1. Introduction
Legume seeds, along with cereals, are one of the most widely consumed foods worldwide [
1] and are a valuable source of plant protein especially in impoverished areas where meat, fish and dairy are economically unavailable [
2]. They are traditionally included in the diets of various cultures, appropriate in a variety of diets and widely studied for their effects on human health [
3,
4,
5,
6]. Soybean (
Glycine max (L.) Merrill) is one of the major oil and protein crops grown worldwide. In terms of acreage under cultivation, it is the world’s fourth crop after wheat, corn, and rice. In 2019, world soybean production was 333.6 million tons; Brazil was the largest producer with a production of 114.27 million tons and a cultivated area of 35.9 million hectares, followed by the USA with a production of 96.8 million tons and an area of 30.4 million hectares [
7].
Soybean seeds contain about 380–450 g kg
−1 protein with favorable amino acid composition, 180–230 g kg
−1 oil, and 200–260 g kg
−1 carbohydrates. In addition, they are a source of many valuable compounds such as fiber, lecithin, mineral salts (P, K, Ca, Mn, Zn, Fe, and B), vitamins (A, B, and D) and antioxidants [
8,
9,
10,
11,
12,
13,
14]. Soy protein contains essential amino acids important in human and animal nutrition [
15,
16,
17], in proportions similar to the reference protein (chicken eggs) [
18]. In addition, soybean seeds are a valuable raw material for the food (oil) and feed (post-extraction meal) industries. Soybean is second after palm (
Elaeis guineensis Jacq.) worldwide as a source for vegetable oil production and consumption [
19]. Approximately 29% of the global supply of consumer vegetable oil is produced from soybeans [
20]. The fatty acid (FA) content of the oil is a quality indicator used to classify, among other things, soybean varieties and soybean-based products and is a key factor in determining its final use [
21,
22]. Soybean oil contains saturated FA (palmitic acid (16:0) and stearic acid (18:0)), unsaturated FA (C18:1n9, linoleic acid (18:2) and 18:3 acids), and polyunsaturated FA (linolenic acid (18:3n6) and C18:3n3 acid [
12,
23]. Soybean oil does not contain cholesterol and 85% of its content is unsaturated FA, which are valuable in human nutrition [
16]. Soybean is low in saturated FA [
23,
24,
25]. Higher concentrations of polyunsaturated FA, such as 18:3 acid, are needed in human nutrition [
26].
A higher concentration of C18:1n9 acid in oil is a desirable characteristic because it affects the long shelf life and stability of oil for industrial purposes [
26]. This FA is not very susceptible to oxidative modifications during processing, storage, and frying. Therefore, the food industry is now interested in producing soybean seeds containing high concentrations of C18:1n9 acid [
27,
28,
29]. Soybean oil with higher levels of this acid is also desirable for biofuel production due to its higher oxidative stability and lubricating properties [
30].
The presence of FAs in the human diet is desirable. C18:1 acid is considered to be effective in lowering cholesterol levels, reduces the incidence of cardiovascular disease, and features anti-diabetic and anti-inflammatory properties [
31]. C18:2n6 acid is an essential omega-6 polyunsaturated FA (PUFA) that can reduce the incidence of diabetes mellitus and will have an effect on lowering blood pressure [
32]. C18:0 acid is a saturated FA and has no effect on blood cholesterol [
33], but when combined with C16:0 acid, it shows antimicrobial properties against
Staphylococcus aureus and
Helicobacter pylori, the latter additionally against
Streptococcus pyogenes [
34].
The formation of nutrients in soybean seeds (protein, oil, fatty acids, soluble sugars, and isoflavones) is strongly influenced by various factors such as genotype, location, climate, water, and earliness group [
12,
35,
36,
37]. Studies on soybean cultivars from different countries show significant differences in FA content and composition. The variable contents include C16:0, C18:3n6, C18:0, C18:1n9, and C18:2n6 acids, but the greatest variation in FA composition was found in C18:3 acid [
12,
23]. This is due to the strong influence of environmental and agro technical conditions on the metabolic pathways in plants [
29,
38,
39,
40].
Very significant intervarietal differences are found in FA composition in different earliness classes of cultivar. Higher contents of C16:0, C 18:0, and C18:1 acids were observed in earlier maturing cultivars, while higher levels of C18:2 and C18:3 acids were observed in late maturing ones [
36]. The greatest variation in FAs composition was found in C18:3 content, and C18:2 acid content varied the most among cultivars [
23]. C18:0 and C18:1 acids showed more variation than C16:0, C18:2, and C18:3 acids [
36]. In the study [
41] all FAs showed differences between cultivars.
In addition to nutritional considerations, the cultivation of legumes, including soybean, provides additional economic and ecological benefits. Further noteworthy is the high phenotypic plasticity of soybean plants depending on thermal and rainfall factors [
42]. The presence of legumes in crop rotations is especially desirable in organic and sustainable farming system due to their ability to fix atmospheric nitrogen in symbiosis with papillary bacteria [
43], which reduces the demand for mineral nitrogen and contributes to improving soil fertility [
44,
45]. This is also supported by other studies showing that
Rhizobium leguminosarum can completely replace chemical fertilizer in common bean [
46], and inoculation of pigeon pea
Cajanus cajan (L.) Huth with
Bradyrhizobium bacteria resulted in the same or higher yield than did the use of mineral nitrogen fertilizer [
47].
Soybean is capable of fixing atmospheric nitrogen in symbiosis with
Bradyrhizobium japonicum bacteria. Since these bacteria are not found in European soils, soybean seeds must be inoculated with
Bradyrhizobium strains before sowing in order to fix nitrogen and realize their yield potential [
48]. Inoculation with papillary bacteria of legumes is a reliable agronomic practice to increase production levels, protect the environment and provide quality food for humans and animals. Inoculation can also cause changes in the chemical composition of seeds of different legume species. It has been shown to increase the antioxidant potential and content of some bioactive compounds such as phenols, flavonoids, organic acids, proteins, and FAs. Therefore, studies on the effect of inoculation with papillary bacteria on the content of bioactive compounds in soybean plants are gaining considerable interest [
10,
49]. Soybean participates in symbiosis with several species of nitrogen-fixing bacteria, mainly belonging to the genus
Bradyrhizobium, including
B. japonicum, which has beneficial effects on plant growth, seed yield, and nitrogen content of this legume [
50,
51,
52]. As a result, soybean has low mineral fertilizer requirements and this further increases the yield of subsequent crops such as cereals [
53].
Inoculation alleviates drought stress and increases yield and fat content in soybean [
54], with plant drought tolerance being associated with nutrient accumulation [
55,
56] and potential improvement in water uptake by plants in symbiosis with papillary bacteria [
55]. Inoculation with
B. japonicum induces metabolic changes in the soybean plant, the most studied of which so far being an increase in protein content [
57]. It also causes an increase in the FA content in the seed [
10]. In field studies, soybean has been shown to be able to fix large amounts of nitrogen, ranging from 0 to 337 kg ha
−1 N [
48,
58], and biological nitrogen fixation by legumes decreases as the proportion of native soil nitrogen supply increases and vice versa [
59]. However, some level of application of nitrogen may be needed during early plant development to overcome nitrogen deficiency at a time when the source of N contained in cotyledons is depleted and plants have not yet formed papillae capable of supplying the plant with symbiotically bound N
2 [
60]. Soybean requires an average of 80 kg N in above-ground dry matter per ton of seed produced [
59,
61]. In practice, nitrogen fertilizer is applied to soybean crops in small amounts as a “starter” at sowing. However, research indicates that in the absence of nitrogen fertilizer, biological N
2 fixation is not sufficient to meet the N demand of the growing crop from early in the season up to the beginning of seed filling, and yield increases in high-yielding soybean production systems require increased biological N
2 fixation, a greater supply of N from soil or fertilizer, or a relaxed trade-off between these two nitrogen sources to meet plant demand [
62,
63].
The effects application of nitrogen fertilizer on soybean seed yield, protein, and oil content have been extensively documented [
64,
65,
66], but there are few studies on oil composition and its response to nitrogen fertilizer that extensively discuss its effects on FA profile [
64,
65,
66]. Some studies have shown that the level of nitrogen fertilizer applied had no effect on the FA composition of soybean seeds [
53], and the content of palmitic (C16:1), oleic (C18:1n9), and linoleic (C18:2n6) acids in seeds did not depend on either years or nitrogen fertilization [
41,
67]. Moreover, varying fertilizer application rates did not modify the fatty acid composition of soybean [
53].
Therefore, an important issue is whether and to what extent inoculation of soybean seeds with symbiotic bacteria combined with varying doses of nitrogen fertilizer can change the FA composition of soybean seeds after harvest and what is the impact of the choice of cultivar.
3. Results and Discussion
Soybean seeds are an important source of FAs [
72,
73,
74]. In addition to environmental conditions, the FA profile in soybean seeds is strongly related to their genetics [
75,
76,
77]. In soybean the following acids can beidentified: lauric acid (C12:0), tridecylic acid (C13:0), myristic acid (C14:0), myristoleic acid (C14:1), pentadecanoic acid (C15:0), cis-10-pentadecenoic (C15:1), C16:0, palmitoleic acid (C16:1), margaric acid (C17:0), heptadecenoic acid (C17:1), C 18:0, C18:1n9, C18:2n6, C18:3n3, C18:3n6, arachidic acid (C20:0), eicosenoic acid (C20:1) eicosedienoic acid (C20:2), dihomo-g-linolenic (C20:3n6), eicosatrienoic acid (C20:3n3), arachidonic acid (C20:4n6), eicosapentaenoic acid (C20:5n3), heneicosanoic acid (C21:0), heneicosylic acid (C21:1), erucic acid (C22:1n9), eicosadienoic acid (C22:2), docosaheptaenoic acid (C22:6n3), tricosanoic acid (C23:0), lignoceric acid (C24:0), nervonic acid (C24:1) [
10,
78]. C16:0, C18:0, C18:1, C18:2n6, and C18:3n3 acids are most commonly considered in studies [
41,
57]. C18:0 and C18:3 acid levels are used for strain selection in crossbreeding selection and lineage evaluation [
79].
In the experiment conducted, a total of 34 fatty acids were identified in soybean seeds, including butyric acid (C4:0), caproic acid (C6:0), caprylic acid (C8:0), capric acid (C10:0), and undecylic acid (C11:0) not reported by the above authors (
Table 3). The manuscript discusses the FAs with higher content and greater importance in seeds.
The experiment showed a significant effect of cultivar on the fatty acid profile of soybean seeds. Out of the FAs identified, the seeds of soybean cultivars accumulated the highest value of C18:2n6 and C18:1n9 acids, which together accounted for 71.8% of the total FAs (
Table 4). Statistical analysis showed a significant effect of cultivar, nitrogen fertilizer, bacterial inoculation of seeds, as well as weather conditions on the profile of FAs in soybean seeds. The seeds of the Annushka cultivar had a significantly higher content of C18:0, C18:2n6, C18:3n3, C16:0, and C20:0 acids and smaller amounts of C18:1n9 and C16:1 acids compared to the Aldana cultivar. Moreover, other authors [
36,
72,
73,
74,
75,
80], indicate that soybean varieties vary in their C18:0, C18:1, and C18:2 acid content.
The ranges of individual fatty acid contents determined in soybean are significant and can range as follows: C16:0 (13.7–68.1g 100 g seeds
−1), C16:1 (0.67–15.2 g 100 g seeds
−1), C 18:0 (3.05–67.1 g 100 g seeds
−1), C18:1 (9.66–63.0 g 100 g seeds
−1), C18:2 (32.5–69.85 g 100 g seeds
−1), and C18:3 (0.90–12.9 g 100 g seeds
−1) [
10,
41,
79,
81].
Abdelghany et al. [
40], evaluating 1025 soybean cultivars of different origins, stressed significant differences in C16:0, C 18:0, C18:1, C18:2, and C18:3 acid contents. The average contents of these acids were 12.2; 3.8; 21.5; 54.2, and 8.3 g 100 g seeds
−1, respectively. On average, higher levels of C16:0 and C18:3 acids were observed in Russian cultivars (12.31 and 8.15 g 100 g seeds
−1, respectively). Higher levels of C 18:0 and C18:1 acids were found in Chinese cultivars (3.95 and 21.95 g 100 g seeds
−1, respectively), while the highest level of C18:2 acid was recorded in cultivars from the USA. In some Polish cultivars, the content of C16:0 acid can amount 10.85–14.1 g 100 g seeds
−1, C 18:0 acid 4.15–5.12 g 100 g seeds
−1, C18:1 acid 21.0–27.18 g 100 g seeds
−1, C18:2 45.3–53.24 g 100 g seeds
−1, and C18:3 acid can be 7.21–9.86 g 100 g seeds
−1 [
78,
82,
83]. This is consistent with the results of the experiment conducted, with lower contents of C 18:0 (3.14–3.28 g 100 g seeds
−1) and C18:1 (20.0–18.1 g 100 g seeds
−1).
Lack of nitrogen fertilizer promoted the accumulation of C18:0, C20:0, and C20:1 acids in seeds, while seeds accumulated less C16:0 and C16:1 acids. Nitrogen fertilizer at the rates of 30 and 60 kg ha−1 N significantly reduced seed acid C20:0 by 12.2 and 12.5%, C20:1 by 14.1%, and C18:0 by 2.5%, respectively. However, application of nitrogen at a rate of 60 kg ha−1 N increased C16:0 count by 5.2% and that of C16:1 acids by 11.3% with respect to the control. However, nitrogen fertilizer had no significant effect on seed accumulation of C18:1n9, C18:2n6, C18:3n6, C18:3n3, and C14:0 acids.
According to [
67], C18:3 unsaturated FA did not show significant changes in response to nitrogen fertilizer and the study of [
41] shows that C16:0, C18:1, and C18:2 acids content in seeds did not depend on nitrogen fertilizer. In a study by Rahim et al. [
66] application rate of 100 kg ha
−1 N significantly increased the C18:2 and C18:1 acid content, while lower rates of 25 and 50 kg ha
−1 N showed no significant differences. Similar observations are reported by [
82], where application of nitrogen fertilizer at rates of 0, 30, 60 kg ha
−1 N did not affect FA composition of soybean. In the study of [
81], application of 75 kg urea per 1 ha increased linoleic acid content by 6.22%, 3.86%, and 0.8% compared to the application of 0 and 25 and 50 kg urea per 1 ha, respectively. There was no significant difference between the application of urea at 50 and 75 kg ha
−1.
In another study [
41] C18:0 acid was the only major FA showing a slight decrease in content from 3.84 g FA 100 g oil
−1 in the cultivar fertilized with 670 kg ha
−1 N rate to 3.63 g FA 100 g oil
−1 in the unfertilized cultivar. Moreover, C18:1n9 and C18:2n6 acid contents varied from 5% to 11% and the ratio of monounsaturated to polyunsaturated FA was 18%, but this was not due to application of nitrogen fertilizer. Only the content of C18:0 acid was significantly modified by application at the rate of 670 kg ha
−1 N.
Silva et al. [
10] reported that inoculation with
B. japonicum increases the FA content of soybean seeds. Taking into account that soybean provides various bioactive compounds, including FAs, which form functional foods included in nutraceutical products [
1,
43,
50,
84,
85,
86,
87,
88,
89,
90] seed grafting is desirable. In the experiment, there was no significant effect of seed inoculation on the content of FAs analyzed, except for 16:0 acid. The seeds inoculated with Nitragina reduced C16:0 acid content by about 3.68% and HiStick
®Soy by 6.6% compared to the variant without inoculation. The study of Rahim et al. [
67] only confirms the decrease in C16:0 acid content under inoculation, while it reports different observations related to decrease in C 18:0 and also an increase in C18:2 and C18:1 acids under inoculation. In addition, the unsaturated FA C18:3 did not show significant changes in response to inoculation. Sharifi et al. [
81] showed that the content of saturated FSs C16:0 and C 18:0 decreased in seeds after
Bradyrhizobium inoculation compared to the variant without inoculation, while unsaturated FAs C18:1, C18:2, and C18:3 increased. Similar results were obtained for particular acids by Rahim at al. [
67] except for C18:3 acid, whose content did not change significantly under inoculation.
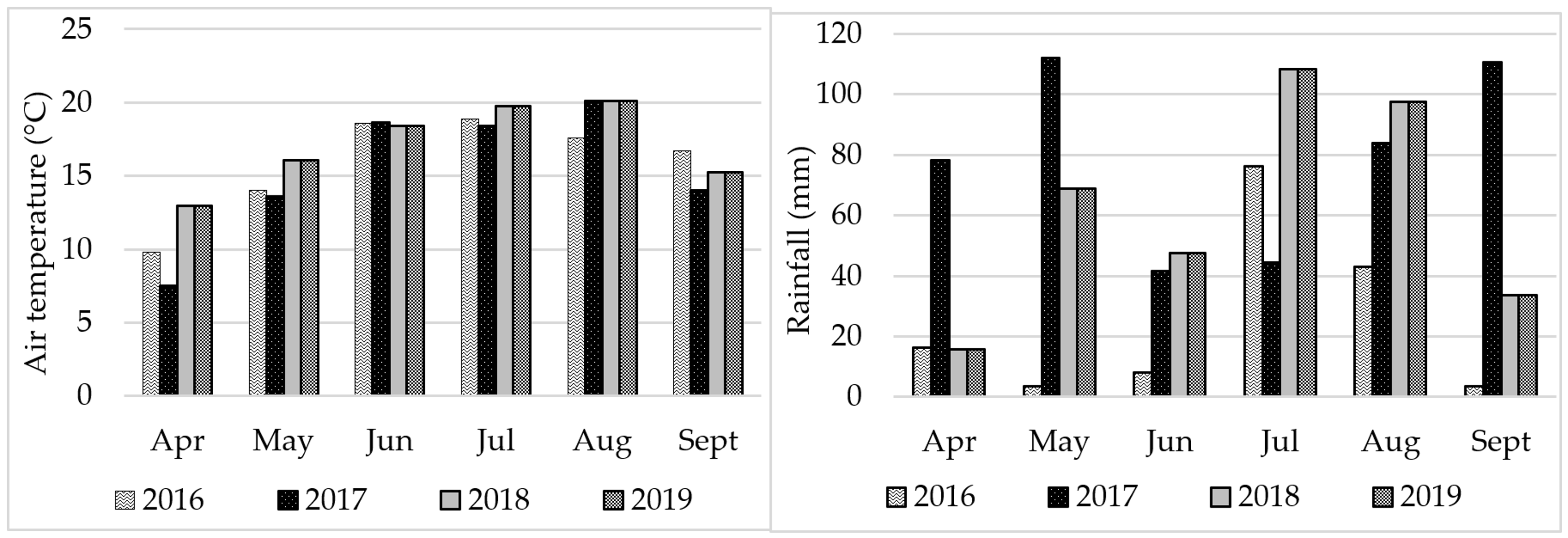
The content of FAs in soybean seeds is modified by the course of weather and environmental conditions [
91,
92], which was confirmed in the experiment that was carried out. Weather patterns strongly modified the fatty acid profile of soybean seeds. Seeds in 2017 contained the most C18:0, C18:3n6, C14:0, C16:0, C16:1, C20:0, and 20:1 acids. Seeds harvested in 2016 had high C18:2n6 acid content, and in 2018. C18:1n9. The C16:1 acid content of the seeds remained similar except for 2016, when it was the lowest by a significant margin. It was observed that a wet and cool year promoted the accumulation of not only C16:0 and C18:0, but also C18:3n6, C14:0, C16:1, sC20:0 and 20:1 acid in soybean seeds. Different results were obtained by [
41], which showed that the C16:0, C18:1 and C18:2 oil content in seeds did not depend on years. Moreover, Abdelghany at al. [
40], evaluating 1025 soybean cultivars collected from different ecoregions and grown in different locations and in different years, showed significant differences in FA content, but different from the experiment presented, they found no differences in saturated C16:0 acid content. In another experiment [
67], not only did C16:0 acid not change, but no significant differences were found in C18:0 acid content either.
Statistical analysis indicates a significant interaction of cultivar and years of experiment in shaping the FA profile of soybean seeds (
Table 5). Significant interaction of these experimental factors was found for six acids: C18:0, C18:1n9, C18:2n6, C18:3n3, C14:0, and C16:0. Seeds of the Annushka cultivar had the highest C18:0 acid content in 2017 and 2019, significantly higher than 2018 by 16.5 and 15%, respectively. Seeds of this cultivar also contained significantly the highest C18:2n6 acid in 2016 and C16:0 in 2017. In contrast, seeds of the cultivar Aldana were distinguished by significantly the highest content of C18:1n9 acid in 2018 and C14:0 acid in 2017.
The cold year 2017 was favorable for the increase in the content of C14:0 saturated acids in the Aldana cultivar, and C16:0 and C18:0 in the Annushka cultivar, while in the warm years higher levels were recorded for the C18:3n3 and C18:2n6 acids in Annushka seeds and C18:1n9 in Aldana seeds. Despite the significance of the interaction cultivar x fertilizer used, no logical and unambiguous relationships were found. There is only a noticeable tendency for cultivars to accumulate saturated acid in seeds in cold years, and unsaturated acids in years with warmer weather conditions.
The experiment also showed a significant effect of interaction between cultivar and inoculation of seeds with
B. japonicum on the FA profile (
Table 6). Such a relationship was found for three FAs: C18:3n6, C14:0, and C20:0. Seed inoculation with HiStick
®Soy resulted in a significant 32.7% increase in C18:3n6 acid content in the Annushka cultivar compared to the bacterial preparation Nitragina. In total 18.3% more C20:0 acid accumulated in the seeds of the Annushka cultivar after inoculation with HiStick
®Soy compared to the seeds of the Aldana cultivar inoculated with Nitragina. It was also reported that the C14:0 acid content in the seeds of the cultivar Aldana after inoculated with Nitragina was significantly higher by 37.6% compared to the variant without inoculation, and also higher by 43.8% compared to the seeds of the cultivar Annushka inoculated with Nitragina.
The research conducted indicates variation in the effect of the interaction of the cultivars and the inoculation preparation used on the content of some fatty acids in soybeans. The Aldana cultivar obtained more favorable results in cooperation with Nitragina, while the Annushka cultivar with the HiStick® Soy preparation. This suggests that more research is needed with different inoculations and different cultivars.
However, statistical analysis of the results of the four-year study, shows no significant effect of the interaction of cultivar and nitrogen fertilizer (
Table S1), nitrogen fertilization and seed inoculation with
B. japonicum (
Table S2), or inoculation and years of study (
Table S3) on the formation of the profile of FAs analyzed.
In the present study, SFA averaged 18.0 g, MUFA 19.5 g, UFA 81.7 g, and PUFA 62.2 g FA 100 g seeds
−1 in soybean seeds (
Table 7). For the years of study, the Aldana cultivar seeds accumulated on average significantly more MUFA (by 9.8%), while the Annushka cultivar seeds contained significantly higher amounts of SFA and PUFA (by 3.3 and 2.1%, respectively). On the other hand, no significant differentiation of cultivars was found in terms of UFA content in seed. Application of the highest dose of nitrogen fertilizer of 60 kg N ha
−1 caused a significant 3.3% increase in the content of SFA in seeds compared to the control, while an opposite relation was obtained for UFA and PUFA. The content of UFA and PUFA in the non-fertilized variant was significantly higher than in the highest nitrogen dose by 5.5 and 9.5%, respectively. However, the rate of nitrogen fertilizer application did not determine the content of MUFA and UFA.
In the current study, inoculation had no effect on UFA, MUFA, PUFA, and the formation of SFA/UFA ratios of soybean seeds. It only had an effect on SFA. The least favorable SFA content occurred in non-treated seeds, and a favorable decrease by 1.09% in its content was observed with Nitragina treatment and 3.3% after HiStick
®Soy treatment. Different results were obtained by Luís et al. [
10], who reported that inoculation with
B. japonicum increases the content of unsaturated fatty acids in soybean seeds. Similar results were reported by Silva et al. [
10] showing that inoculation of
B. japonicum sv
glycinearum, increased the content of total FAs and this was due to an increase in MUFA and PUFA. SFA accounted for 73% and 65% of the total FA content in the inoculated and control samples, respectively. Among them, C18:0 acid was the major compound in both samples, accounting for 55% and 34% of the total SFA content in inoculated and control seeds, respectively [
10].
Soybean seeds had the highest SFA content in 2017, MUFA and UFA in 2018 and PUFA in 2016.
The cultivar, nitrogen fertilizer application and inoculation had no effect on the formation of SFA/UFA and C18:2n6/C18:3n3 ratios. The experiment showed that the cultivar did not determine these ratios, but the proportion of MUFA and the proportion of PUFA were significant in cultivars. Similarly, this was also seen in another experiment where the percentage of MUFA differed between cultivars by more than 27% and the percentage of PUFA ranged from 59.61–60.12% and differed cultivars by 3.5% [
82].
C18:2 and C18:3 acids are essential fatty acids in the human diet, and the ratio between them determines the nutritional value [
28,
29]. In our study, there was no effect of inoculation on this relationship and the average ratio was 5:1, which was more favorable than that calculated in the study by Pisulewska et al. [
78], which was 7:1.
The course of weather conditions during the study years had a significant impact on these matters. In 2017, the value of SFA/UFA ratio in soybean seeds was significantly higher from 8,0% to 20% compared to the other study years, while the value of the C18:2n6/C18:3n3 ratio was then significantly lower than in 2017 and 2018 (by 13.9 and 14.2%, respectively). A relatively humid and cold 2017 year contributed to an increase in the SFA/UFA ratio, while the value of the C18:2n6/C18:3n3 acid ratio was significantly lower in warm years with optimal humidity or relatively humid years. In the experiment of Tamagno et al. [
41], the ratio of C18:1n9 acid to PUFA did not vary with year.
In the experiment, significant interaction between cultivar and years of testing on MUFA and PUFA, as well as the ratio of C18:2n6/C18:3n3 acids content in soybean seeds was noted (
Table 8). Seeds of the cultivar Aldana contained significantly more MUFA (by 33.9%) and less PUFA (by 9,1%) in 2018 compared to seeds of the cultivar Annushka collected in 2016 and also showed a significantly lower value of C18:2n6/C18:3n3 ratio compared to seeds of both cultivars obtained in 2019. However, soybean seeds of both cultivars in 2017 and 2018 contained—the significantly lowest amount of PUFAs.
However, based on the experiment, there was no significant interaction between cultivar and nitrogen fertilizer application (
Table S4), cultivar and
B. japonicum inoculation (
Table S5), inoculation and years of testing (
Table S6) on SFA content, MUFA, UFA, and PUFA, as well as the formation of SFA/UFA and C18:2n6/C18:3n3 acids ratios.
The multiple regression method with backward selection was used to evaluate the effect of selected fatty acids on the measured FA content. The possibility of eliminating irrelevant variables in stepwise multiple regression calculations allows one to narrow down the number of independent variables. Therefore, the results of the calculations allow us to determine which fatty acids interacted with the levels of C16:0, C18:0, C18:1n9, C18:2n6, and C18:3n3 acids and to what extent (
Table 9). The models showed good correlation with the explanatory variables. In the equations presented, the values indicate the significance of the estimated regression parameters. The equations obtained have high coefficients of determination R2. This allows us to conclude that the amount of C20:2 and C21:0 acid accumulated in soybeans has the greatest effect on C14:0 acid content. The amount of accumulated C16:0 acid is most affected by C15:1 and C24:0. C18:1n9 acid has the least effect on C16:1 content. A 1% increase in C20:1 acid will cause a 3.20 unit decrease in C18:0 acid, while a one unit increase in C15:1 acid will cause a 2.718 unit decrease in C18:3n3/C18:1n9 acids which is described by 99% of the independent variables.