Overexpression of GhMPK3 from Cotton Enhances Cold, Drought, and Salt Stress in Arabidopsis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Gene Cloning, Sequence Analysis, Phylogenetic Analysis, and Cis-Regulatory Element Analysis
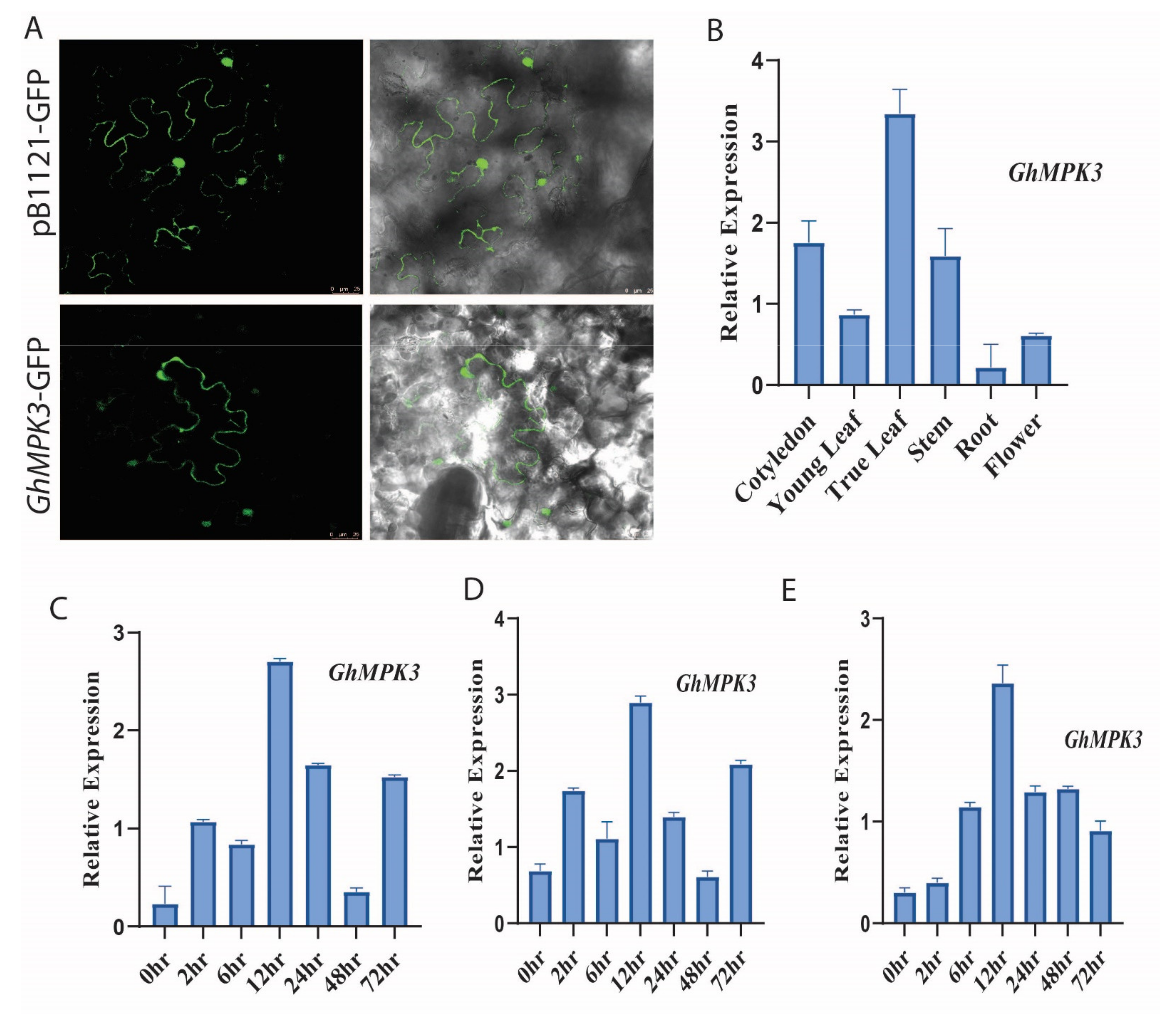
2.3. Subcellular Localization
2.4. Expression Analysis
2.5. Overexpressed of GhMPK3 in Arabidopsis Plants
2.5.1. Construction of Vector and Genetic Transformation of Overexpressed Plants
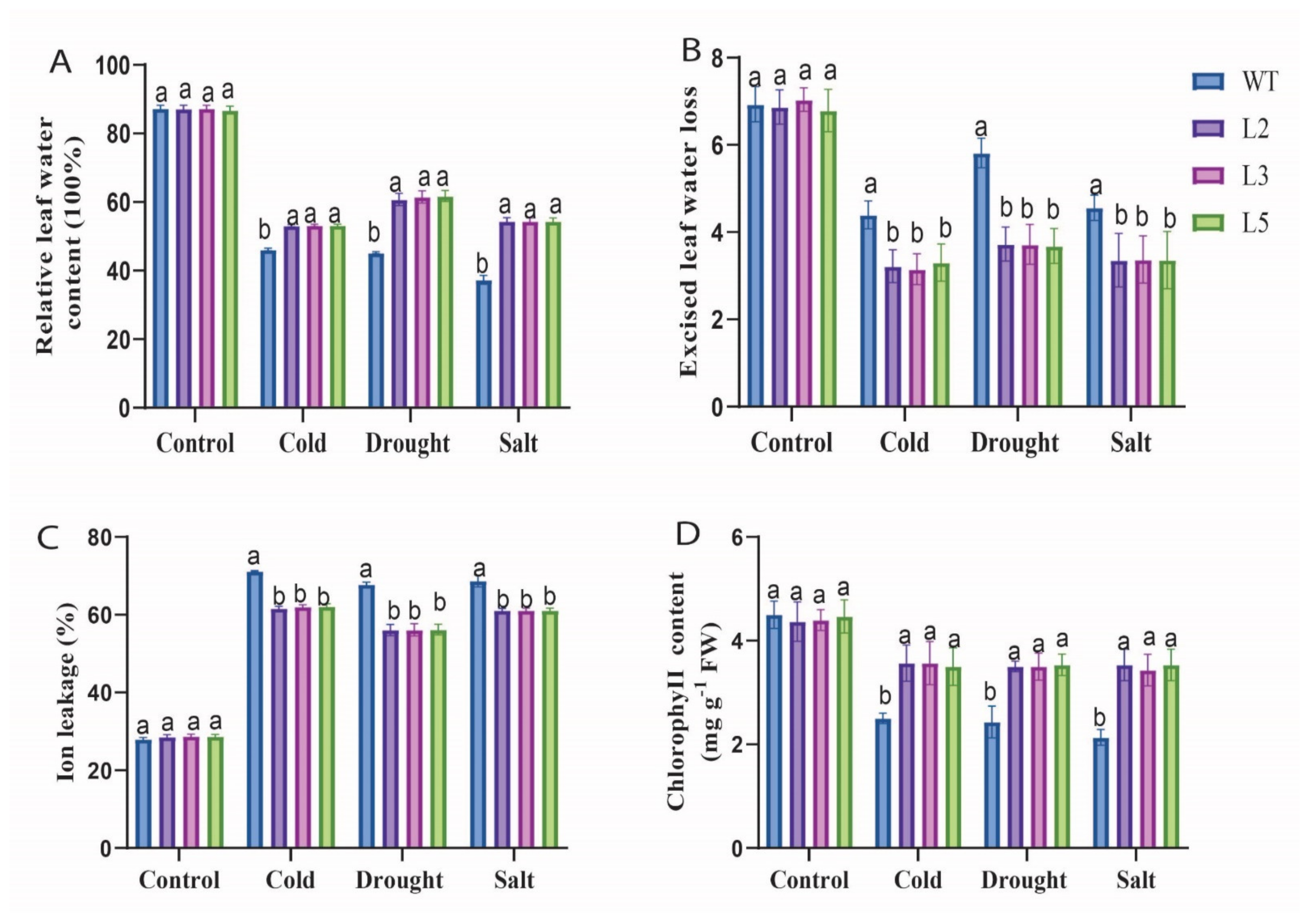
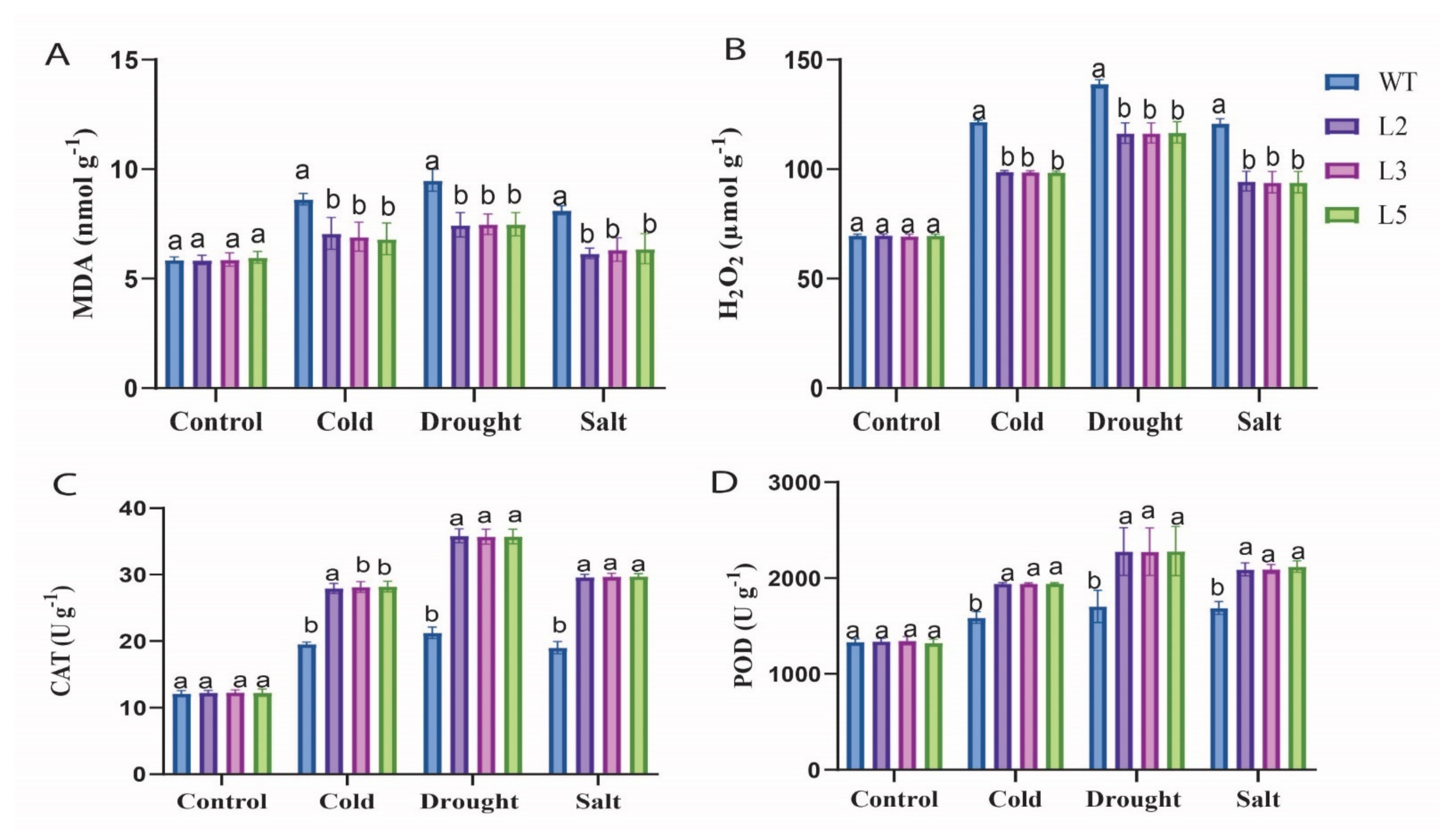
2.5.2. Stress Treatment, Physiological Parameters Measurement, Oxidant and Antioxidant Determination in Overexpressed and Wild-Type Plants
2.6. Expression Level of Stress-Responsive Genes in Transgenic and Wild-Type Plants under Cold, Drought, and Salt Stress
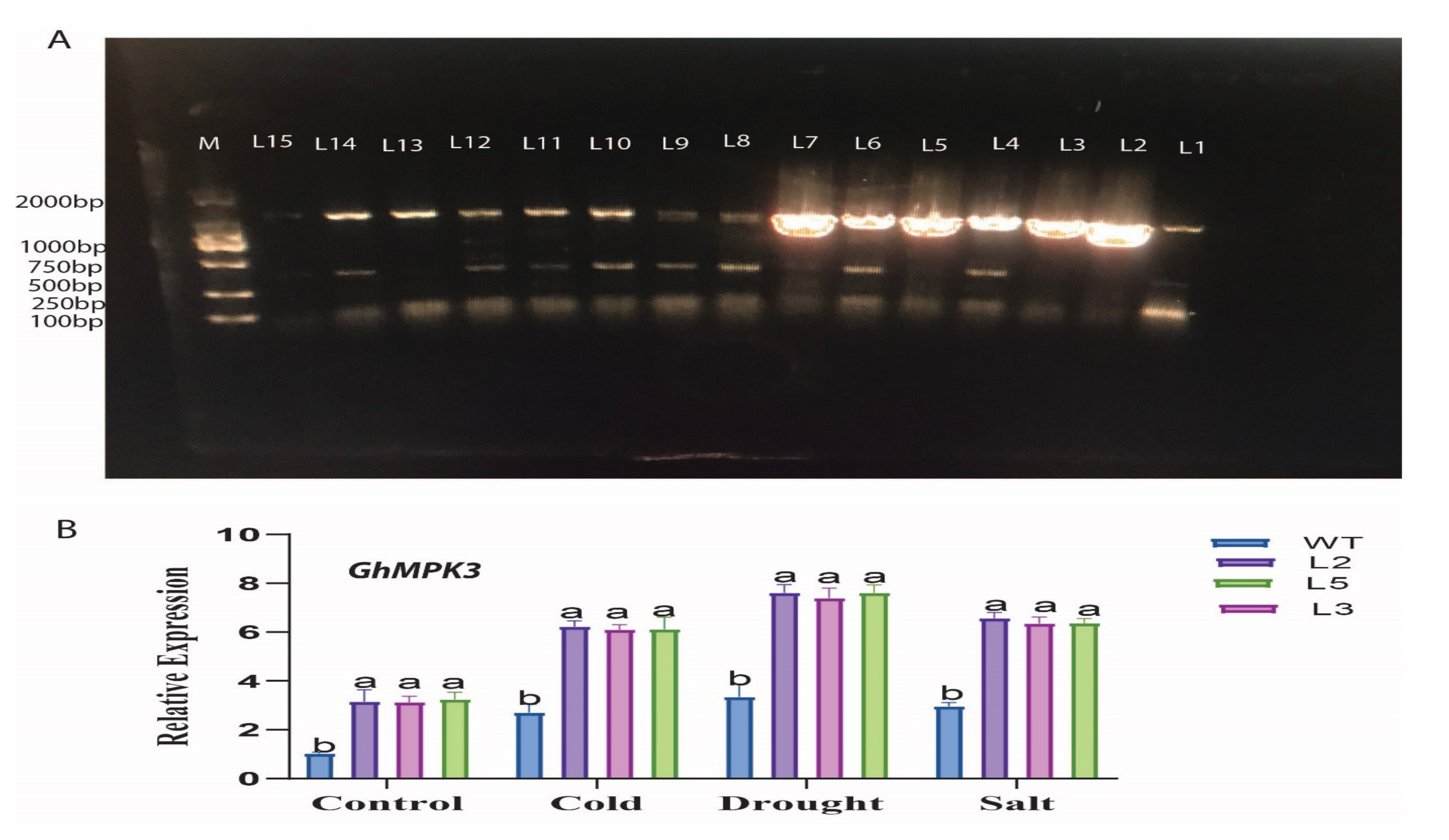
2.7. Virus-Induced Gene Silencing Analysis of GhMPK3
2.8. Transactivation Assay of GhMPK3
2.9. Statistical Analysis
3. Results
3.1. Cloning and Characterization of GhMPK3
3.2. Profiling Expression of Cotton GhMPK3
3.3. Overexpression of Gh_D05G3876 Enhances Cold, Drought, and Salt Stress Tolerance
3.4. Physiological Characteristics and Enzymatic Activity of Evaluated under Cold, Drought, and Salt Stress Conditions
3.5. Expression of Stress-Responsive Genes in WT and GhMPK3-Overexpressed Arabidopsis under Cold, Drought, and Salt Treatments
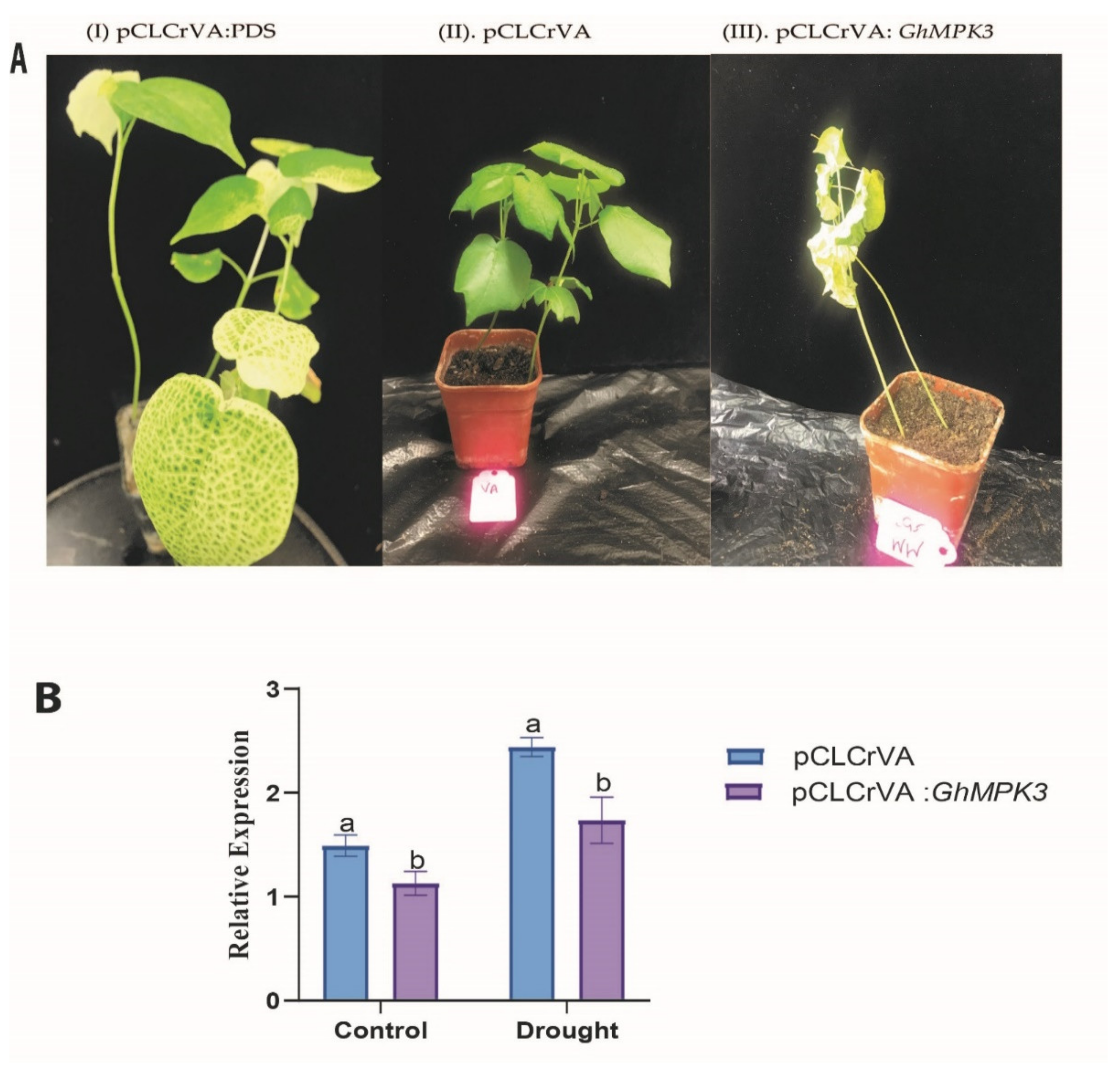
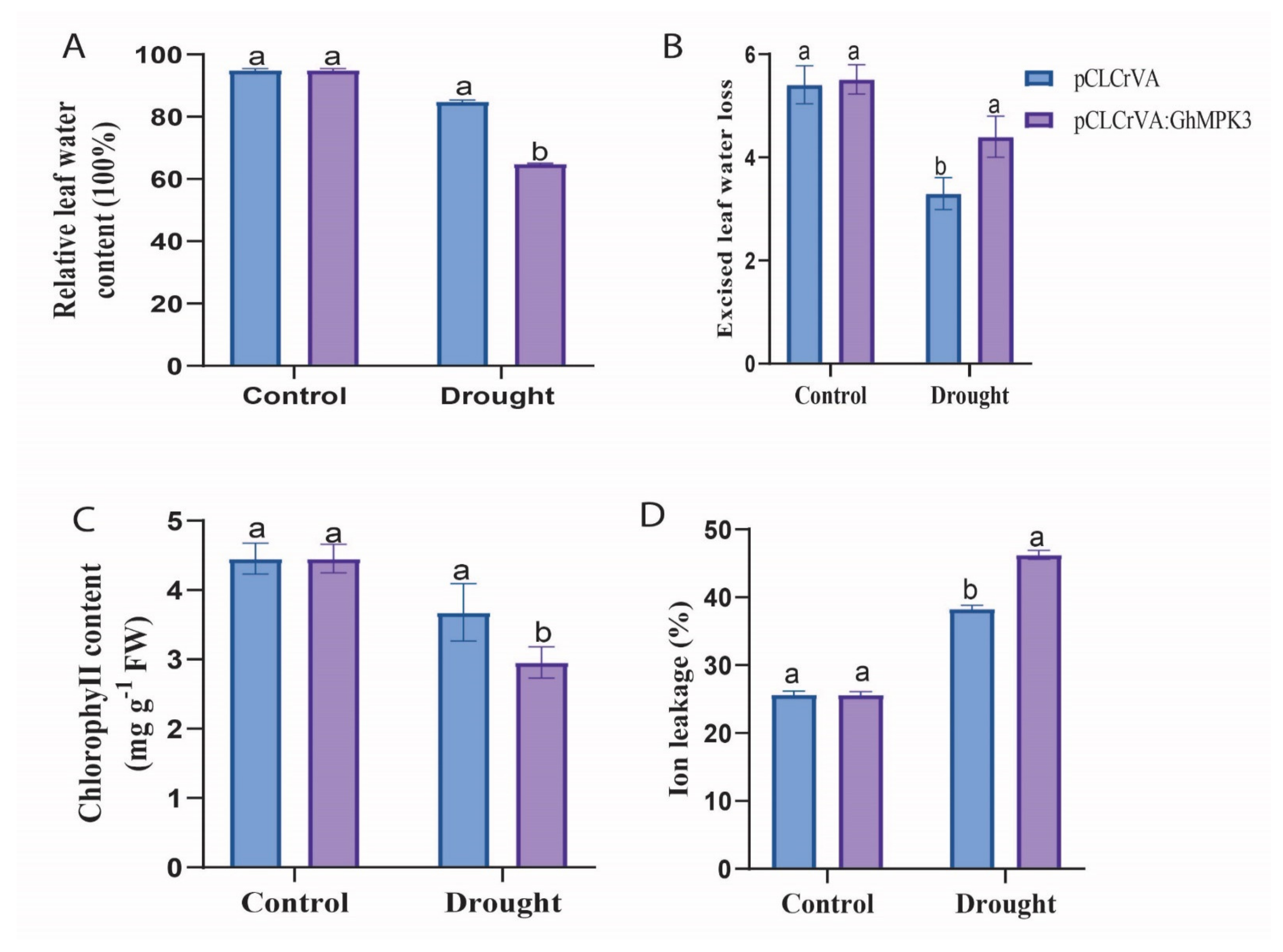
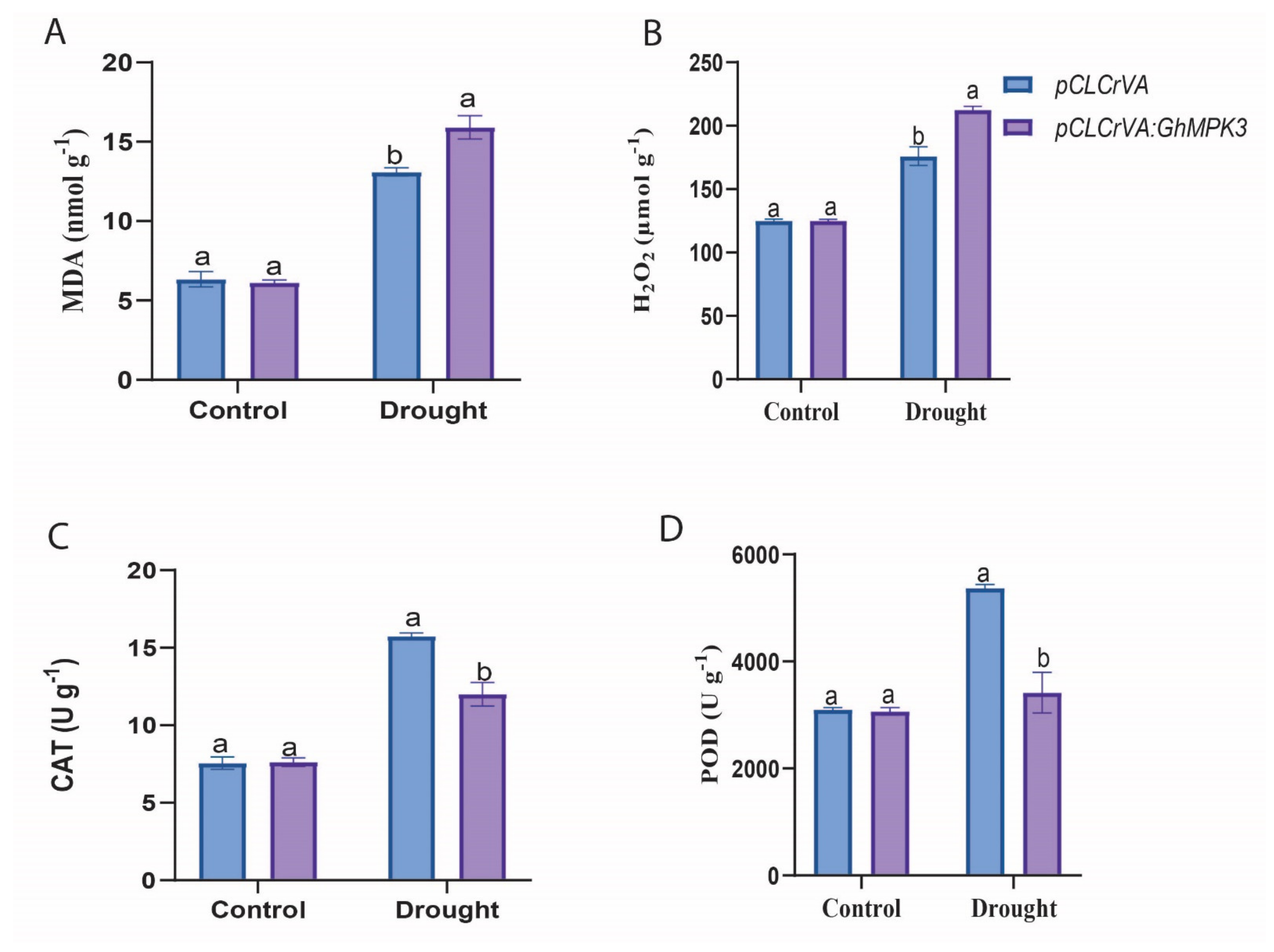
3.6. GhMPK3 Silenced Cotton Displays Sensitivity to Drought Tolerance
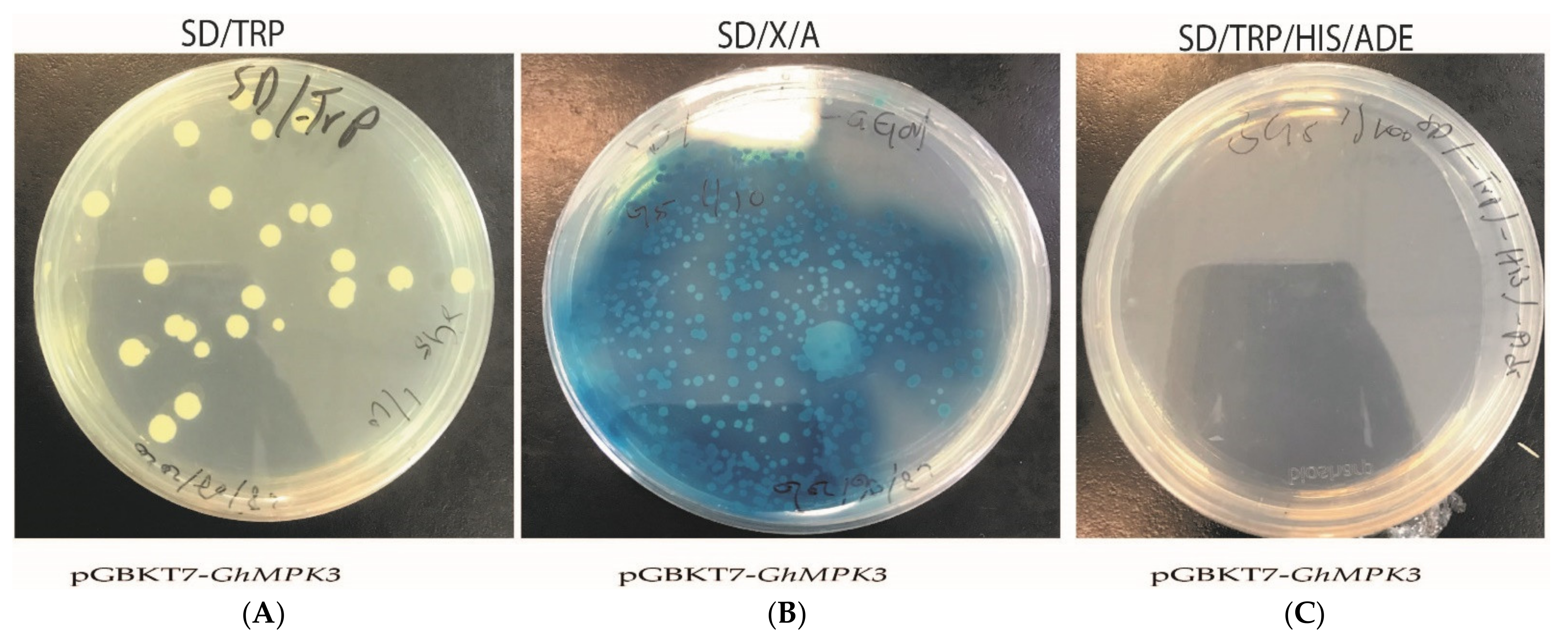
3.7. Transcriptional Activation Assay of GhMPK3
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, K.; Chen, J.; Wang, Q.; Yang, Y. Direct Phosphorylation and Activation of a Mitogen-Activated Protein Kinase by a Calcium-Dependent Protein Kinase in Rice. Plant Cell 2014, 26, 3077–3089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuteja, N. Abscisic Acid and Abiotic Stress Signaling. Plant Signal. Behav. 2007, 2, 135–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Yang, Y.; Chen, S.; Ning, N.; Hu, H. Arabidopsis IAR4 Modulates Primary Root Growth under Salt Stress through ROS-Mediated Modulation of Auxin Distribution. Front. Plant Sci. 2019, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J. Update on Stress Signaling Genetic Analysis of Plant Salt Tolerance Using Arabidopsis. Plant Physiol. 2020, 124, 941–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, P.; Magwanga, R.O.; Kirungu, J.N.; Hu, Y.; Dong, Q.; Cai, X.; Zhou, Z.; Wang, X.; Zhang, Z.; Hou, Y.; et al. Overexpression of Cotton a DTX/MATE Gene Enhances Drought, Salt, and Cold Stress Tolerance in Transgenic Arabidopsis. Front. Plant Sci. 2019, 10, 299. [Google Scholar] [CrossRef] [Green Version]
- Yamazakia, T.; Kawamura, Y.; Uemura, M.; Yamazaki, T. Extracellular freezing-induced mechanical stress and surface area regulation on the plasma membrane in cold-acclimated plant cells. Plant Signal. Behav. 2009, 4, 231–233. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Wang, C.; Yan, Y.; Jia, H.; Guo, X. Overexpression of Cotton GhMPK11 Decreases Disease Resistance through the Gibberellin Signaling Pathway in Transgenic Nicotiana benthamiana. Front. Plant Sci. 2016, 7, 689. [Google Scholar] [CrossRef] [Green Version]
- Cristina, M.; Petersen, M.; Mundy, J. Mitogen-Activated Protein Kinase Signaling in Plants. Annu. Rev. Plant Biol. 2010, 61, 621–649. [Google Scholar] [CrossRef]
- Nakagami, H.; Pitzschke, A.; Hirt, H. Emerging MAP kinase pathways in plant stress signalling. Trends Plant Sci. 2005, 10, 339–346. [Google Scholar] [CrossRef]
- Pitzschke, A.; Schikora, A.; Hirt, H. MAPK cascade signalling networks in plant defence. Curr. Opin. Plant Biol. 2009, 12, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Klessig, D.F. MAPK cascades in plant defense signaling. Trends Plant Sci. 2001, 6, 520–527. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Yue, H.; Zhao, X.; Wang, M.; Song, W.; Nie, X. Genome-Wide Identification and Analysis of MAPK and MAPKK Gene Families in Bread Wheat (Triticum aestivum L.). Genes 2017, 8, 284. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Xu, X.; Cai, C.; Guo, W. Genome-wide identification of mitogen-activated protein kinase gene family in Gossypium raimondii and the function of their corresponding orthologs in tetraploid cultivated cotton. BMC Plant Biol. 2014, 14, 345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, T.; Wang, G.; Yan, Y.; Xia, Q. Cloning and evolutionary analysis of tobacco MAPK gene family. Mol. Biol. Rep. 2012, 40, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xi, D.; Li, S.; Gao, Z.; Zhao, S.; Shi, J.; Wu, C.; Guo, X. A cotton group C MAP kinase gene, GhMPK2, positively regulates salt and drought tolerance in tobacco. Plant Mol. Biol. 2011, 77, 17–31. [Google Scholar] [CrossRef]
- Grover, C.E.; Kim, H.; Wing, R.A.; Paterson, A.H.; Wendel, J.F. Incongruent Patterns of Local and Global Genome Size Evolution in Cotton. Genome Res. 2004, 14, 1474–1482. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Lu, W.; He, X.; Wang, F.; Zhou, Y.; Guo, X.; Guo, X. The CottonMitogen-Activated Protein Kinase Kinase 3Functions in Drought Tolerance by Regulating Stomatal Responses and Root Growth. Plant Cell Physiol. 2016, 57, 1629–1642. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Hao, L.; Guo, X.; Liu, S.; Yan, Y.; Guo, X. A Raf-like MAPKKK gene, GhRaf19, negatively regulates tolerance to drought and salt and positively regulates resistance to cold stress by modulating reactive oxygen species in cotton. Plant Sci. 2016, 252, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Kohel, R.J.; Richmond, T.R.; Lewis, C.F. Texas Marker-1. Description of a Genetic Standard for Gossypium hirsutum L. 1. Crop. Sci. 1970, 10, 670–671. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, N.; Ma, L.; Lu, J.; Wang, H.; Wang, C.; Yu, S.; Wei, H. The Cotton BEL1-Like Transcription Factor GhBLH7-D06 Negatively Regulates the Defense Response against Verticillium dahliae. Int. J. Mol. Sci. 2020, 21, 7126. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xue, Q. Computational identification and phylogenetic analysis of the MAPK gene family in Oryza sativa. Plant Physiol. Biochem. 2007, 45, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van De Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Elasad, M.; Wei, H.; Wang, H.; Su, J.; Ondati, E.; Yu, S. Genome- Wide Analysis and Characterization of the TRX Gene Family in Upland Cotton. Trop. Plant Biol. 2018, 11, 119–130. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Ghtrx, C.; Gene, C.; Elasad, M.; Ahmad, A.; Wang, H.; Ma, L.; Yu, S.; Wei, H. Tolerance in Arabidopsis. Plants 2020, 9, 1388. [Google Scholar]
- Wang, N.-N.; Zhao, L.; Lu, R.; Li, Y.; Li, X.-B. Cotton mitogen-activated protein kinase4 (GhMPK4) confers the transgenic Arabidopsis hypersensitivity to salt and osmotic stresses. Plant Cell Tissue Organ Cult. 2015, 123, 619–632. [Google Scholar] [CrossRef]
- McCaig, T.N.; Romagosa, I. Measurement and Use of Excised-Leaf Water Status in Wheat. Crop. Sci. 1989, 29, 1140–1145. [Google Scholar] [CrossRef]
- McKay, H. Root electrolyte leakage and root growth potential as indicators of spruce and larch establishment. Silva Fenn. 1998, 32, 241–252. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.T.; Pang, C.; Fan, S.; Song, M.; Arain, S.; Yu, S. Isolation and expression profiling of GhNAC transcription factor genes in cotton (Gossypium hirsutum L.) during leaf senescence and in response to stresses. Gene 2013, 531, 220–234. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, X.; Wang, Y.; Lebwohl, M. Inhibition of ultraviolet light-induced oxidative events in the skin and internal organs of hairless mice by isoflavone genistein. Cancer Lett. 2002, 185, 21–29. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium Deficiency and High Light Intensity Enhance Activities of Superoxide Dismutase, Ascorbate Peroxidase, and Glutathione Reductase in Bean Leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [Green Version]
- Magwanga, R.O.; Kirungu, J.N.; Lu, P.; Yang, X.; Dong, Q.; Cai, X.; Xu, Y.; Wang, X.; Zhou, Z.; Hou, Y.; et al. Genome wide identification of the trihelix transcription factors and overexpression of Gh_A05G2067 (GT-2), a novel gene contributing to increased drought and salt stresses tolerance in cotton. Physiol. Plant. 2019, 167, 447–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, L.; Wei, H.; Wang, H.; Su, J.; Yu, S. Characterization and functional analysis of GhWRKY42, a group IId WRKY gene, in upland cotton (Gossypium hirsutum L.). BMC Genet. 2018, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Gao, W.; Xu, L.; Liu, M.; Luo, X.; He, X.; Yang, X.; Zhang, X.; Zhu, L. GbMPK3, a mitogen-activated protein kinase from cotton, enhances drought and oxidative stress tolerance in tobacco. Plant Cell Tissue Organ Cult. 2013, 116, 153–162. [Google Scholar] [CrossRef]
- Magwanga, R.O.; Lu, P.; Kirungu, J.N.; Dong, Q.; Hu, Y.; Zhou, Z.; Cai, X.; Wang, X.; Hou, Y.; Wang, K.; et al. Cotton Late Embryogenesis Abundant (LEA2) Genes Promote Root Growth and Confer Drought Stress Tolerance in Transgenic Arabidopsis thaliana. G3 Genes Genomes Genet. 2018, 8, 2781–2803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, W.Y.; Peng, S.P.; Shao, C.Y.; Shao, H.B.; Yang, H.C. Ethylene glycol tetra-acetic acid and salicylic acid improve anti-oxidative ability of maize seedling leaves under heavy-metal and polyethylene glycol 6000-simulated drought stress. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2014, 148, 96–108. [Google Scholar] [CrossRef] [Green Version]
- Ben Rejeb, I.; Pastor, V.; Mauch-Mani, B. Plant Responses to Simultaneous Biotic and Abiotic Stress: Molecular Mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Lu, P.; Magwanga, R.O.; Kirungu, J.N.; Dong, Q.; Cai, X.; Zhou, Z.; Wang, X.; Xu, Y.; Hou, Y.; Peng, R.; et al. Genome-wide analysis of the cotton G-coupled receptor proteins (GPCR) and functional analysis of GTOM1, a novel cotton GPCR gene under drought and cold stress. BMC Genom. 2019, 20, 651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhang, L.; Wang, X.; Zhang, W.; Hao, L.; Chu, X.; Guo, X. CottonGhMPK6anegatively regulates osmotic tolerance and bacterial infection in transgenicNicotiana benthamiana, and plays a pivotal role in development. FEBS J. 2013, 280, 5128–5144. [Google Scholar] [CrossRef] [PubMed]
- Glory, E.; Murphy, R.F. Automated Subcellular Location Determination and High-Throughput Microscopy. Dev. Cell 2007, 12, 7–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zou, D.; Li, Y.; Sun, X.; Wang, N.-N.; Gong, S.-Y.; Zheng, Y.; Li, X.-B. GhMPK17, a Cotton Mitogen-Activated Protein Kinase, Is Involved in Plant Response to High Salinity and Osmotic Stresses and ABA Signaling. PLoS ONE 2014, 9, e95642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brock, A.K.; Willmann, R.; Kolb, D.; Grefen, L.; Lajunen, H.M.; Bethke, G.; Lee, J.; Nürnberger, T.; Gust, A.A. The Arabidopsis Mitogen-Activated Protein Kinase Phosphatase PP2C5 Affects Seed Germination, Stomatal Aperture, and Abscisic Acid-Inducible Gene Expression. Plant Physiol. 2010, 153, 1098–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahlfors, R.; Macioszek, V.; Rudd, J.; Brosché, M.; Schlichting, R.; Scheel, D.; Kangasjärvi, J. Stress hormone-independent activation and nuclear translocation of mitogen-activated protein kinases in Arabidopsis thaliana during ozone exposure. Plant J. 2004, 40, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, H.; Virk, N.; Cheng, X.; Bhatti, M.F. In Silico Analysis Reveals Multiple Genes of Mapk, Mapkk and Mapkkk Gene Families of the Mapk Cascade. PeerJ 2017, 5, e3255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, K.; Rao, K.P.; Sharma, P.; Sinha, A.K. Differential regulation of rice mitogen activated protein kinase kinase (MKK) by abiotic stress. Plant Physiol. Biochem. 2008, 46, 891–897. [Google Scholar] [CrossRef]
- Ning, J.; Li, X.; Hicks, L.M.; Xiong, L. A Raf-Like MAPKKK Gene DSM1 Mediates Drought Resistance through Reactive Oxygen Species Scavenging in Rice. Plant Physiol. 2010, 152, 876–890. [Google Scholar] [CrossRef] [Green Version]
- Venditti, P.; Di Stefano, L.; Di Meo, S. Mitochondrial metabolism of reactive oxygen species. Mitochondrion 2013, 13, 71–82. [Google Scholar] [CrossRef]
- Zong, X.-J.; Li, D.-P.; Gu, L.-K.; Li, D.-Q.; Liu, L.-X.; Hu, X.-L. Abscisic acid and hydrogen peroxide induce a novel maize group C MAP kinase gene, ZmMPK7, which is responsible for the removal of reactive oxygen species. Planta 2009, 229, 485–495. [Google Scholar] [CrossRef]
- Samuel, M.A.; Ellis, B.E. Double Jeopardy: Both Overexpression and Suppression of a Redox-Activated Plant Mito-gen-Activated Protein Kinase Render Tobacco Plants Ozone Sensitive. Plant Cell 2002, 14, 2059–2069. [Google Scholar] [CrossRef] [Green Version]
- Yadav, D.; Ahmed, I.; Shukla, P.; Boyidi, P.; Kirti, P.B. Overexpression of Arabidopsis AnnAt8 Alleviates Abiotic Stress in Transgenic Arabidopsis and Tobacco. Plants 2016, 5, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frugoli, J.A.; Zhong, H.H.; Nuccio, M.L.; McCourt, P.; McPeek, M.A.; Thomas, T.L.; McClung, C.R. Catalase Is Encoded by a Multigene Family in Arabidopsis thaliana (L.) Heynh. Plant Physiol. 1996, 112, 327–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, M.; Fujita, Y.; Noutoshi, Y.; Takahashi, F.; Narusaka, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Crosstalk between abiotic and biotic stress responses: A current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol. 2006, 9, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci. 2005, 10, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Msanne, J.; Lin, J.; Stone, J.M.; Awada, T. Characterization of abiotic stress-responsive Arabidopsis thaliana RD29A and RD29B genes and evaluation of transgenes. Planta 2011, 234, 97–107. [Google Scholar] [CrossRef] [PubMed]
- García, M.N.M.; Cortelezzi, J.I.; Fumagalli, M.; Capiati, D.A. Expression of the Arabidopsis ABF4 gene in potato increases tuber yield, improves tuber quality and enhances salt and drought tolerance. Plant Mol. Biol. 2018, 98, 137–152. [Google Scholar] [CrossRef]
- Chutipaijit, S. Changes in physiological and antioxidant activity of indica rice seedlings in response to mannitol-induced osmotic stress. Chil. J. Agric. Res. 2016, 76, 455–462. [Google Scholar] [CrossRef]
- Tähtiharju, S.; Sangwan, V.; Monroy, A.F.; Dhindsa, R.S.; Borg, M. The induction of kin genes in cold-acclimating Arabidopsis thaliana. Evidence of a role for calcium. Planta 1997, 203, 442–447. [Google Scholar] [CrossRef]
- Su, W.; Huang, L.; Ling, H.; Mao, H.; Huang, N.; Su, Y.; Ren, Y.; Wang, D.; Xu, L.; Muhammad, K.; et al. Sugarcane calcineurin B-like (CBL) genes play important but versatile roles in regulation of responses to biotic and abiotic stresses. Sci. Rep. 2020, 10, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Ishitani, M.; Kim, C.; Zhu, J.-K. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc. Natl. Acad. Sci. USA 2000, 97, 6896–6901. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadau, S.B.; Ahmad, A.; Tajo, S.M.; Ibrahim, S.; Kazeem, B.B.; Wei, H.; Yu, S. Overexpression of GhMPK3 from Cotton Enhances Cold, Drought, and Salt Stress in Arabidopsis. Agronomy 2021, 11, 1049. https://doi.org/10.3390/agronomy11061049
Sadau SB, Ahmad A, Tajo SM, Ibrahim S, Kazeem BB, Wei H, Yu S. Overexpression of GhMPK3 from Cotton Enhances Cold, Drought, and Salt Stress in Arabidopsis. Agronomy. 2021; 11(6):1049. https://doi.org/10.3390/agronomy11061049
Chicago/Turabian StyleSadau, Salisu Bello, Adeel Ahmad, Sani Muhammad Tajo, Sani Ibrahim, Bello Babatunde Kazeem, Hengling Wei, and Shuxun Yu. 2021. "Overexpression of GhMPK3 from Cotton Enhances Cold, Drought, and Salt Stress in Arabidopsis" Agronomy 11, no. 6: 1049. https://doi.org/10.3390/agronomy11061049





