Use of New BTH Derivative as Supplement or Substitute of Standard Fungicidal Program in Strawberry Cultivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tested Substance
2.2. Experiment 2019
2.3. Experiment 2020
2.4. Plant Size
2.5. Storage Stability
2.6. Assessment of Fruit Health in the Field and during Storage
2.7. The Firmness of Fruits
2.8. The Measurement of Color
2.9. Total Soluble Solids (TSSs)
2.10. Fresh and Dry Weight
2.11. Determination of the Content of Ascorbic Acid
2.12. Determination of the Content of Phenolic Compounds
2.13. Determination of the Content of Anthocyanin Compounds
2.14. Fruit Respiration
2.15. Statistical Analysis
3. Results
3.1. Overview of the Experiments
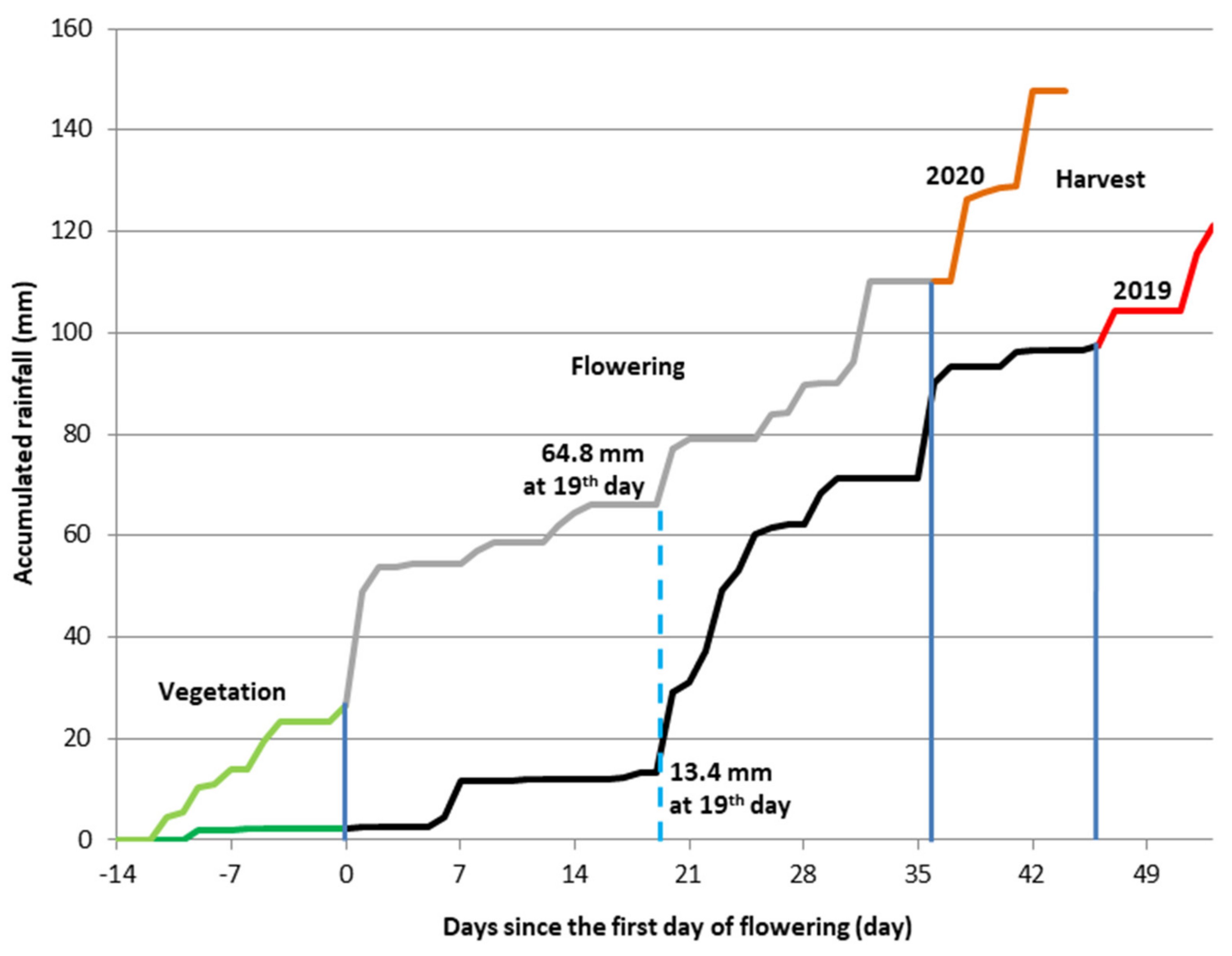
3.2. Weather Conditions
3.3. Experiment 2019
3.3.1. The Plant Size
3.3.2. The Yield
3.3.3. Plant Infections in the Field and During Storage
3.3.4. Physicochemical Parameters
3.4. Experiment 2020
3.4.1. The Plant Size
3.4.2. The Yield
3.4.3. Plant Infections in the Field and during Storage
3.4.4. Physicochemical Parameters
3.4.5. The Storage
3.5. Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission (EC). The European Biodiversity Strategy 2030: Bringing Nature Back into Our Lives; European Commission: Brussels, Belgium, 2020; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1590574123338&uri=CELEX:52020DC0380 (accessed on 26 August 2020).
- Klessig, D.F.; Choi, H.W.; Dempsey, D.A. Systemic acquired resistance and salicylic acid: Past, present, and future. Mol. Plant-Microbe Interact. 2018, 31, 871–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunz, W.; Schurter, R.; Maetzke, T. The chemistry of benzothiadiazole plant activators. Pestic. Sci. 1997, 50, 275–282. [Google Scholar] [CrossRef]
- Gutierrez, H.A.Q.; Meller-Harel, Y.; David, D.R.; Borenshtein, M.; Shulchany, R.; Elad, Y. Effect of climate parameters on induced resistance in strawberry powdery mildew. IOBC/WPRS Bull. 2012, 78, 239–243. [Google Scholar]
- Meller-Harel, Y.; Kolton, M.; Elad, Y.; Rav-David, D.; Cytryn, E.; Ezra, D.; Borenstein, M.; Shulchani, R.; Graber, E.R. Induced systemic resistance in strawberry (Fragaria× ananassa) to powdery mildew using various control agents. IOBC/WPRS Bull. 2011, 71, 47–51. [Google Scholar]
- Poppy, G.M.; Wilkinson, M.J. Gene Flow from GM Plants; Blackwell Publishing: Oxford, UK, 2005; p. 239. [Google Scholar]
- Luna, E.; Bruce, T.J.A.; Roberts, M.R.; Flors, V.; Ton, J. Next-generation systemic acquired resistance. Plant Physiol. 2012, 158, 844–853. [Google Scholar] [CrossRef] [Green Version]
- Smiglak, M.; Kukawka, R.; Lewandowski, P.; Pospieszny, H. Cationic derivatives of the plant resistance inducer benzo(1,2,3)thiadiazole-7-carbothioic acid S-methyl ester (BTH) as bifunctional ionic liquids. Tetrahedron Lett. 2014, 55, 3565–3568. [Google Scholar] [CrossRef]
- Canet, J.V.; Dobón, A.; Ibáñez, F.; Perales, L.; Tornero, P. Resistance and biomass in Arabidopsis: A new model for Salicylic Acid perception. Plant Biotechnol. J. 2010, 8, 126–141. [Google Scholar] [CrossRef] [Green Version]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth-defense tradeoffs in plants: A balancing act to optimize fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef] [Green Version]
- Van Butselaar, T.; van den Ackerveken, G. Salicylic acid steers the growth–Immunity tradeoff. Trends Plant Sci. 2020, 25, 566–576. [Google Scholar] [CrossRef]
- Groszmann, M.; Gonzalez-Bayon, R.; Lyons, R.L.; Greaves, I.K.; Kazan, K.; Peacock, W.J.; Dennis, E.S. Hormone-regulated defense and stress response networks contribute to heterosis in Arabidopsis F1 hybrids. Proc. Natl. Acad. Sci. USA 2015, 112, E6397–E6406. [Google Scholar] [CrossRef] [Green Version]
- Hanssoon, A.S.; Abduljabbar, I.A. Review on the role of salicylic acid in plants. In Sustainable Crop Production, 1st ed.; Hasanuzzaman, M., Ed.; IntechOpen: London, UK, 2020; Chapter 4; pp. 1–6. [Google Scholar] [CrossRef] [Green Version]
- Najafian, S.; Khoshkhui, M.; Vahid, T. Effect of salicylic acid and salinity in rosemary (Rosmarinus officinalis L.): Investigation on changes in gas exchange, water relations, and membrane stabilization. Adv. Environ. Biol. 2009, 3, 322–328. [Google Scholar]
- Feder-Kubis, J.; Czerwoniec, P.; Lewandowski, P.; Pospieszny, H.; Smiglak, M. Ionic liquids with natural origin component: A path to new plant protection products. ACS Sust. Chem. Eng. 2020, 8, 842–852. [Google Scholar] [CrossRef]
- Smiglak, M.; Lewandowski, P.; Kukawka, R.; Budziszewska, M.; Krawczyk, K.; Obrępalska-Stęplowska, A.; Pospieszny, H. Dual functional salts of Benzo[1.2.3]thiadiazole-7-carboxylates as a highly efficient weapon against viral plant diseases. ACS Sust. Chem. Eng. 2017, 5, 4197–4204. [Google Scholar] [CrossRef]
- Smiglak, M.; Kukawka, R.; Lewandowski, P.; Budziszewska, M.; Obrepalska-Steplowska, A.; Krawczyk, K.; Zwolińska, A.; Pospieszny, H. New dual functional salts based on cationic derivative of plant resistance inducer–Benzo[1.2.3]thiadiazole-7-carbothioic acid, S-Methyl ester. ACS Sust. Chem. Eng. 2016, 4, 3344–3351. [Google Scholar] [CrossRef]
- Kukawka, R.; Czerwoniec, P.; Lewandowski, P.; Pospieszny, H.; Smiglak, M. New ionic liquids based on systemic acquired resistance inducers combined with the phytotoxicity reducing cholinium cation. N. J. Chem. 2018, 42, 11984–11990. [Google Scholar] [CrossRef]
- Czerwoniec, P.; Lewandowski, P.; Smiglak, M. Derivatives of isonicotinic acid as new efficient systemic acquired resistance (SAR) inducers. ChemistrySelect 2020, 5, 10759–10764. [Google Scholar] [CrossRef]
- Frackowiak, P.; Pospieszny, H.; Smiglak, M.; Obrępalska-Stęplowska, A. Assessment of the efficacy and mode of action of Benzo(1,2,3)-Thiadiazole-7-Carbothioic Acids-Methyl ester (BTH) and its derivatives in plant protection against viral disease. Int. J. Mol. Sci. 2019, 20, 1598. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.; Nguyen, A.; Betts, N.M.; Lyons, T.J. Strawberry as a functional food: An evidence-based review. Crit. Rev. Food Sci. Nutr. 2014, 54, 790–806. [Google Scholar] [CrossRef]
- Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Alvarez-Suarez, J.M.; Afrin, S.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Strawberry as a health promoter: An evidence based review. Food Funct. 2015, 6, 1386–1398. [Google Scholar] [CrossRef] [Green Version]
- Cervantes, L.; Ariza, M.T.; Miranda, L.; Lozano, D.; Medina, J.J.; Soria, C.; Martínez-Ferri, E. Stability of fruit quality traits of different strawberry varieties under variable environmental conditions. Agronomy 2020, 10, 1242. [Google Scholar] [CrossRef]
- Worldwide Production of Strawberry in 2017. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwi_z82j39rvAhXi-SoKHUPVBGgQFjAAegQIBBAD&url=https%3A%2F%2Fedepot.wur.nl%2F503212&usg=AOvVaw1JQyvZ6iuymtK-pwkMhl82 (accessed on 1 March 2021).
- Statistics Poland, Production of Agricultural and Horticultural Crops in 2019. Available online: https://stat.gov.pl/download/gfx/portalinformacyjny/en/defaultaktualnosci/3332/2/4/1/produkcja_upraw_rolnych_i_ogrodniczych_w_2019_r.pdf (accessed on 1 March 2021).
- Paszko, D.; Pawlak, J.; Wróblewska, W. Yield of two strawberry cultivars depending on the cropping method on the example of a commercial plantation in a specialized horticultural farm. Acta Sci. Pol. Hortorum Cultus 2014, 13, 149–159. [Google Scholar]
- Fagherazzi, A.F.; Suek Zanin, D.; Soares dos Santos, M.F.; Martins de Lima, J.; Welter, P.D.; Francis Richter, A.; Regianini Nerbass, F.; Anneliese Kretzschmar, A.; Rufato, L.; Baruzzi, G. Initial crown diameter influences on the fruit yield and quality of strawberry pircinque. Agronomy 2021, 11, 184. [Google Scholar] [CrossRef]
- Meyers, K.J.; Watkins, C.B.; Pritts, M.P.; Liu, R.H. Antioxidant and antiproliferative activities of strawberries. J. Agric. Food Chem. 2003, 5, 6887–6892. [Google Scholar] [CrossRef]
- Seeram, N.P.; Lee, R.; Scheuller, S.; Heber, D. Identification of phenolic compounds in strawberries by liquid chromatography electrosprayionization mass spectroscopy. Food Chem. 2006, 97, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Tulipani, S.; Mezzetti, B.; Capocasa, F.; Bompadre, S.; Beekwilder, J.; de Vos, C.H.R.; Capanoglu, E.; Bovy, A.; Battino, M. Antioxidants, phenoliccompounds, and nutritional quality of different strawberry genotypes. J. Agric. Food Chem. 2008, 56, 696–704. [Google Scholar] [CrossRef]
- Ghaouth, A.E.; Arul, J.; Ponnampalam, R.; Boulet, M. Chitosan coating effect on storability and quality of fresh strawberries. J. Food Sci. 1991, 56, 1618–1620. [Google Scholar] [CrossRef]
- Koike, S.T. Crown rot of strawberry caused by Macrophomina phaseolina in California. Plant Dis. 2008, 92, 1253. [Google Scholar] [CrossRef]
- Petrasch, S.; Knapp, S.J.; Van Kan, J.A.L.; Blanco-Ulate, B. Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Path. 2019, 20, 877–892. [Google Scholar] [CrossRef] [Green Version]
- Parker, C. Strawberry fields forever: Can consumers see pesticides and sustainability as an issue? Sustain. Sci. 2015, 10, 285–303. [Google Scholar] [CrossRef]
- Friends of the Earth Australia. Available online: https://www.foe.org.au/sites/default/files/TheDoseMakesThePoisonFeb2012_0.pdf (accessed on 1 March 2021).
- Seufert, V.; Ramankutty, N.; Foley, J. Comparing the yields of organic and conventional agriculture. Nature 2012, 485, 229–232. [Google Scholar] [CrossRef]
- Smiglak, M.; Pospieszny, H.; Kukawka, R.; Lewandowski, P.; Stolarska, O.; Maciejewski, H. Application of 7-Carboxybenzo(1,2,3)Thiadiazole Amides as Plant Stimulants. Patent Application No. WO/2017/017626, 2 February 2017. [Google Scholar]
- Tsao, R.; Yang, R. Optimization of a new mobile phase to know the complex and real polyphenolic composition: Towards a total phenolic index using high-performance liquid chromatography. J. Chromatogr. A. 2003, 1018, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In Handbook of Food Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2004; Volume 2, Chapter 18; pp. 19–31. [Google Scholar] [CrossRef]
- Schurter, R.; Kunz, W.; Nyfeler, R. Process and a Composition for Immunizing Plants Against Diseases. U.S. Patent 4,931,581, 6 June 1990. [Google Scholar]
- Walters, D.R.; Ratsep, J.; Havis, N.D. Controlling crop diseases using induced resistance: Challenges for the future. J. Exp. Bot. 2013, 64, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Amil-Ruiz, F.; Blanco-Portales, R.; Munŏz-Blanco, J.; Caballero, J.L. The strawberry plant defense mechanism. A molecular review. Plant Cell Physiol. 2011, 52, 1873–1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayres, P.G.; Woolacott, B. Effects of soil water level on the development of adult plant resistance to powdery mildew in barley. Ann. Appl. Biol. 1980, 94, 255–263. [Google Scholar] [CrossRef]
- Dietrich, R.; Ploss, K.; Heil, M. Growth responses and fitness costs after induction of pathogen resistance depend on environmental conditions. Plant Cell. Environ. 2004, 28, 211–222. [Google Scholar] [CrossRef]
- Aschehoug, E.T.; Brooker, R.; Atawater, D.Z.; Maron, J.L.; Callaway, R.M. The mechanisms and consequences of interspecific competition among plants. Annu. Rev. Ecol. Evol. S. 2016, 47, 263–281. [Google Scholar] [CrossRef] [Green Version]
- Ji, P.; Yin, J.; Kone, D. Application of acibenzolar- S-methyl and standard fungicides for control of Phytophthora blight on squash. Crop Prot. 2011, 30, 1601–1605. [Google Scholar] [CrossRef]
- Mazaro, S.M.; Deschamps, C.; May De Mio, L.L.; Biasi, L.A.; De Gouvea, A.; Sautter, C.K. Post harvest behavior of strawberry fruits after pre harvest treatment with chitosan and acibenzolar-s-methyl. Rev. Bras. Frutic. 2008, 30, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Azami-Sardooei, Z.; Seifi, H.S.; de Vleesschauwer, D.; Höfte, M. Benzothiadiazole (BTH)-induced resistance against Botrytis cinerea is inversely correlated with vegetative and generative growth in bean and cucumber, but not in tomato. Australas. Plant Pathol. 2013, 42, 485–490. [Google Scholar] [CrossRef]
- Petit, A.-N.; Fontaine, F.; Vatsa, P.; Clément, C.; Vaillant-Gaveau, N. Fungicide impacts on photosynthesis in crop plants. Photosynth. Res. 2012, 111, 315–326. [Google Scholar] [CrossRef]
- Saladin, G.; Magne, C.; Clement, C. Effects of fludioxonil and pyrimethanil, two fungicides used against Botrytis cinerea, on carbohydrate physiology in Vitis vinifera L. Pest. Manag. Sci. 2003, 59, 1083–1092. [Google Scholar] [CrossRef]
- Petit, A.-N.; Fontaine, F.; Clement, C.; Vaillant-Gaveau, N. Photosynthesis limitations of grapevine after treatment with the fludioxonil fungicide. J. Agric. Food Chem. 2008, 56, 6761–6767. [Google Scholar] [CrossRef]
- Bektas, Y.; Eulgem, T. Synthetic plant defense elicitors. Front. Plant Sci. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Romanazzi, G.; Sanzani, S.M.; Bi, Y.; Tian, S.; Martínez, P.G.; Alkan, N. Induced resistance to control postharvest decay of fruit and vegetables. Postharvest Biol. Technol. 2006, 122, 82–94. [Google Scholar] [CrossRef]
- EL-Metwally, M.A.; Tarabih, M.E.; EL-Eryan, E.E. Effect of application of β-aminobutyric acid on maintaining quality of crimson seedless grape and controlling postharvest diseases under cold storage conditions. Plant Pathol. J. 2014, 13, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Hu, Z.; Zheng, Y.; Yang, Z.; Lu, B. Effect of BTH on antioxidant enzymes, radical-scavenging activity and decay in strawberry fruit. Food Chem. 2011, 125, 145–149. [Google Scholar] [CrossRef]
- Hukkanen, A.; Kostamo, K.; Karenlampi, S.; Kokko, H. Impact of agrochemicals on peronospora sparsa and phenolic profiles in three rubus arcticus cultivars. J. Agric. Food Chem. 2008, 56, 1008–1016. [Google Scholar] [CrossRef]
- Achuo, E.A.; Audenaert, K.; Meziane, H.; Höfte, M. The salicylic acid-dependent defence pathway is effective against different pathogens in tomato and tobacco. Plant Path. 2004, 53, 65–72. [Google Scholar] [CrossRef]
- Govrin, E.M.; Levine, A. Infection of Arabidopsis with a necrotrophic pathogen, Botrytis cinerea, elicits various defense responses but does not induce systemic acquired resistance (SAR). Plant Mol. Biol. 2012, 48, 267–276. [Google Scholar] [CrossRef]
- Hou, R.; Shi, J.; Ma, X.; Wei, H.; Hu, J.; Tsang, Y.F.; Gao, M.-T. Effect of phenolic acids derived from rice straw on Botrytis cinerea and infection on tomato. Waste Biomass Valorization 2020, 11, 6555–6563. [Google Scholar] [CrossRef]
- Baysal, O.; Turgut, C.; Mao, G. Acibenzolar-S-methyl induced resistance to Phytophthora capsici in pepper leaves. Biol. Plant. 2005, 49, 599–604. [Google Scholar] [CrossRef]
- Lin, T.C.; Ishizaka, M.; Ishii, H. Acibenzolar-S-methyl-induced systemic resistance against anthracnose and powdery mildew diseases on cucumber plants without accumulation of phytoalexins. J. Phytopathol. 2009, 157, 40–50. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Zhang, B. Induced resistance in peach fruit as treated by Pichia guilliermondii and their possible mechanism. Int. J. Food Prop. 2020, 23, 34–51. [Google Scholar] [CrossRef] [Green Version]
- Cocco, C.; Magnani, S.; Maltoni, M.L.; Quacquarelli, I.; Cacchi, M.; Antunes, L.E.C.; D’Antuono, L.F.; Faedi, W.; Baruzzi, G. Effects of site and genotype on strawberry fruits quality traits and bioactive compounds. J. Berry Res. 2015, 5, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Bi, Y.; Wang, J.; Dong, B.; Li, H.; Gong, D.; Zhao, Y.; Tang, Y.; Yu, X.; Shang, Q. BTH treatment caused physiological, biochemical and proteomic changes of muskmelon (Cucumis melo L.) fruit during ripening. J. Proteom. 2015, 120, 179–193. [Google Scholar] [CrossRef]
- Terry, L.A.; Joyce, D.C. Suppression of grey mould on strawberry fruit with the chemical plant activator acibenzolar. Pest Manag. Sci. 2000, 56, 989–992. [Google Scholar] [CrossRef]

| Factor | Description |
|---|---|
| Name | N-methoxy-N-methylbenzo(1.2.3)thiadiazole-7-carboxamide (BTHWA) |
| Chemical Formula | C9H9N3O2S |
| Molar mass | 223.25 g/moL |
| Appearance | Beige crystalline powder |
| Melting point (°C) | 125.1 |
| Solubility in water (20 °C) | 25 mg/L |
| Abbreviation of Treatment Variant | Description of Treatment |
|---|---|
| UTC | Untreated control |
| SFP | Standard Fungicide Program |
| 40 × 9/0F | BTHWA 40 mg/L, 9 treatments, no fungicide treatment |
| 20 × 9/0F | BTHWA 20 mg/L, 9 treatments, no fungicide treatment |
| 20 × 9/1F | BTHWA 20 mg/L, 9 treatments, 1 fungicide treatment |
| 20 × 9/SFP | BTHWA 20 mg/L, 9 treatments, Standard Fungicide Program |
| Variant of Treatment | Date of Application and BBCH Scale | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 05.04 03 | 19.04 13 | 26.04 61 | 02.05 65 | 09.05 65/67 | 17.05 65/67 | 21.05 71 | 28.05 71 | 05.06 72/81 | |
| UTC | |||||||||
| SFP | D | E | D | E | D | ||||
| 40 × 9/0F | A | A | A | A | A | A | A | A | A |
| 20 × 9/0F | C | C | C | C | C | C | C | C | C |
| 20 × 9/1F | C | C | C + D | C | C | C | C | C | C |
| 20 × 9/SFP | C | C | C + D | C + E | C + D | C + E | C + D | C | C |
| Abbreviation of Treatment Variant | Description of Treatment |
|---|---|
| UTC | Untreated control |
| SFP | Standard Fungicide Program |
| 20 × 4/0F | BTHWA 20 mg/L, 4 treatments, no fungicide treatment |
| 20 × 4/1F | BTHWA 20 mg/L, 4 treatments, 1 fungicide treatment |
| 20 × 4/SFP | BTHWA 20 mg/L, 4 treatments, Standard Fungicide Program |
| 30 × 4/0F | BTHWA 30 mg/L, 4 treatments, no fungicide treatment |
| 30 × 4/1F | BTHWA 30 mg/L, 4 treatments, 1 fungicide treatment |
| 30 × 4/SFP | BTHWA 30 mg/L, 4 treatments, Standard Fungicide Program |
| Variant of Treatment | Date of Application and BBCH SCALE | |||||
|---|---|---|---|---|---|---|
| 30.04 56 | 13.05 61 | 21.05 65 | 29.05 67 | 04.06 81 | 10.06 81 | |
| UTC | ||||||
| SFP | D | E | D | E | D | |
| 20 × 4/0F | C | C | C | C | ||
| 20 × 4/1F | C | C + E | C | C | ||
| 20 × 4/SFP | C | C + D | E | C + D | E | C + D |
| 30 × 4/0F | B | B | B | B | ||
| 30 × 4/1F | B | B + E | B | B | ||
| 30 × 4/SFP | B | B + D | E | B + D | E | B + D |
| Parameter | Variant of Treatment | |||||
|---|---|---|---|---|---|---|
| UTC | SFP | 40 × 9/0F | 20 × 9/0F | 20 × 9/0F | 20 × 9/SFP | |
| Height (cm) | 27.85 a | 28.18 a | 27.88 a | 28.62 a | 30.42 b | 27.98 a |
| Diameter (cm) | 28.44 | 27.42 | 26.38 | 28.95 | 27.74 | 27.51 |
| Plant area (cm2) | 648.1 | 606.2 | 599.4 | 677.3 | 615.8 | 628.2 |
| Volume (dm3) | 6.007 | 5.6286 | 5.7149 | 6.4602 | 6.249 | 5.933 |
| Parameter | Variant of Treatment | |||||
|---|---|---|---|---|---|---|
| UTC | SFP | 40 × 9/0F | 20 × 9/0F | 20 × 9/0F | 20 × 9/SFP | |
| Marketable yield (pcs./m2) | 52.6 | 56.03 | 63.11 | 58.24 | 59.71 | 56.31 |
| Non-marketable yield (pcs./m2) | 0.032 | 0.032 | 0.032 | 0.0641 | 0.032 | 0 |
| Total yield (pcs./m2) | 52.63 | 56.06 | 63.14 | 58.3 | 59.74 | 56.31 |
| Marketable yield (kg/m2) | 0.0004 | 0.0004 | 0.0004 | 0.0007 | 0.0004 | 0 |
| Non-marketable yield (kg/m2) | 0.4171 | 0.4379 | 0.5184 | 0.4667 | 0.5206 | 0.4535 |
| Total yield (kg/m2) | 7.91 | 7.85 | 8.29 | 8.14 | 8.75 | 8.06 |
| Mean weight of marketable fruit (g) | 52.6 | 56.03 | 63.11 | 58.24 | 59.71 | 56.31 |
| Parameter | Variant of Treatment | |||||
|---|---|---|---|---|---|---|
| UTC | SFP | 40 × 9/0F | 20 × 9/0F | 20 × 9/0F | 20 × 9/SFP | |
| FIELD | ||||||
| Botrytis cinerea (pcs./m2) | 0 | 0.032 | 0 | 0 | 0.032 | 0 |
| Phytophtora sp. (pcs./m2) | 0 | 0 | 0 | 0 | 0 | 0 |
| Colletotrichum sp. (pcs./m2) | 0 | 0 | 0 | 0 | 0 | 0 |
| Rhizopus stolonifer (pcs./m2) | 0.032 | 0 | 0.032 | 0.0641 | 0 | 0 |
| Botrytis cinerea (%) | 0 | 0.0608 | 0 | 0 | 0.047 | 0 |
| Phytophtora sp. (%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Colletotrichum sp. (%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Rhizopus stolonifer (%) | 0.062 | 0 | 0.0408 | 0.0938 | 0 | 0 |
| Fruits Infected (%) | 0.062 | 0.0608 | 0.0408 | 0.0938 | 0.047 | 0 |
| STORAGE | ||||||
| Botrytis cinerea (%) | 1.4166 b | 0.25 a | 0.8333 ab | 0.6666 ab | 1.0833 ab | 0.4166 ab |
| Phytophtora sp. (%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Colletotrichum sp. (%) | 0.1667 | 0 | 0 | 0.0833 | 0.5 | 0.0833 |
| Rhizopus stolonifer (%) | 0.0833 | 0.5833 | 0.8333 | 0.4167 | 0 | 0.1667 |
| Fruits infected (%) | 1.6667 | 0.8334 | 1.6667 | 1.1667 | 1.5833 | 0.6667 |
| Parameter | Variant of Treatment | |||||
|---|---|---|---|---|---|---|
| UTC | SFP | 40 × 9/0F | 20 × 9/0F | 20 × 9/0F | 20 × 9/SFP | |
| Anthocyans (mg/100 g of fruits) | 57.82 a | 62.94 b | 56.45 a | 68.84 c | 61.22 b | 61.22 b |
| Polyphenols (mg/100 g of fruits) | 385.4 | 390.4 | 389.4 | 397.3 | 372.3 | 393.9 |
| Ascorbic acid (mg/100 g of fruits) | 63.8 d | 58.72 c | 56.1 b | 55.06 ab | 54.58 a | 63.21 d |
| Firmness (N) | 1.1230 | 1.1717 | 1.0550 | 1.0730 | 1.0720 | 0.8433 |
| Fruit color—L (darkness/lightness) | 36.97 c | 35.87 bc | 33.66 a | 35.78 bc | 36.12 bc | 33.48 a |
| Fruit color—a (green/red) | 15.24 ab | 15.03 ab | 16.44 ab | 14.15 a | 21.93 c | 18.09 b |
| Fruit color—b (blue/yellow) | 11.73 | 11.61 | 11.40 | 10.28 | 14.67 | 11.04 |
| BTHWA | Type of Fungicide Treatment | Mean | |||
|---|---|---|---|---|---|
| 0F | 1F | SFP | |||
| Height (cm) | 20 × 4 | 25.78 b° | 24.39 a | 23.90 a | 24.69 |
| 30 × 4 | 26.70 b*° | 23.91 a | 22.70 a* | 24.44 | |
| Mean | 26.24 B*° | 24.15 A | 23.30 A | ||
| Diameter (cm) | 20 × 4 | 28.46 b*° | 27.50 b*° | 25.36 a* | 27.11 *° |
| 30 × 4 | 28.60 b*° | 25.49 a* | 25.11 a* | 26.40 *° | |
| Mean | 28.53 C*° | 26.49 B*° | 25.24 A* | ||
| Plant area (cm2) | 20 × 4 | 649.0 b*° | 604.7 b*° | 515.3 a* | 589.6 *° |
| 30 × 4 | 653.4 b*° | 520.0 a* | 501.4 a | 558.3 *° | |
| Mean | 651.2 C*° | 562.3 B*° | 508.3 A* | ||
| Volume (dm3) | 20 × 4 | 5.681 cd*° | 4.975 bc*° | 4.185 ab | 4.947 *° |
| 30 × 4 | 5.959 d*° | 4.222 ab | 3.804 a | 4.662 *° | |
| Mean | 5.820 C*° | 4.599 B*° | 3.995 A | ||
| Parameter | Variant of Treatment | |
|---|---|---|
| UTC | SFP | |
| Height (cm) | 24.55 | 23.85 |
| Diameter (cm) | 23.53 | 24.39 |
| Plant area (cm2) | 444.01 | 474.03 |
| Volume (dm3) | 3.72 | 3.81 |
| BTHWA | Type of Fungicide Treatment | Mean | |||
|---|---|---|---|---|---|
| 0F | 1F | SFP | |||
| Marketable yield (pcs./m2) | 20 × 4 | 37.01 ° | 52.17 | 73.14 * | 54.11 |
| 30 × 4 | 39.04 ° | 61.29 | 77.38 * | 59.23 | |
| Mean | 38.03 A° | 56.73 AB | 75.46 B* | ||
| Non-marketable yield (pcs./m2) | 20 × 4 | 16.00 b° | 14.32 b*° | 6.39 a* | 12.25 * |
| 30 × 4 | 13.91 b*° | 15.84 b° | 4.99 a* | 11.52 * | |
| Mean | 14.96 B*° | 14.99 B*° | 5.69 A* | ||
| Total yield (pcs./m2) | 20 × 4 | 53.02 | 66.49 | 79.54 | 66.35 |
| 30 × 4 | 52.95 | 76.94 | 82.37 | 70.75 | |
| Mean | 52.99 A | 71.71 AB | 80.95 B | ||
| Marketable yield (kg/m2) | 20 × 4 | 0.3412 | 0.4294 | 0.5783 | 0.4484 |
| 30 × 4 | 0.3425 | 0.5307 | 0.5642 | 0.4790 | |
| Mean | 0.3398 A° | 0.4801 AB | 0.5712 B | ||
| Non-marketable yield (kg/m2) | 20 × 4 | 0.1337 b° | 0.1107 b*° | 0.0459 a* | 0.0968 * |
| 30 × 4 | 0.1055 b*° | 0.1146 b*° | 0.0325 a* | 0.0842 * | |
| Mean | 0.1197 B*° | 0.1127 B*° | 0.0392 A* | ||
| Total yield (kg/m2) | 20 × 4 | 0.4715 | 0.5401 | 0.6243 | 0.5453 |
| 30 × 4 | 0.4476 | 0.6653 | 0.5977 | 0.5632 | |
| Mean | 0.4514 | 0.5927 | 0.6105 | ||
| Mean weight of marketable fruit (g) | 20 × 4 | 8.99 | 8.23 | 7.79 | 8.34 |
| 30 × 4 | 8.84 | 8.63 | 7.29 | 8.25 | |
| Mean | 8.92 B | 8.43 B | 7.54 A | ||
| Parameter | Variant of Treatment | |
|---|---|---|
| UTC | SFP | |
| Marketable yield (pcs./m2) | 42.707 | 68.051 |
| Non-marketable yield (pcs./m2) | 22.562 β | 4.991 α |
| Total yield (pcs./m2) | 65.277 | 73.042 |
| Marketable yield (kg/m2) | 0.384 | 0.55 |
| Non-marketable yield (kg/m2) | 0.182 β | 0.0.034 α |
| Total yield (kg/m2) | 0.566 | 0.58 |
| Mean weight of marketable fruit (g) | 7.98 | 7.82 |
| BTHWA | Type of Fungicide Treatment | Mean | |||
|---|---|---|---|---|---|
| 0F | 1F | SFP | |||
| FIELD | |||||
| Botrytis cinerea (pcs./m2) | 20 × 4 | 2.0275 | 2.4175 ° | 0.7801 | 1.7412 |
| 30 × 4 | 1.8977 | 2.4694 ° | 0.7274 | 1.6982 | |
| Mean | 1.9626 ° | 2.4435 ° | 0.7542 | ||
| Phytophtora sp. (pcs./m2) | 20 × 4 | 13.9086 ° | 11.9054 * | 5.5634 * | 10.4619 * |
| 30 × 4 | 11.9865 * | 12.9678 ° | 4.2658 * | 9.7401 * | |
| Mean | 12.9435 B*° | 12.4407 B*° | 4.9146 A* | ||
| Colletotrichum sp. (pcs./m2) | 20 × 4 | 0.0260 * | 0.0000 * | 0.0519 * | 0.0260 * |
| 30 × 4 | 0.0260 * | 0.1039 * | 0.0000 * | 0.0433 * | |
| Mean | 0.0260 * | 0.0519 * | 0.0260 * | ||
| Rhizopus stolonifer (pcs./m2) | 20 × 4 | 0.0519 | 0.0000 | 0.0000 | 0.0173 |
| 30 × 4 | 0.0000 | 0.1039 | 0.0000 | 0.0346 | |
| Mean | 0.0260 | 0.0641 | 0.0000 | ||
| Botrytis cinerea (%) | 20 × 4 | 3.3946 ° | 3.2051 ° | 1.0142 | 2.538 |
| 30 × 4 | 3.7351 ° | 3.1554 ° | 0.8389 | 2.5764 ° | |
| Mean | 3.5649 B° | 3.1802 B° | 0.9266 A* | ||
| Phytophtora sp. (%) | 20 × 4 | 27.77 b° | 19.95 ab° | 7.7471 a* | 18.49 |
| 30 × 4 | 22.46 ab° | 18.24 ab | 4.8862 a* | 15.2 * | |
| Mean | 25.12 B° | 19.1 B*° | 6.3166 A* | ||
| Colletotrichum sp. (%) | 20 × 4 | 0.06 * | 0 * | 0.0485 * | 0.0362 * |
| 30 × 4 | 0.059 * | 0.1283 * | 0 * | 0.0624 * | |
| Mean | 0.0595 * | 0.0641 * | 0.0243 * | ||
| Rhizopus stolonifer (%) | 20 × 4 | 0.1029 | 0 | 0 | 0.0343 |
| 30 × 4 | 0 | 0.1645 | 0 | 0.0548 | |
| Mean | 0.0514 | 0.0822 | 0 | ||
| Fruits infected (%) | 20 × 4 | 31.33 b° | 23.16 b° | 8.8098 a* | 21.1 ° |
| 30 × 4 | 26.25 b° | 21.69 b*° | 5.7251 a* | 17.89 * | |
| Mean | 28.79 B° | 22.42 B*° | 7.2674 A* | ||
| STORAGE | |||||
| Botrytis cinerea (%) | 20 × 4 | 1.5833 | 2.5834 | 0.1667 | 1.4444 |
| 30 × 4 | 3 | 2.5 | 1.5834 | 2.3611 | |
| Mean | 2.2917 | 2.5417 | 0.875 | ||
| Phytophtora sp. (%) | 20 × 4 | 4.3333 | 6.9167 | 6.1667 | 5.8056 |
| 30 × 4 | 8.0833 | 5.3334 | 6 | 6.4722 | |
| Mean | 6.2083 | 6.125 | 6.0833 | ||
| Colletotrichum sp. (%) | 20 × 4 | 0 | 0 | 0 | 0 |
| 30 × 4 | 0.1667 | 0.1667 | 0 | 0.1111 | |
| Mean | 0.0833 | 0.0833 | 0 | ||
| Rhizopus stolonifer (%) | 20 × 4 | 0 | 0.25 | 0.0833 | 0.1111 |
| 30 × 4 | 1.1667 | 0.5 | 0 | 0.5556 | |
| Mean | 0.5833 | 0.375 | 0.0417 | ||
| Fruits infected (%) | 20 × 4 | 5.9167 | 9.75 | 6.4167 | 7.3611 |
| 30 × 4 | 12.42 | 8.5 | 7.5833 | 9.5 | |
| Mean | 9.1667 | 9.125 | 7 | ||
| Parameter | Variant of Treatment | |
|---|---|---|
| UTC | SFP | |
| FIELD | ||
| Botrytis cinerea (pcs./m2) | 2.6923 | 0.3526 |
| Phytophtora sp. (pcs./m2) | 24.7756 β | 5.6731 α |
| Colletotrichum sp. (pcs./m2) | 0.3526 β | 0.1282 α |
| Rhizopus stolonifer (pcs./m2) | 0.0000 | 0.0000 |
| Botrytis cinerea (%) | 3.1579 β | 0.3704 α |
| Phytophtora sp. (%) | 30.6883 β | 6.1383 α |
| Colletotrichum sp. (%) | 0.4508 β | 0.1360 α |
| Rhizopus stolonifer (%) | 0.0000 | 0.0000 |
| Fruits infected (%) | 34.2970 β | 6.6446 α |
| STORAGE | ||
| Botrytis cinerea (%) | 1.6667 | 0.6667 |
| Phytophtora sp. (%) | 8.1667 | 5.8333 |
| Colletotrichum sp. (%) | 0.1666 | 0.0000 |
| Rhizopus stolonifer (%) | 0.0833 | 0.5834 |
| Fruits infected (%) | 10.0833 | 7.0834 |
| BTHWA | Type of Fungicide Treatment | Mean | |||
|---|---|---|---|---|---|
| 0F | 1F | SFP | |||
| Anthocyans (mg/100 g of fruits) | 20 × 4 | 47.3745 b* | 43.0980 a° | 45.0921 b | 45.1882 A |
| 30 × 4 | 52.4836 c*° | 46.2983 b | 45.2830 b | 48.0216 B | |
| Mean | 49.9290 B* | 44.6981 A | 45.1875 A | ||
| Polyphenols (mg/100 g of fruits) | 20 × 4 | 298.6946 b* | 284.3074 a | 298.0145 b* | 293.6722 A |
| 30 × 4 | 306.2806 c*° | 286.0339 a | 296.0526 b* | 296.1223 B | |
| Mean | 302.4876 C*° | 285.1706 A | 297.0335 B* | ||
| Ascorbic acid (mg/100 g of fruits) | 20 × 4 | 44.8829 c*° | 39.1063 a*° | 45.5021 c*° | 43.1638 A° |
| 30 × 4 | 45.4299 c*° | 42.7957 b° | 43.6239 b° | 43.9498 B° | |
| Mean | 45.1564 B° | 40.9510 A° | 44.5630 B° | ||
| Firmness (N) | 20 × 4 | 1.5005 b° | 0.9467 a* | 0.9477 a* | 1.1317 |
| 30 × 4 | 1.4504 b° | 1.2467 ab° | 1.1365 ab | 1.2779 ° | |
| Mean | 1.4755 B° | 1.0967 A* | 1.0421 A* | ||
| Fruit color—L (darkness/lightness) | 20 × 4 | 26.39 | 26.46 | 27.41 | 26.75 |
| 30 × 4 | 27.93 ° | 26.7 | 26.14 | 26.92 | |
| Mean | 27.16 | 26.58 | 26.77 | ||
| Fruit color—a (green/red) | 20 × 4 | 20.05 ab | 18.75 a*° | 20.35 ab | 19.72 A |
| 30 × 4 | 22.85 c | 21.61 bc | 18.71 a*° | 21.06 B | |
| Mean | 21.45 B | 20.18 AB | 19.53 A | ||
| Fruit color—b (blue/yellow) | 20 × 4 | 12.05 a | 11.51 a | 11.64 a | 11.73 |
| 30 × 4 | 14.36 b | 12.2 a | 10.46 a*° | 12.34 | |
| Mean | 13.21 B | 11.85 AB | 11.05 A*° | ||
| Parameter | Variant of Treatment | |
|---|---|---|
| UTC | SFP | |
| Anthocyans (mg/100 g of fruits) | 44.39 α | 46.90 β |
| Polyphenols (mg/100 g of fruits) | 282.7 α | 291.3 β |
| Ascorbic acid (mg/100 g of fruits) | 42.11 α | 48.93 β |
| Firmness (N) | 1.3672 β | 0.9209 α |
| Fruit color—L (darkness/lightness) | 26.94 | 26.03 |
| Fruit color—a (green/red) | 21.09 | 21.40 |
| Fruit color—b (blue/yellow) | 12.94 | 13.32 |
| BTHWA | Type of Fungicide Treatment | Mean | |||
|---|---|---|---|---|---|
| 0F | 1F | SFP | |||
| Respiration rate (mgCO2/kg) | 20 × 4 | - | 41.7524 a* | 41.1259 a* | 41.4391 A* |
| 30 × 4 | - | 50.7497 b*° | 45.7430 ab* | 48.0963 B*° | |
| Mean | - | 46.2510 *° | 43.2844 * | ||
| Dry weight of fruits (%) | 20 × 4 | - | 7.6075 b*° | 7.2892 b* | 7.4484 B*° |
| 30 × 4 | - | 6.584 a*° | 7.1664 b* | 6.8752 A* | |
| Mean | - | 7.0958 * | 7.2278 * | ||
| Brix (◦) | 20 × 4 | - | 7.2078 b*° | 7.4089 c*° | 7.3083 B* |
| 30 × 4 | - | 7.2356 b*° | 6.8877 a* | 7.0616 A* | |
| Mean | - | 7.2217 * | 7.1483 * | ||
| Parameter | Variant of Treatment | |
|---|---|---|
| UTC | SFP | |
| Respiration rate (mgCO2/kg) | 61.3769 β | 36.7526 α |
| Dry weight of fruits (%) | 8.4603 β | 6.8968 α |
| Brix (◦) | 8.1311 β | 6.9078 α |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spychalski, M.; Kukawka, R.; Krzesiński, W.; Spiżewski, T.; Michalecka, M.; Poniatowska, A.; Puławska, J.; Mieszczakowska-Frąc, M.; Panasiewicz, K.; Kocira, A.; et al. Use of New BTH Derivative as Supplement or Substitute of Standard Fungicidal Program in Strawberry Cultivation. Agronomy 2021, 11, 1031. https://doi.org/10.3390/agronomy11061031
Spychalski M, Kukawka R, Krzesiński W, Spiżewski T, Michalecka M, Poniatowska A, Puławska J, Mieszczakowska-Frąc M, Panasiewicz K, Kocira A, et al. Use of New BTH Derivative as Supplement or Substitute of Standard Fungicidal Program in Strawberry Cultivation. Agronomy. 2021; 11(6):1031. https://doi.org/10.3390/agronomy11061031
Chicago/Turabian StyleSpychalski, Maciej, Rafal Kukawka, Włodzimierz Krzesiński, Tomasz Spiżewski, Monika Michalecka, Anna Poniatowska, Joanna Puławska, Monika Mieszczakowska-Frąc, Katarzyna Panasiewicz, Anna Kocira, and et al. 2021. "Use of New BTH Derivative as Supplement or Substitute of Standard Fungicidal Program in Strawberry Cultivation" Agronomy 11, no. 6: 1031. https://doi.org/10.3390/agronomy11061031






