Life Cycle Assessment of Biomass Production from Lignocellulosic Perennial Grasses under Changing Soil Nitrogen and Water Content in the Mediterranean Area
Abstract
:1. Introduction
2. Materials and Methods
2.1. Agronomic Data
2.2. Life Cycle Assessment
2.3. Statistical Analysis
3. Results
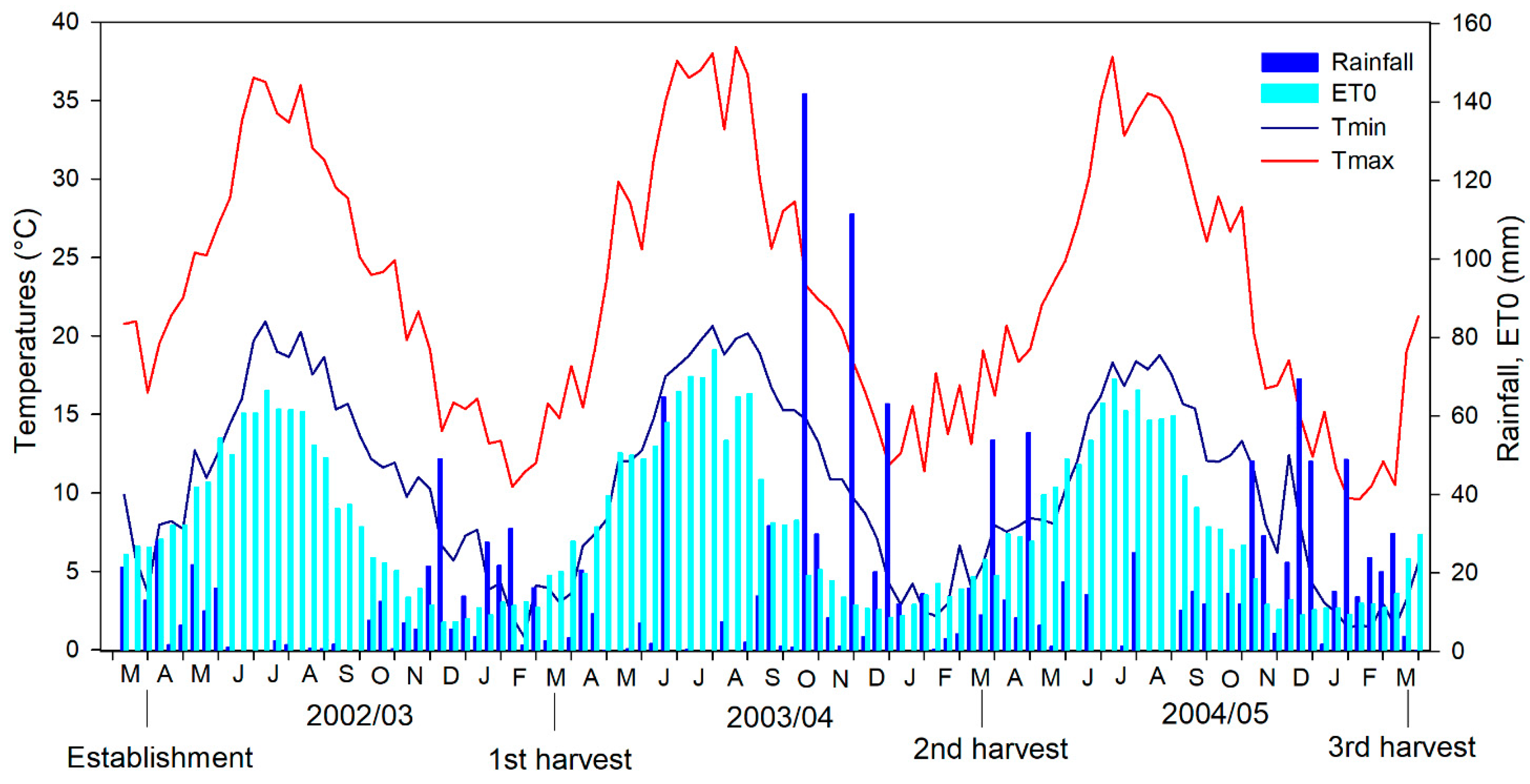
3.1. Meteorological Conditions
3.2. Biomass Yield and Aboveground CO2-Sequestration
3.3. Life Cycle Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DIRECTIVE (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the Promotion of the Use of Energy from Renewable Sources (Recast). Official Journal of the European Union, L 328/82. Available online: https://eur-lex.europa.eu/eli/dir/2018/2001/oj (accessed on 15 March 2021).
- Von Cossel, M.; Lewandowski, I.; Elbersen, B.; Staritsky, I.; Van Eupen, M.; Iqbal, Y.; Mantel, S.; Scordia, D.; Testa, G.; Cosentino, S.L.; et al. Marginal agricultural land low-input systems for biomass production. Energies 2019, 12, 3123. [Google Scholar] [CrossRef] [Green Version]
- Scordia, D.; Cosentino, S.L. Perennial Energy Grasses: Resilient Crops in a Changing European Agriculture. Agriculture 2019, 9, 169. [Google Scholar] [CrossRef] [Green Version]
- Lewandowski, I.; Scurlock, J.M.; Lindvall, E.; Christou, M. The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe. Biomass Bioenergy 2003, 25, 335–361. [Google Scholar] [CrossRef]
- Monti, A.; Fazio, S.; Venturi, G. Cradle-to-farm gate life cycle assessment in perennial energy crops. Eur. J. Agron. 2009, 31, 77–84. [Google Scholar] [CrossRef]
- Schmidt, T.; Fernando, A.L.; Monti, A.; Rettenmaier, N. Life Cycle Assessment of Bioenergy and Bio-Based Products from Perennial Grasses Cultivated on Marginal Land in the Mediterranean Region. Bioenergy Res. 2015, 8, 1548–1561. [Google Scholar] [CrossRef]
- Kim, S.; Dale, B.E. Life cycle assessment of various cropping systems utilized for producing biofuels: Bioethanol and biodiesel. Biomass Bioenergy 2005, 29, 426–439. [Google Scholar] [CrossRef]
- Von Blottnitz, H.; Curran, M.A. A review of assessments conducted on bio-ethanol as a transportation fuel from a net energy, greenhouse gas, and environmental life cycle perspective. J. Clean. Prod. 2007, 15, 607–619. [Google Scholar] [CrossRef]
- Pimentel, D.; Patzek, T. Ethanol production using corn, switchgrass, and wood; biodiesel production using soybean and sunflower. Nat. Resour. Res. 2005, 14, 65–76. [Google Scholar] [CrossRef]
- Farrell, A.E.; Plevin, R.J.; Turner, B.T.; Jones, A.D.; O’Hare, M.; Kammen, D.M. Ethanol can contribute to energy and environmental goals. Science 2006, 311, 506–508. [Google Scholar] [CrossRef] [Green Version]
- Cherubini, F.; Bird, N.D.; Cowie, A.; Jungmeier, G.; Schlamadinger, B.; Woess-Gallasch, S. Energy- and greenhouse gas-based LCA of biofuel and bioenergy systems: Key issues, ranges and recommendations. Resources. Conserv. Recycl. 2009, 53, 434–447. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Intergovernmental Panel on Climate Change, Cambridge University Press: Cambridge, UK; Cambridge, CA, USA, 2013.
- Loizidou, M.; Giannakopoulos, C.; Bindi, M.; Moustakas, K. Climate change impacts and adaptation options in the Mediterranean basin. Reg. Environ. Chang. 2016, 16, 1859–1861. [Google Scholar] [CrossRef] [Green Version]
- Ayangbenro, A.S.; Babalola, O.O. Reclamation of arid and semi-arid soils: The role of plant growth-promoting archaea and bacteria. Curr. Plant Biol. 2021, 25, 100173. [Google Scholar] [CrossRef]
- Von Cossel, M.; Wagner, M.; Lask, J.; Magenau, E.; Bauerle, A.; Cossel, V.V.; Warrach-Sagi, K.; Elbersen, B.; Staritsky, I.; van Eupen, M.; et al. Prospects of bioenergy cropping systems for a more social-ecologically sound bioeconomy. Agronomy 2019, 9, 605. [Google Scholar] [CrossRef] [Green Version]
- Panoutsou, C.; Chiaramonti, D. Socio-Economic Opportunities from Miscanthus Cultivation in Marginal Land for Bioenergy. Energies 2020, 13, 2741. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Scordia, D.; Sanzone, E.; Testa, G.; Copani, V. Response of giant reed (Arundo donax L.) to nitrogen fertilization and soil water availability in semi-arid Mediterranean environment. Eur. J. Agron. 2014, 60, 22–32. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Riggi, E.; Testa, G.; Scordia, D.; Copani, V. Evaluation of European developed fibre hemp genotypes (Cannabis sativa L.) in semi-arid Mediterranean environment. Ind. Crops Prod. 2013, 50, 312–324. [Google Scholar] [CrossRef]
- Scordia, D.; van den Berg, D.; van Sleen, P.; Alexopoulou, E.; Cosentino, S.L. Are herbaceous perennial grasses suitable feedstock for thermochemical conversion pathways? Ind. Crops Prod. 2016, 91, 350–357. [Google Scholar] [CrossRef]
- Doorenbos, J.; Pruitt, W.O. Guidelines for predicting crop water requirements. In Irrigation and Drainage Paper No. 24; FAO: Rome, Italy, 1977; Volume 179. [Google Scholar]
- ISO 14040:2006. Environmental Management-Life cycle Assessment-Principles and Framework; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- ISO 14044:2006. Environmental Management-Life Cycle Assessment-Requirements and Guidelines; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- Institute for Energy and Environmental Research. Available online: https://www.ifeu.de/en/project/biofit-fair/ (accessed on 15 March 2021).
- Alexopoulou, E.; Zanetti, F.; Scordia, D.; Zegada-Lizarazu, W.; Christou, M.; Testa, G.; Cosentino, S.L.; Monti, A. Long-Term Yields of Switchgrass, Giant Reed, and Miscanthus in the Mediterranean Basin. Bioenergy Res. 2015, 8, 1492–1499. [Google Scholar] [CrossRef]
- Mantineo, M.; D’Agosta, G.M.; Copani, V.; Patanè, C.; Cosentino, S.L. Biomass yield and energy balance of three perennial crops for energy use in the semi-arid Mediterranean environment. Field Crops Res. 2009, 114, 204–213. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Boumans, L.J.M.; Batjes, N.H. Modeling global annual N2O and NO emissions from fertilized fields. Glob. Biogeochem. Cycles 2002, 16, 1080. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Boumans, L.J.M.; Batjes, N.H. Estimation of global NH3 volatilization loss from synthetic fertilizers and animal manure applied to arable lands and grasslands. Glob. Biogeochem. Cycles 2002, 16, 1024. [Google Scholar] [CrossRef]
- IPCC (Intergovernmental Panel on Climate Change). Guidelines for National Greenhouse Gas Inventories; Egglestone, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; Volume 4: Agriculture, Forestry and Other Land Use; Prepared by the National Greenhouse Gas Inventories Program; IGES: Kanagawa, Japan, 2006.
- Peter, C.; Fiore, A.; Hagemann, U.; Nendel, C.; Xiloyannis, C. Improving the accounting of field emissions in the carbon footprint of agricultural products: A comparison of default IPCC methods with readily available medium-effort modeling approaches. Int. J. Life Cycle Assess 2016, 21, 791–805. [Google Scholar] [CrossRef] [Green Version]
- Di, H.J.; Cameron, K.C. Calculating nitrogen leaching losses and critical nitrogen application rates in dairy pasture systems using a semi-empirical model. N. Z. J. Agric. Res. 2000, 43, 139–147. [Google Scholar] [CrossRef]
- Huijbregts, M.A.J.; Steinmann, Z.J.N.; Elshout, P.M.F.; Stam, G.; Verones, F.; Vieira, M.; Zijp, M.; Hollander, A.; Van Zelm, R. ReCiPe2016. A harmonized life cycle impact assessment method at midpoint and endpoint level. Int. J. Life Cycle Assess 2017, 22, 138–147. [Google Scholar] [CrossRef]
- Habersatter, K.; Fecker, I.; Dall’Acqua, S.; Fawer, M.; Fallscheer, F.; Förster, R. Ökoinventare für Verpackungen Vol I + II, Swiss Agency for the Environment, Forests and Landscape (BUWAL), Schriftenreihe Umwelt Nr 250/ I+II, 2nd Corrected and Updated Version; Korrigierte und Aktualisierte Auflage: Bern, Switzerland, 1998. [Google Scholar]
- Houghton, J.T.; Meira Filho, L.G.; Bruce, J.; Lee, H.; Callander, B.A.; Haites, E.; Harris, N.; Maskell, K. Climate change 1994. In Radiative Forcing of Climate Change and an Evaluation of the IPCC IS92 Emission Scenarios; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Houghton, J.T.; Meira Filho, L.G.; Callander, B.A.; Harris, N.; Kattenberg, A.; Maskell, K. Climate change 1995. In The Science of Climate Change; Contribution OF Wgi to the Second Assessment Report OF the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Wenzel, H.; Hauschild, M.; Alting, L. Environmental assessment of products. In Volume 1: Methodology, Tools and Case Studies in Products Development; Chapman & Hall: London, UK, 1997. [Google Scholar]
- Hauschild, M.; Wenzel, H. Environmental Assessment of Products; Chapman & Hall: London, UK, 1998. [Google Scholar]
- Weidema, B.P. Multi-user test of the data quality matrix for product life cycle inventory data. Int. J. Life Cycle Assess. 1998, 3, 259–265. [Google Scholar] [CrossRef]
- Monti, A.; Zegada-Lizarazu, W. Sixteen-year biomass yield and soil carbon storage of giant reed (Arundo donax L.) grown under variable nitrogen fertilization rates. Bioenergy Res. 2016, 9, 248–256. [Google Scholar] [CrossRef]
- Copani, V.; Cosentino, S.L.; Testa, G.; Scordia, D. Agamic propagation of giant reed (Arundo donax L.) in semi-arid Mediterranean environment. Ital. J. Agron. 2013, 8, 18–24. [Google Scholar]
- Scordia, D.; Zanetti, F.; Varga, S.S.; Alexopoulou, E.; Cavallaro, V.; Monti, A.; Copani, V.; Cosentino, S.L. New Insights into the Propagation Methods of Switchgrass, Miscanthus and Giant Reed. Bioenergy Res. 2015, 8, 1480–1491. [Google Scholar] [CrossRef]
- Lewandowski, I.; Clifton-Brown, J.; Trindade, L.M.; van der Linden, G.C.; Schwarz, K.U.; Müller-Sämann, K.; Anisimov, A.; Chen, C.L.; Dolstra, O.; Donnison, I.; et al. Progress on optimizing miscanthus biomass production for the European bioeconomy: Results of the EU FP7 project OPTIMISC. Front. Plant Sci. 2016, 18, 1620. [Google Scholar] [CrossRef] [Green Version]
- Lewandowski, I.; Heinz, A. Delayed harvest of miscanthus—influences on biomass quantity and quality and environmental impact of energy production. Eur. J. Agron. 2003, 19, 45–63. [Google Scholar] [CrossRef]
- Cherubini, E.; Franco, D.; Zanghelini, G.M.; Soares, S.R. Uncertainty in LCA case study due to allocation approaches and life cycle impact assessment methods. Int. J. Life Cycle Assess. 2018, 23, 2055–2070. [Google Scholar] [CrossRef]



| Source | DF | DMY | CO2-Sequestration |
|---|---|---|---|
| Adj MS | |||
| Harvest | 2 | 5427.01 *** | 15,079.6 *** |
| Species (S) | 1 | 2259.60 *** | 6088.6 *** |
| Irrigation (I) | 1 | 530.46 *** | 1476.2 *** |
| Fertilization (F) | 1 | 26.49 ns | 73.1 ns |
| S × I | 1 | 0.20 ns | 0.30 ns |
| S × F | 1 | 21.51 ns | 59.4 ns |
| I × F | 1 | 3.53 ns | 9.8 ns |
| S × I × F | 1 | 1.49 ns | 4.1 ns |
| Error | 62 | 33.99 | 93.5 |
| Source | DF | NREU | GWP | OD | AC | EU |
|---|---|---|---|---|---|---|
| Adj MS | ||||||
| Harvest | 2 | 697,594 *** | 1,714,927 *** | 15,174,406 *** | 2,975,953 *** | 3,713,964 *** |
| Species (S) | 1 | 157,300 ** | 371,154 ** | 1,175,013 ** | 567,909 *** | 743,625 *** |
| Irrigation (I) | 1 | 39,919 ns | 189,045 * | 1,271,968 ** | 359,438 ** | 462,952 ** |
| Fertilization (F) | 1 | 6637 ns | 72,523 ns | 106,865 ** | 25,114 ns | 11,859 * |
| S × I | 1 | 24,204 ns | 72,719 ns | 3888 ns | 126,065 ns | 165,414 ns |
| S × F | 1 | 178.0 ns | 839.2 ns | 195,575 ns | 727.5 ns | 3191 ns |
| I × F | 1 | 83.2 ns | 4284.1 ns | 83,364 ns | 253.4 ns | 70.4 ns |
| S × I × F | 1 | 712.1 ns | 277.6 ns | 5819.9 ns | 3039.2 ns | 5881 ns |
| Error | 62 | 10,987 | 27,258 | 130,675 | 42,094 | 54,596 |
| NREU | GWP | OD | AC | EU | |
|---|---|---|---|---|---|
| Species | |||||
| Arundo | −870.26a | −691.44a | 820.45b | 339.44b | 429.16b |
| Miscanthus | −776.78b | −547.85b | 1075.95a | 517.06a | 632.42a |
| Irrigation | |||||
| I25 | −799.97a | −568.40b | 1081.12a | 498.91a | 610.98a |
| I75 | −847.07a | −670.89a | 815.29b | 357.59b | 450.61b |
| Nitrogen | |||||
| N50 | −833.12a | −651.38a | 826.37b | 409.57a | 507.96b |
| N100 | −813.92a | −587.91a | 1070.03a | 446.92a | 553.62a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scordia, D.; D’Agosta, G.M.; Mantineo, M.; Testa, G.; Cosentino, S.L. Life Cycle Assessment of Biomass Production from Lignocellulosic Perennial Grasses under Changing Soil Nitrogen and Water Content in the Mediterranean Area. Agronomy 2021, 11, 988. https://doi.org/10.3390/agronomy11050988
Scordia D, D’Agosta GM, Mantineo M, Testa G, Cosentino SL. Life Cycle Assessment of Biomass Production from Lignocellulosic Perennial Grasses under Changing Soil Nitrogen and Water Content in the Mediterranean Area. Agronomy. 2021; 11(5):988. https://doi.org/10.3390/agronomy11050988
Chicago/Turabian StyleScordia, Danilo, Giuseppina Marina D’Agosta, Mariadaniela Mantineo, Giorgio Testa, and Salvatore Luciano Cosentino. 2021. "Life Cycle Assessment of Biomass Production from Lignocellulosic Perennial Grasses under Changing Soil Nitrogen and Water Content in the Mediterranean Area" Agronomy 11, no. 5: 988. https://doi.org/10.3390/agronomy11050988






