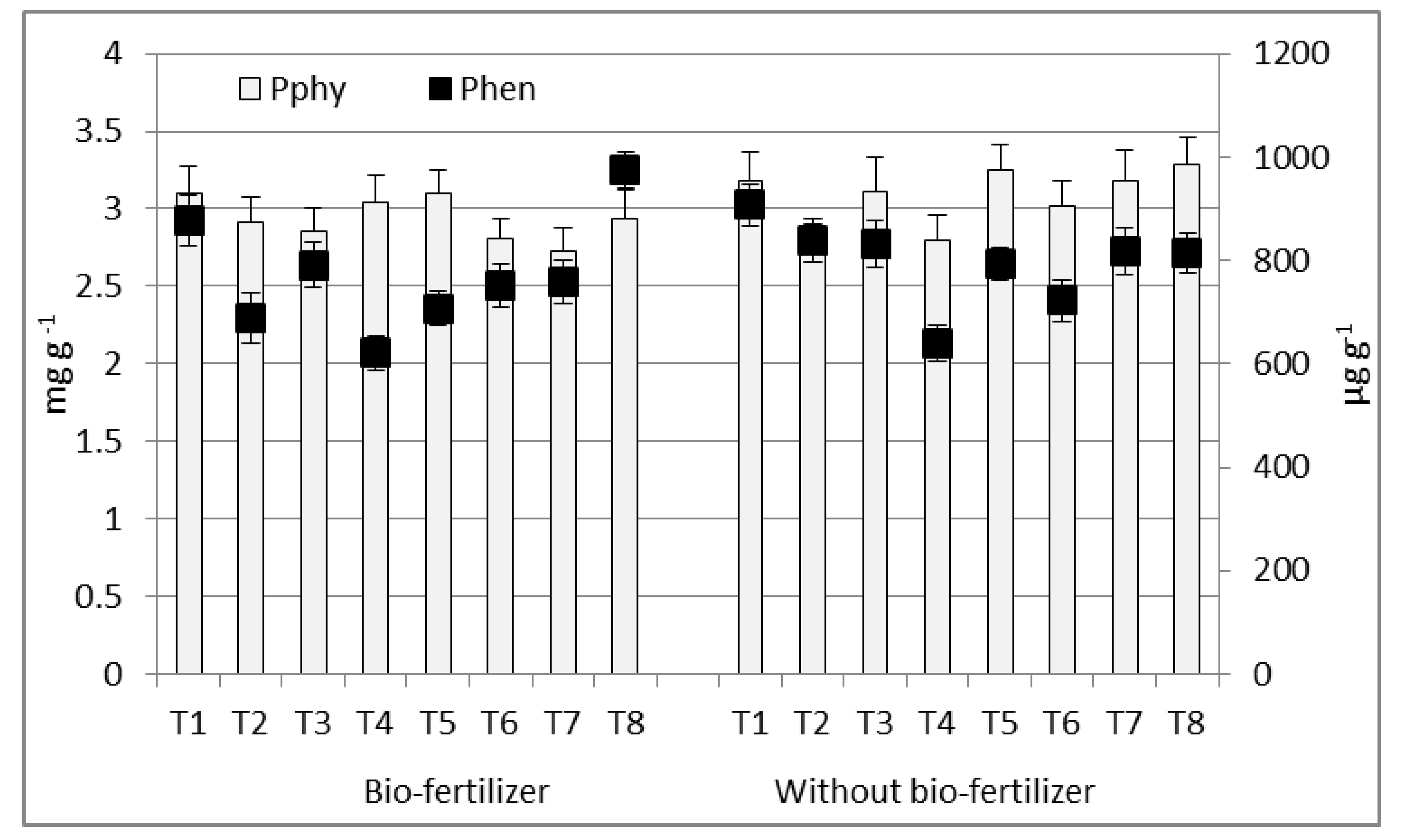
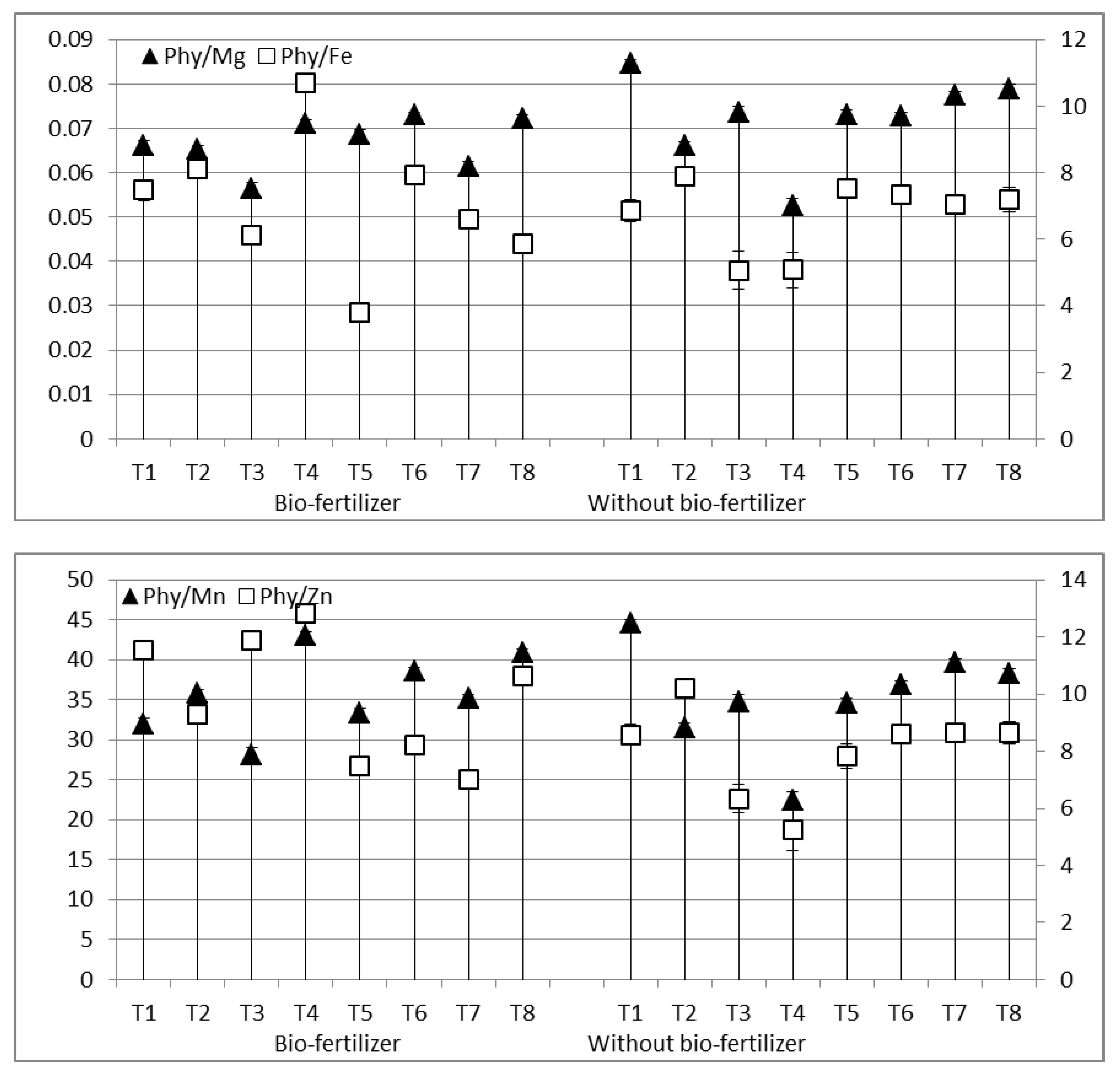
3.3. Potential Bio-Availability of Essential Elements
4. Discussion
4.1. Cover Crops Biomass and Sweet Maize Kernel Yield
4.2. Variability in Chemical Composition of Sweet Maize Kernel
4.3. Potential Bio-Availability of Essential Elements
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Garnett, T. Food sustainability: Problems, perspectives and solutions. Proc. Nutr. Soc. 2013, 72, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Gillespie, S.; Bold, M.V.D. Agriculture, Food Systems, and Nutrition: Meeting the Challenge. Glob. Chall. 2017, 1, 1600002. [Google Scholar] [CrossRef]
- Restovich, S.B.; Andriulo, A.E.; Portela, S.I. Introduction of cover crops in a maize–soybean rotation of the Humid Pampas: Effect on nitrogen and water dynamics. Field Crop. Res. 2012, 128, 62–70. [Google Scholar] [CrossRef]
- Reberg-Horton, S.C.; Grossman, J.M.; Kornecki, T.S.; Meijer, A.D.; Price, A.J.; Place, G.T.; Webster, T.M. Utilizing cover crop mulches to reduce tillage in organic systems in the southeastern USA. Renew. Agric. Food Syst. 2011, 27, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Baijukya, F.; de Ridder, N.; Giller, K. Nitrogen Release from Decomposing Residues of Leguminous Cover Crops and their Effect on Maize Yield on Depleted Soils of Bukoba District, Tanzania. Plant Soil 2006, 279, 77–93. [Google Scholar] [CrossRef]
- Deguchi, S.; Uozumi, S.; Tawaraya, K.; Kawamoto, H.; Tanaka, O. Living Mulch with White Clover Improves Phosphorus Nutrition of Maize of Early Growth Stage. Soil Sci. Plant Nutr. 2005, 51, 573–576. [Google Scholar] [CrossRef] [Green Version]
- Salmerón, M.; Isla, R.; Cavero, J. Effect of winter cover crop species and planting methods on maize yield and N availability under irrigated Mediterranean conditions. Field Crop. Res. 2011, 123, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Kabir, Z.; Koide, R.T. Effect of autumn and winter mycorrhizal cover crops on soil properties, nutrient uptake and yield of sweet corn in Pennsylvania, USA. Plant Soil 2002, 238, 205–215. [Google Scholar] [CrossRef]
- Rosa, R. Quality of sweet corn yield depending on winter catch crops and weed control method. Hortorum Cultus 2015, 14, 59–74. [Google Scholar]
- Janoševic, B.; Dolijanovic, Z.; Dragicevic, V.; Simic, M.; Dodevska, M.; Djordjevic, S.; Moravcevic, D.; Miodragovic, R. Cover crop effects on the fate of N in sweet maize (Zea mays L. saccharata Sturt.) production in a semiarid region. Int. J. Plant. Prod. 2017, 11, 285–294. [Google Scholar]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Factories 2014, 13, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maris, S.; Capra, F.; Ardenti, F.; Chiodini, M.; Boselli, R.; Taskin, E.; Puglisi, E.; Bertora, C.; Poggianella, L.; Amaducci, S.; et al. Reducing N Fertilization without Yield Penalties in Maize with a Commercially Available Seed Dressing. Agronomy 2021, 11, 407. [Google Scholar] [CrossRef]
- Hussain, A.; Zahir, Z.A.; Ditta, A.; Tahir, M.U.; Ahmad, M.; Mumtaz, M.Z.; Hayat, K.; Hussain, S. Production and Implication of Bio-Activated Organic Fertilizer Enriched with Zinc-Solubilizing Bacteria to Boost up Maize (Zea mays L.) Production and Biofortification under Two Cropping Seasons. Agronomy 2019, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Swapna, G.; Jadesha, G.; Mahadevu, P. Assessment of Correlation and Path Coefficient Analysis for Yield and it’s Attributing Traits in Rice (Oryza sativa L.) Genotypes. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 3859–3865. [Google Scholar] [CrossRef]
- Khanduri, A.; Prasanna, B.M.; Firoz, H.; Lakhera, P.C. Genetic analyses and association studies of yield components and kernel sugar concentration in sweet corn. Indian J. Genet. Plant. Breed. 2010, 70, 257–263. [Google Scholar]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldivar, S.O. Chapter 14—Minor constituents and phytochemicals of the kernel. Corn Chem. Technol. 2019, 369–403. [Google Scholar] [CrossRef]
- Platel, K.; Srinivasan, K. Bioavailability of Micronutrients from Plant Foods: An Update. Crit. Rev. Food Sci. Nutr. 2015, 56, 1608–1619. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Stockmann, R.; Ng, K.; Ajlouni, S. Opportunities for plant-derived enhancers for iron, zinc, and calcium bioavailability: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 652–685. [Google Scholar] [CrossRef]
- Watanabe, F.S.; Olsen, S.R. Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from the soil. Soil Sci. Soc. Am. J. 1965, 29, 677–678. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Dragičević, V.D.; Sredojević, S.D.; Perić, V.A.; Nišavić, A.R.; Srebrić, M.B. Validation study of a rapid colorimetric method for the determination of phytic acid and inorganic phosphorus from seeds. Acta Period. Technol. 2011, 11–21. [Google Scholar] [CrossRef]
- Sari-Gorla, M.; Ferrario, S.; Rossini, L.; Frova, C.; Villa, M. Developmental expression of glutathione-S-transferase in maize and its possible connection with herbicide tolerance. Euphytica 1993, 67, 221–230. [Google Scholar] [CrossRef]
- Simić, A.; Sredojevic, S.; Todorovic, M.; Đukanović, L.; Radenović, Č. Studies on the relationship between the content of total phenolics in exudates and germination ability of maize seed during accelerated aging. Seed Sci. Technol. 2004, 32, 213–218. [Google Scholar] [CrossRef]
- Vancetovic, J.; Zilic, S.; Bozinovic, S.; Ignjatovic-Micic, D. Simulating of Top-Cross system for enhancement of antioxidants in maize grain. Span. J. Agric. Res. 2014, 12, 467. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, G.; Wretling, S. Determination of fructose, glucose and sucrose in foods (Bestämningavfruktos, glukosochsackarosil-ivsmedel). Our Food 1979, 31, 435–439. [Google Scholar]
- Rikovski, I.; Džamić, M.; Rajković, M. Practicum of Analytical Chemistry; Construction Book; Faculty of Agriculture: Belgrade, Serbia, 1989. (In Serbian) [Google Scholar]
- Wallace, J.M.; Williams, A.; Liebert, J.A.; Ackroyd, V.J.; Vann, R.A.; Curran, W.S.; Keene, C.L.; VanGessel, M.J.; Ryan, M.R.; Mirsky, S.B. Cover Crop-Based, Organic Rotational No-Till Corn and Soybean Production Systems in the Mid-Atlantic United States. Agriculture 2017, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, D.C.; Lyon, D.J.; Hergert, G.W.; Higgins, R.K.; Holman, J.D. Cover Crop Biomass Production and Water Use in the Central Great Plains. Agron. J. 2015, 107, 2047–2058. [Google Scholar] [CrossRef]
- Finney, D.M.; White, C.M.; Kaye, J.P. Biomass Production and Carbon/Nitrogen Ratio Influence Ecosystem Services from Cover Crop Mixtures. Agron. J. 2016, 108, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Boselli, R.; Fiorini, A.; Santelli, S.; Ardenti, F.; Capra, F.; Maris, S.C.; Tabaglio, V. Cover crops during transition to no-till maintain yield and enhance soil fertility in intensive agro-ecosystems. Field Crop. Res. 2020, 255, 107871. [Google Scholar] [CrossRef]
- Appelgate, S.R.; Lenssen, A.W.; Wiedenhoeft, M.H.; Kaspar, T.C. Cover Crop Options and Mixes for Upper Midwest Corn-Soybean Systems. Agron. J. 2017, 109, 968–984. [Google Scholar] [CrossRef] [Green Version]
- Kramberger, B.; Gselman, A.; Kristl, J.; Lešnik, M.; Šuštar, V.; Muršec, M.; Podvršnik, M. Winter cover crop: The effects of grass–clover mixture proportion and biomass management on maize and the apparent residual N in the soil. Eur. J. Agron. 2014, 55, 63–71. [Google Scholar] [CrossRef]
- Kara, B.; Atar, B.; Gul, H. Effects of different sowing dates on protein, sugar and dry matter of sweet corn. Res. Crops 2012, 13, 493–497. [Google Scholar]
- Păcurar, L.; Apahidean, M.; Haş, V. Morpho-Productive and Chemical Composition of Local and Foreign Sweet Corn Hybrids Grown in the Conditions of Transylvania Plateau. Bull. Univ. Agric. Sci. Vet. Med. Cluj Napoca Hortic. 2017, 74, 168. [Google Scholar] [CrossRef] [Green Version]
- Dragicevic, V.; Oljaca, S.; Simic, M.; Dolijanovic, Z.; Kresovic, B.; Brankov, M. Content of some antioxidants in intercropped maize and soybean grain. J. Agric. Sci. 2017, 62, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Dragicevic, V.; Oljaca, S.; Stojiljkovic, M.; Simic, M.; Dolijanovic, Z.; Kravic, N. Effect of the maize–soybean intercropping system on the potential bioavailability of magnesium, iron and zinc. Crop. Pasture Sci. 2015, 66, 1118. [Google Scholar] [CrossRef]
- Jin, J.; Wang, G.; Liu, X.; Pan, X.; Herbert, S.J.; Tang, C. Interaction Between Phosphorus Nutrition and Drought on Grain Yield, and Assimilation of Phosphorus and Nitrogen in Two Soybean Cultivars Differing in Protein Concentration in Grains. J. Plant Nutr. 2006, 29, 1433–1449. [Google Scholar] [CrossRef]
- Adamczewska-Sowińska, K.; Sowiński, J. Reaction of Sweet Maize to the Use of Polyethylene Film and Polypropylene Non-Woven Fabric in the Initial Growth Phase. Agronomy 2020, 10, 141. [Google Scholar] [CrossRef] [Green Version]
- Mesarović, J.; Srdić, J.; Mladenović-Drinić, S.; Dragičević, V.; Simić, M.; Brankov, M.; Milojković-Opsenica, D. Evaluation of the nutritional profile of sweet maize after herbicide and foliar fertilizer application. J. Cereal Sci. 2019, 87, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Šimić, D.; Drinić, S.M.; Zdunić, Z.; Jambrović, A.; Ledenčan, T.; Brkić, J.; Brkić, A.; Brkić, I. Quantitative Trait Loci for Biofortification Traits in Maize Grain. J. Hered. 2011, 103, 47–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragicevic, V.; Mladenovic-Drinic, S.; Stojiljkovic, M.; Filipovic, M.; Nikolic, B.; Babic, V.; Kravic, N. Maize inbreds from different heterotic groups as favorable sources for increased potential bioavailability of magnesium, iron, manganese and zinc. Chil. J. Agric. Res. 2016, 76, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Hallama, M.; Pekrun, C.; Lambers, H.; Kandeler, E. Hidden miners—The roles of cover crops and soil microorganisms in phosphorus cycling through agroecosystems. Plant Soil 2019, 434, 7–45. [Google Scholar] [CrossRef] [Green Version]
| Average Temperature (°C) | Precipitation Sum (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Months | 2015/16 | 2016/17 | 2017/18 | 1991–2019 | 2015/16 | 2016/17 | 2017/18 | 1991–2019 |
| November | 9.2 | 7.4 | 7.9 | 8.3 | 63.4 | 58.8 | 42.3 | 51.1 |
| December | 4.3 | 0.2 | 4.5 | 3.0 | 3.8 | 0.6 | 17.4 | 55.6 |
| January | 2.5 | −1.8 | 5.0 | 2.0 | 46.3 | 6.8 | 31.9 | 47.6 |
| February | 9.0 | 5.3 | 1.9 | 3.8 | 38.5 | 18.1 | 41.7 | 43.2 |
| March | 9.1 | 11 | 6.4 | 8.2 | 102.6 | 21.3 | 32.4 | 48.8 |
| April | 15.5 | 12.4 | 18 | 13.6 | 53.9 | 47.1 | 24.6 | 52.5 |
| Aver./Sum | 8.3 | 5.7 | 7.3 | 6.5 | 308.5 | 152.7 | 190.3 | 298.8 |
| May | 17.5 | 18.6 | 21.7 | 18.1 | 71.3 | 49.2 | 39.0 | 72.8 |
| June | 22.5 | 24.4 | 22.7 | 21.9 | 152.2 | 39 | 150.1 | 92.0 |
| July | 24.4 | 25.5 | 23.6 | 23.8 | 3.0 | 26.7 | 61.9 | 67.9 |
| August | 22.3 | 25.8 | 25.7 | 23.8 | 60.8 | 23.7 | 44.0 | 55.0 |
| Aver./Sum | 21.7 | 23.6 | 23.4 | 20.2 | 287.3 | 138.6 | 295.0 | 340.2 |
| Treatment | 2015/16 | 2016/17 | 2017/18 | Aver. |
|---|---|---|---|---|
| T1 | 21,400 d | 45,600 l | 18,310 b | 28,437 b |
| T2 | 38,440 hi | 38,240 h | 20,760 cd | 32,480 c |
| T3 | 16,610 a | 29,240 e | 19,675 c | 21,842 a |
| T4 | 43,515 k | 54,040 n | 31,175 f | 42,910 e |
| T5 | 39,500 i | 44,500 kl | 41,150 j | 41,717 d |
| T6 | 36,100 g | 57,150 o | 49,860 m | 47,703 f |
| Aver. | 32,594 b | 44,795 c | 30,155 a | |
| Source of variation | CC | Y | CC × Y | |
| df. | 5 | 2 | 10 | |
| Anova (p values) | 0.069 |
| CC | BF | Y | CC × BF | CC × Y | BF × Y | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BF | Without BF | 2016 | 2017 | 2018 | 2016 | 2017 | 2018 | ||||||||
| T1 | 5446.8 a | BF | 5920.0 b | 2016 | 3733.7 a | T1 | 5535.9 ab | 5357.8 a | 3972.2 b | 4070.4 b | 8297.9 ef | BF | 3617.2 a | 5372.1 b | 8770.8 c |
| T2 | 6050.3 b | Without BF | 5717.1 a | 2017 | 5224.0 b | T2 | 6575.2 b | 5525.4 ab | 3796.4 b | 5612.5 cd | 8742.0 ef | Without BF | 3855.1 a | 5076 b | 8220.1 c |
| T3 | 5600.3 ab | 2018 | 8495.5 c | T3 | 5759.3 ab | 5441.4 a | 3196 ab | 5589.1 cd | 8015.9 e | ||||||
| T4 | 6489.6 b | T4 | 6284.6 b | 6694.5 b | 4941.1c | 6261.7 d | 8265.9 ef | ||||||||
| T5 | 5410.2 a | T5 | 5636.9 ab | 5196.7 a | 2977.4a | 4538.5 bc | 8714.5 ef | ||||||||
| T6 | 5519.7 a | T6 | 5234.7 a | 5804.7 ab | 2732.9 a | 5433.4 c | 8392.7 ef | ||||||||
| T7 | 6173.9 b | T7 | 6787.8 b | 5560.1 ab | 4446.4 bc | 5367.6 c | 8707.7 ef | ||||||||
| T8 | 5851.0 ab | T8 | 5545.9 ab | 6156.2 b | 3807.0 b | 4919.2 c | 8826.8 f | ||||||||
| df. | 7 | 1 | 2 | 7 | 14 | 2 | |||||||||
| Anova (p values) | 0.000 ** | 0.040 * | 0.000 ** | 0.000 ** | 0.000 ** | 0.005 * | |||||||||
| Sucrose | Glucose | Fructose | Pphy | Phenolics | GSH | YP | Vitam. C | Mg | Fe | Mn | Zn | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % DM | mg g−1 | µg g−1 | nmol g−1 | µg g−1 | mg 100 g−1 | µg g−1 | |||||||
| T1 | 1.39 b | 1.65 ab | 1.23 a | 3.14 c | 892 e | 1383 a | 11.74 b | 23.47 a | 1555 c | 45.65 g | 7.00 b | 36.15 ab | |
| T2 | 1.18 a | 1.64 a | 1.39 b | 2.83 a | 763 bc | 1629 e | 11.57 a | 27.57 c | 1588 d | 35.40 c | 7.01 b | 38.26 ab | |
| T3 | 1.16 a | 1.67 ab | 1.27 a | 2.98 b | 812 d | 1468 b | 11.58 a | 26.25 b | 1711 f | 36.26 d | 7.94 c | 41.04 b | |
| T4 | 1.22 a | 1.63 a | 1.24 a | 2.92 b | 631 a | 1513 c | 12.66 d | 26.06 b | 1770 g | 32.41 a | 8.15 d | 34.21 ab | |
| T5 | 1.38 b | 1.7 b | 1.44 c | 3.18 c | 750 b | 1519 c | 12.63 d | 28.55 d | 1651 e | 36.67 d | 7.77 c | 37.49 ab | |
| T6 | 1.14 a | 1.63 a | 1.36 b | 2.91 b | 737 b | 1397 a | 12.29 c | 30.31 e | 1473 a | 40.45 f | 6.43 a | 32.42 ab | |
| T7 | 1.50 c | 1.64 a | 1.52 d | 2.96 b | 789 c | 1704 f | 11.71 b | 28.45 d | 1575 cd | 34.01 b | 6.57 a | 30.68 a | |
| T8 | 1.23 a | 1.74 b | 1.46 c | 3.11 c | 894 e | 1599 d | 12.35 c | 28.55 d | 1513 b | 39.48 e | 6.55 a | 39.36 ab | |
| BF | 1.25 a | 1.71 b | 1.37 | 2.93 a | 771 a | 1518 a | 11.60 a | 28.37 b | 1628 b | 37.16 b | 6.92 a | 31.70 | |
| Without BF | 1.3 b | 1.61 a | 1.36 | 3.07 b | 796 b | 1536 b | 12.53 b | 26.44 a | 1581 a | 29.95 a | 7.44 b | 28.89 | |
| 2016 | 1.29 b | 1.71 b | 1.41 b | 2.52 a | 1299 c | 1379 a | 26.25 c | 27.04 a | 2426 c | 69.59 c | 12.29 c | 68.36 c | |
| 2017 | 1.29 b | 1.71 b | 1.41 b | 3.29 c | 595 b | 1545 b | 1.85 a | 27.04 a | 533 a | 5.62 a | 0.84 a | 5.33 a | |
| 2018 | 1.26 a | 1.56 a | 1.26 a | 3.19 b | 456 a | 1656 c | 8.09 b | 28.12 b | 1855 b | 35.02 b | 8.40 b | 31.37 b | |
| Source of variation | df. | Anova (p values) | |||||||||||
| CC | 7 | 0.000 ** | 0.000 ** | 0.09 | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.055 * | 0.000 ** | 0.042 * |
| BF | 1 | 0.000 ** | 0.000 ** | 0.164 ns | 0.000 ** | 0.003 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.322 ns |
| Y | 2 | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** |
| CC × BF | 7 | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.005 ** |
| CC × Y | 14 | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** |
| BF × Y | 2 | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.097 ns |
| CC × BF× Y | 14 | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.002 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragicevic, V.; Dolijanović, Ž.; Janosevic, B.; Brankov, M.; Stoiljkovic, M.; Dodevska, M.S.; Simić, M. Enhanced Nutritional Quality of Sweet Maize Kernel in Response to Cover Crops and Bio-Fertilizer. Agronomy 2021, 11, 981. https://doi.org/10.3390/agronomy11050981
Dragicevic V, Dolijanović Ž, Janosevic B, Brankov M, Stoiljkovic M, Dodevska MS, Simić M. Enhanced Nutritional Quality of Sweet Maize Kernel in Response to Cover Crops and Bio-Fertilizer. Agronomy. 2021; 11(5):981. https://doi.org/10.3390/agronomy11050981
Chicago/Turabian StyleDragicevic, Vesna, Željko Dolijanović, Biljana Janosevic, Milan Brankov, Milovan Stoiljkovic, Margarita S. Dodevska, and Milena Simić. 2021. "Enhanced Nutritional Quality of Sweet Maize Kernel in Response to Cover Crops and Bio-Fertilizer" Agronomy 11, no. 5: 981. https://doi.org/10.3390/agronomy11050981
APA StyleDragicevic, V., Dolijanović, Ž., Janosevic, B., Brankov, M., Stoiljkovic, M., Dodevska, M. S., & Simić, M. (2021). Enhanced Nutritional Quality of Sweet Maize Kernel in Response to Cover Crops and Bio-Fertilizer. Agronomy, 11(5), 981. https://doi.org/10.3390/agronomy11050981







