Effects of Phosphorus Ensembled Nanomaterials on Nutrient Uptake and Distribution in Glycine max L. under Simulated Precipitation
Abstract
1. Introduction
2. Experimental Section
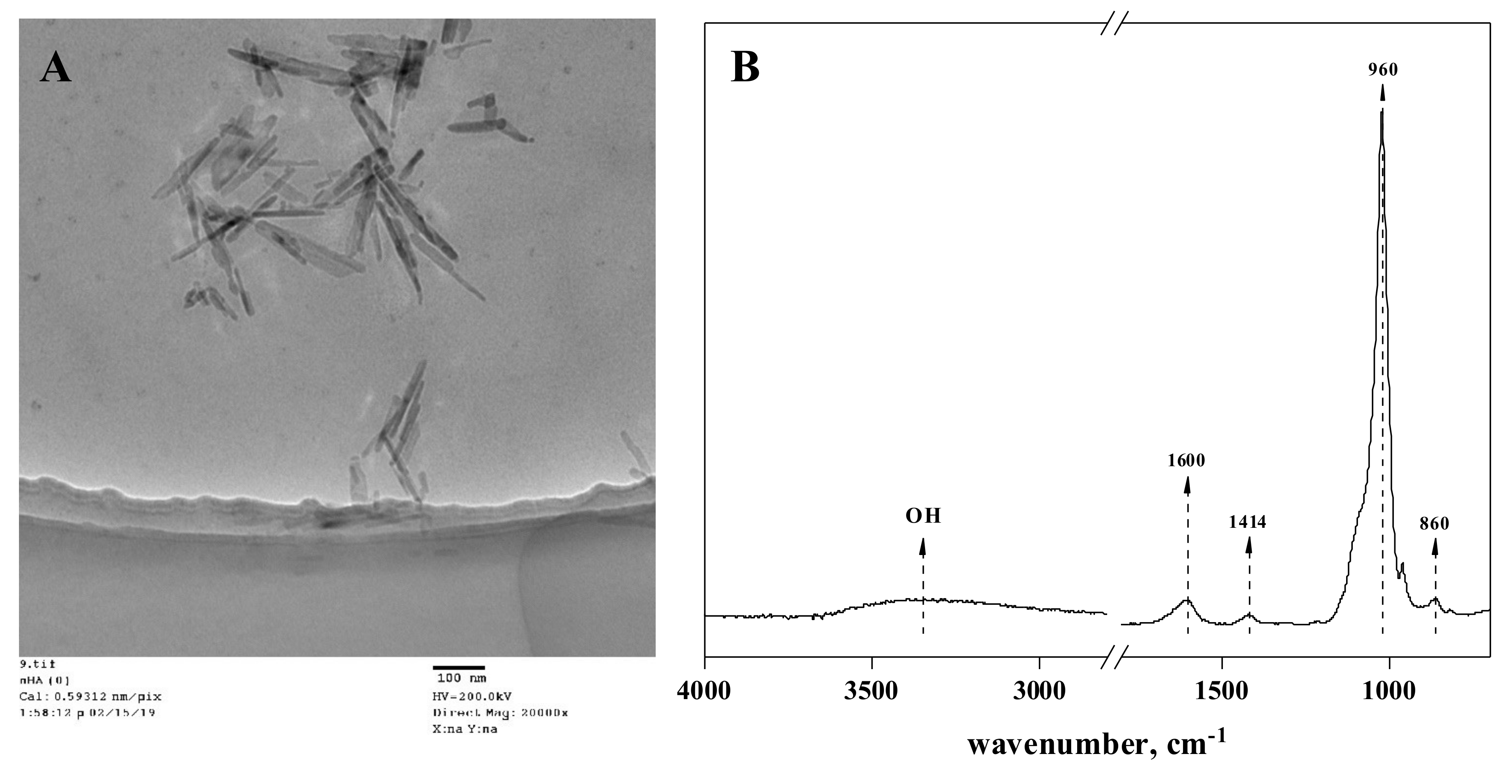
2.1. Synthesis and Characterization of Hydroxyapatite Nanoparticles
2.2. Greenhouse Cultivation
2.3. Simulated Precipitation Experiment
2.4. Wash-Off Test from Different Heights
2.5. Plant Harvest
2.5.1. Pigment Measurement
2.5.2. Elemental Analysis in Plant Tissues
2.5.3. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Synthesized Nano-Hydroxyapatites
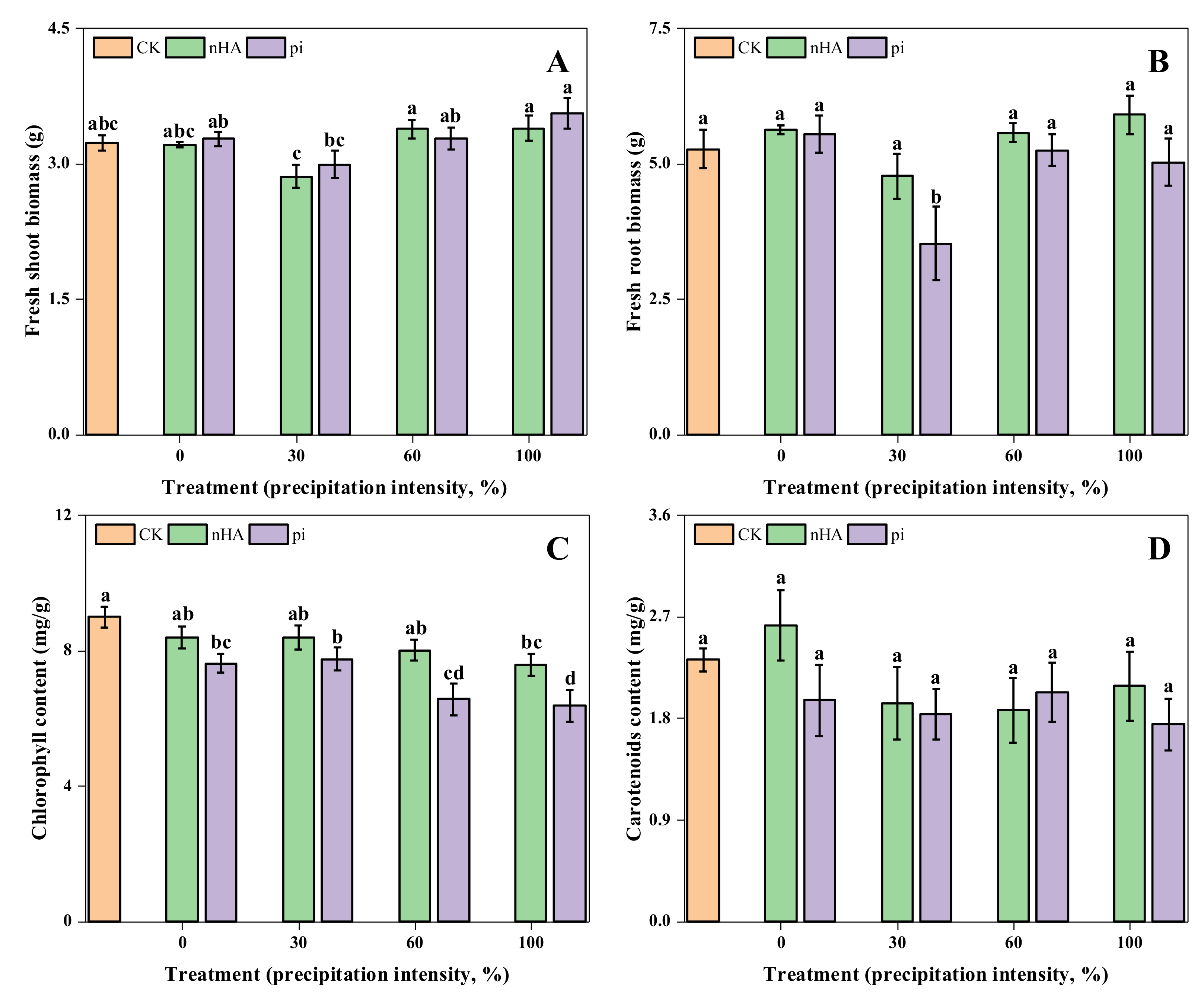
3.2. Physiological Responses of nHA- and pi-Treated Soybean under Simulated Precipitation and Wash-Off Conditions
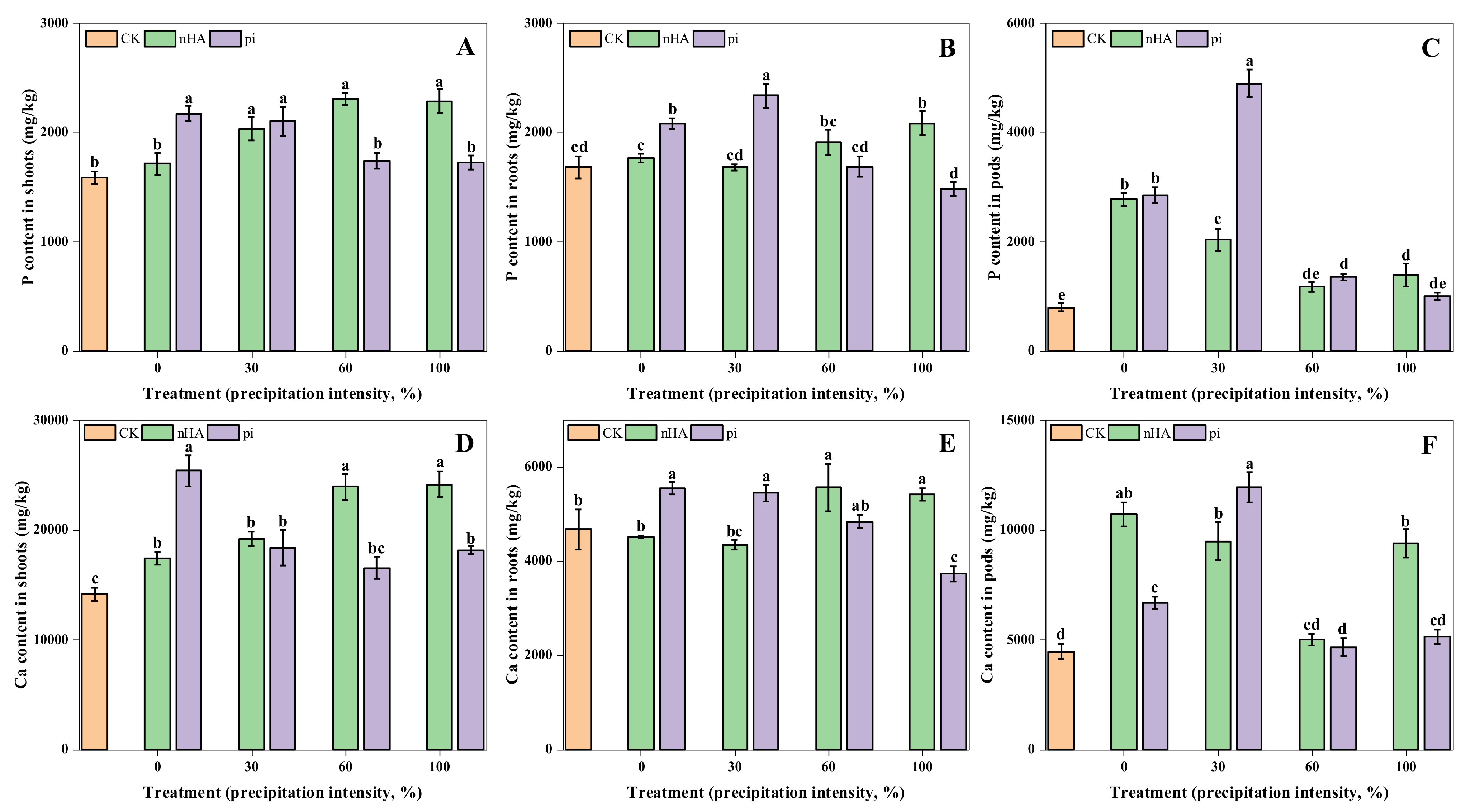
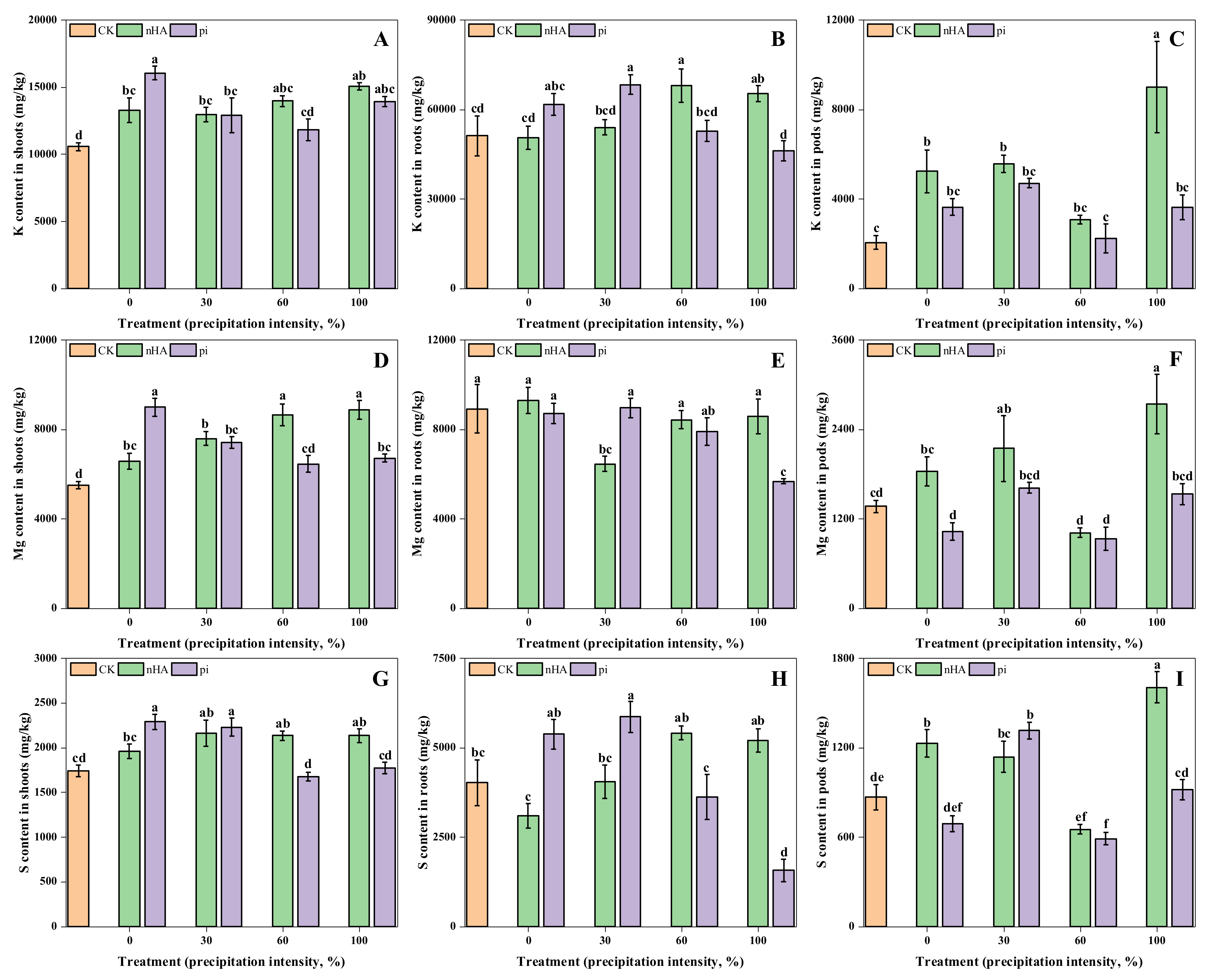
3.3. Nutrient Contents in Soybean
3.3.1. Macronutrient Contents in Shoots, Roots, and Soil
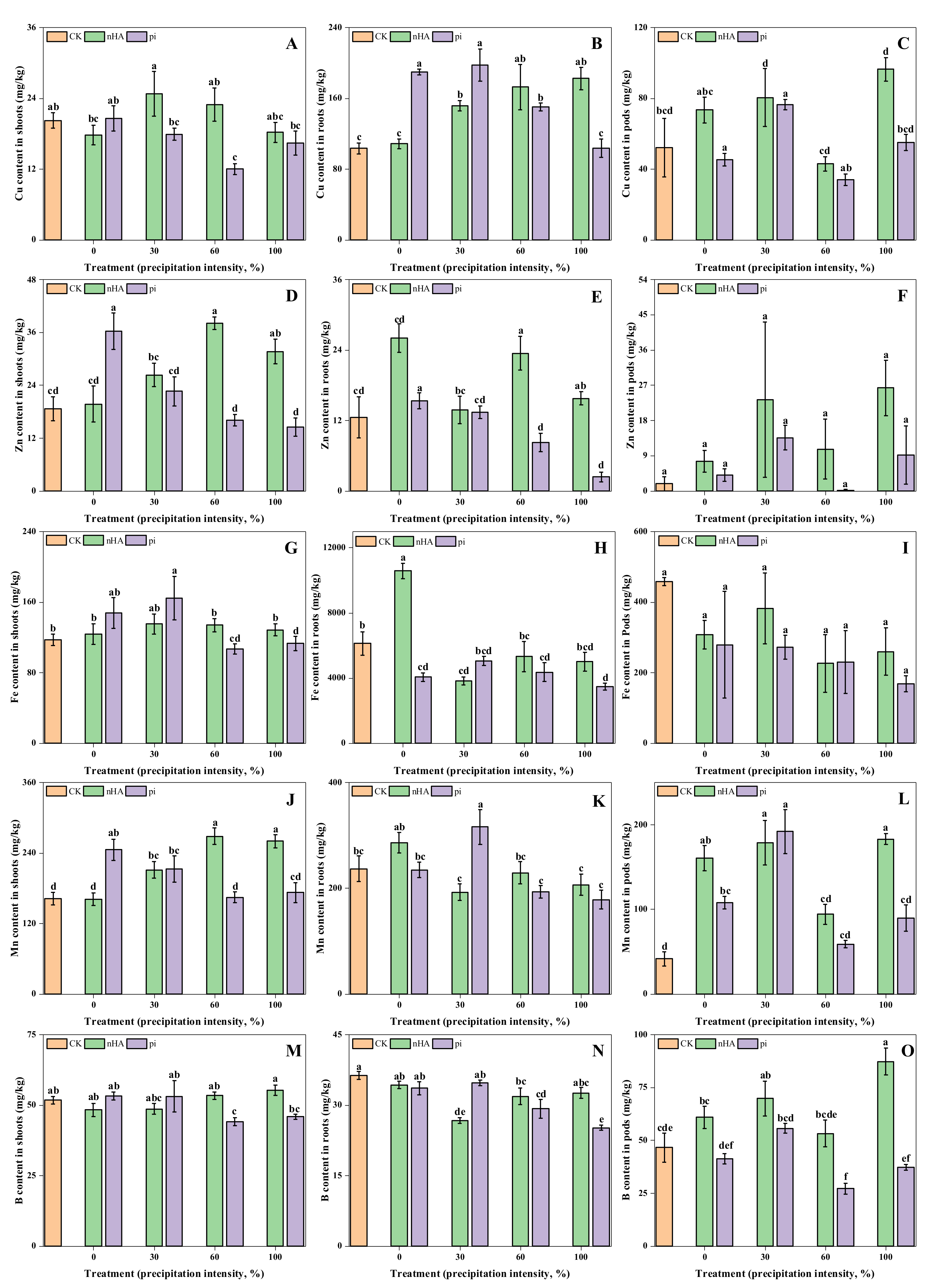
3.3.2. Micronutrient Contents in Shoots, Roots and Soil
3.3.3. Nutrient Contents in Edible Tissues
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; ESA Working paper No.12-03; FAO: Rome, Italy, 2012. [Google Scholar]
- United Nations, Department of Economic and Social Affairs. World Population Prospects: The 2017 Revision, Key Findings and Advance Tables; ESA/P/WP/248; United Nations: New York, NY, USA, 2017. [Google Scholar]
- United Nations, Department of Economic and Social Affairs. World Population Prospects 2019: Highlights; ST/ESA/SER.A/423; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Mueller, N.D.; Gerber, J.S.; Johnston, M.; Ray, D.K.; Ramankutty, N.; Foley, J.A. Closing yield gaps through nutrient and water management. Nature 2012, 490, 254. [Google Scholar] [CrossRef]
- FAO. World Fertilizer Trends and Outlook to 2022; FAO: Rome, Italy, 2019. [Google Scholar]
- FAO. The State of Food and Agriculture: Moving forward on Food Loss and Waste Reduction; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Springmann, M.; Clark, M.; Mason-D’Croz, D.; Wiebe, K.; Bodirsky, B.L.; Lassaletta, L.; de Vries, W.; Vermeulen, S.J.; Herrero, M.; Carlson, K.M. Options for keeping the food system within environmental limits. Nature 2018, 562, 519. [Google Scholar] [CrossRef]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 2018, 13, 677. [Google Scholar] [CrossRef]
- Clark, M.; Tilman, D. Comparative analysis of environmental impacts of agricultural production systems, agricultural input efficiency, and food choice. Environ. Res. Lett. 2017, 12, 064016. [Google Scholar] [CrossRef]
- Guo, T.; Johnson, L.T.; LaBarge, G.A.; Penn, C.J.; Stumpf, R.P.; Baker, D.B.; Shao, G. Less Agricultural Phosphorus Applied in 2019 Led to Less Dissolved Phosphorus Transported to Lake Erie. Environ. Sci. Technol. 2021, 55, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Saia, S.M.; Carrick, H.J.; Buda, A.R.; Regan, J.M.; Walter, M.T. Critical Review of Polyphosphate and Polyphosphate Accumulating Organisms for Agricultural Water Quality Management. Environ. Sci. Technol. 2021, 55, 2722–2742. [Google Scholar] [CrossRef]
- Ceulemans, T.; Bodé, S.; Bollyn, J.; Harpole, S.; Coorevits, K.; Peeters, G.; Van Acker, K.; Smolders, E.; Boeckx, P.; Honnay, O. Phosphorus resource partitioning shapes phosphorus acquisition and plant species abundance in grasslands. Nat. Plants 2017, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hawkesford, M.J.; Kopriva, S.; De Kok, L.J. Nutrient Use Efficiency in Plants; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Dobermann, A. Nutrient use efficiency–measurement and management. In Fertilizer Best Management Practices; IFA: Paris, France, 2007. [Google Scholar]
- Wang, J.; Bouwman, A.F.; Liu, X.; Beusen, A.H.; Van Dingenen, R.; Dentener, F.; Yao, Y.; Glibert, P.M.; Ran, X.; Yao, Q. Harmful Algal Blooms in Chinese Coastal Waters Will Persist Due to Perturbed Nutrient Ratios. Environ. Sci. Technol. Lett. 2021, 8, 276–284. [Google Scholar] [CrossRef]
- Némery, J.; Garnier, J. The fate of phosphorus. Nat. Geosci. 2016, 9, 343–344. [Google Scholar] [CrossRef]
- Vaccari, D.A.; Powers, S.M.; Liu, X. Demand-driven model for global phosphate rock suggests paths for phosphorus sustainability. Environ. Sci. Technol. 2019, 53, 10417–10425. [Google Scholar] [CrossRef]
- Liu, W.; Ciais, P.; Liu, X.; Yang, H.; Hoekstra, A.Y.; Tang, Q.; Wang, X.; Li, X.; Cheng, L. Global Phosphorus Losses from Croplands under Future Precipitation Scenarios. Environ. Sci. Technol. 2020, 54, 14761–14771. [Google Scholar] [CrossRef]
- Alewell, C.; Ringeval, B.; Ballabio, C.; Robinson, D.A.; Panagos, P.; Borrelli, P. Global phosphorus shortage will be aggravated by soil erosion. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Lowry, G.V.; Ghoshal, S.; Tufenkji, N.; Brambilla, D.; Dutcher, J.R.; Gilbertson, L.M.; Giraldo, J.P.; Kinsella, J.M.; Landry, M.P. Technology readiness and overcoming barriers to sustainably implement nanotechnology-enabled plant agriculture. Nat. Food 2020, 1, 416–425. [Google Scholar] [CrossRef]
- Johnston, A.E.; Poulton, P.R.; Fixen, P.E.; Curtin, D. Phosphorus: Its efficient use in agriculture. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2014; Volume 123, pp. 177–228. [Google Scholar]
- Group, A.C. Innovative Approach Taken to Phosphorus Nanofertilizer Research. Available online: https://www.agchemigroup.eu/en/blog/post/innovative-approach-taken-phosphorus-nanofertilizer-research (accessed on 24 May 2018).
- Lowry, G.V.; Avellan, A.; Gilbertson, L.M. Opportunities and challenges for nanotechnology in the agri-tech revolution. Nat. Nanotechnol. 2019, 14, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wang, Y.; Gilbertson, L.M. Opportunities to advance sustainable design of nano-enabled agriculture identified through a literature review. Environ. Sci. Nano 2018, 5, 11–26. [Google Scholar] [CrossRef]
- Vázquez-Núñez, E.; López-Moreno, M.L.; de la Rosa Álvarez, G.; Fernández-Luqueño, F. Incorporation of Nanoparticles into Plant Nutrients: The Real Benefits. In Agricultural Nanobiotechnology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 49–76. [Google Scholar]
- Subramanian, K.S.; Manikandan, A.; Thirunavukkarasu, M.; Rahale, C.S. Nano-fertilizers for balanced crop nutrition. In Nanotechnologies in Food and Agriculture; Springer: Berlin/Heidelberg, Germany, 2015; pp. 69–80. [Google Scholar]
- Raliya, R.; Saharan, V.; Dimkpa, C.; Biswas, P. Nanofertilizer for precision and sustainable agriculture: Current state and future perspectives. J. Agric. Food. Chem. 2017, 66, 6487–6503. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Bindraban, P.S. Nanofertilizers: New products for the industry? J. Agric. Food. Chem. 2017, 66, 6462–6473. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Huang, Y.; Keller, A.A. Comparative metabolic response between cucumber (Cucumis sativus) and corn (Zea mays) to a Cu (OH) 2 nanopesticide. J. Agric. Food. Chem. 2017, 66, 6628–6636. [Google Scholar] [CrossRef]
- Chariou, P.L.; Ortega-Rivera, O.A.; Steinmetz, N.F. Nanocarriers for the Delivery of Medical, Veterinary, and Agricultural Active Ingredients. ACS Nano 2020, 14, 2678–2701. [Google Scholar] [CrossRef]
- Talreja, N.; Chauhan, D.; Rodríguez, C.A.; Mera, A.C.; Ashfaq, M. Nanocarriers: An Emerging Tool for Micronutrient Delivery in Plants. In Plant Micronutrients; Springer: Berlin/Heidelberg, Germany, 2020; pp. 373–387. [Google Scholar]
- Lew, T.T.S.; Koman, V.B.; Silmore, K.S.; Seo, J.S.; Gordiichuk, P.; Kwak, S.-Y.; Park, M.; Ang, M.C.-Y.; Khong, D.T.; Lee, M.A. Real-time detection of wound-induced H 2 O 2 signalling waves in plants with optical nanosensors. Nat. Plants 2020, 6, 404–415. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Kumar, S.; Jasrotia, P.; Singh, D.; Singh, G.P. Nanosensors for plant disease diagnosis: Current understanding and future perspectives. In Nanoscience for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2019; pp. 189–205. [Google Scholar]
- Srivastava, A.K.; Dev, A.; Karmakar, S. Nanosensors and nanobiosensors in food and agriculture. Environ. Chem. Lett. 2018, 16, 161–182. [Google Scholar] [CrossRef]
- Gilbertson, L.M.; Pourzahedi, L.; Laughton, S.; Gao, X.; Zimmerman, J.B.; Theis, T.L.; Westerhoff, P.; Lowry, G.V. Guiding the design space for nanotechnology to advance sustainable crop production. Nat. Nanotechnol. 2020, 15, 801–810. [Google Scholar] [CrossRef]
- Ditta, A.; Mehmood, S.; Imtiaz, M.; Rizwan, M.S.; Islam, I. Soil fertility and nutrient management with the help of nanotechnology. In Nanomaterials for Agriculture and Forestry Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 273–287. [Google Scholar]
- Liu, B.; Fan, Y.; Li, H.; Zhao, W.; Luo, S.; Wang, H.; Guan, B.; Li, Q.; Yue, J.; Dong, Z. Control the entire journey of pesticide application on superhydrophobic plant surface by dynamic covalent trimeric surfactant coacervation. Adv. Funct. Mater. 2021, 31, 2006606. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Synthetic apatite nanoparticles as a phosphorus fertilizer for soybean (Glycine max). Sci. Rep. 2014, 4, 5686. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, M. Preparation and properties of chitosan-coated NPK compound fertilizer with controlled-release and water-retention. Carbohydr. Polym. 2008, 72, 240–247. [Google Scholar] [CrossRef]
- Tang, S.; Fei, X. Refractory Calcium Phosphate-Derived Phosphorus Fertilizer Based on Hydroxyapatite Nanoparticles for Nutrient Delivery. ACS Appl. Nano Mater. 2021, 4, 1364–1376. [Google Scholar] [CrossRef]
- Soliman, A.S.; Hassan, M.; Abou-Elella, F.; Ahmed, A.H.; El-Feky, S.A. Effect of nano and molecular phosphorus fertilizers on growth and chemical composition of baobab (Adansonia digitata L.). J. Plant Sci. 2016, 11, 52–60. [Google Scholar] [CrossRef]
- Ma, C.; Borgatta, J.; De La Torre Roche, R.; Zuverza-Mena, N.; White, J.C.; Hamers, R.J.; Elmer, W. Time-dependent transcriptional response of tomato (Solanum lycopersicum L.) to Cu nanoparticle exposure upon infection with Fusarium oxysporum f. sp. lycopersici. ACS Sustain. Chem. Eng. 2019, 7, 10064–10074. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Noureen, S.; Anwar, S.; Ali, B.; Naveed, M.; Abd_Allah, E.F.; Alqarawi, A.A.; Ahmad, P. Combined use of biochar and zinc oxide nanoparticle foliar spray improved the plant growth and decreased the cadmium accumulation in rice (Oryza sativa L.) plant. Environ. Sci. Pollut. Res. 2019, 26, 11288–11299. [Google Scholar] [CrossRef]
- Rui, M.; Ma, C.; Hao, Y.; Guo, J.; Rui, Y.; Tang, X.; Zhao, Q.; Fan, X.; Zhang, Z.; Hou, T. Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front. Plant Sci. 2016, 7, 815. [Google Scholar] [CrossRef]
- Pradhan, S.; Patra, P.; Das, S.; Chandra, S.; Mitra, S.; Dey, K.K.; Akbar, S.; Palit, P.; Goswami, A. Photochemical modulation of biosafe manganese nanoparticles on Vigna radiata: A detailed molecular, biochemical, and biophysical study. Environ. Sci. Technol. 2013, 47, 13122–13131. [Google Scholar] [CrossRef] [PubMed]
- Jassby, D.; Su, Y.; Kim, C.; Ashworth, V.; Adeleye, A.S.; Rolshausen, P.; Roper, C.; White, J. Delivery, Uptake, Fate, and Transport of Engineered Nanoparticles in Plants: A Critical Review and Data Analysis. Environ. Sci. Nano 2019, 6, 2311–2331. [Google Scholar]
- Carnovale, C.; Bryant, G.; Shukla, R.; Bansal, V. Identifying Trends in Gold Nanoparticle Toxicity and Uptake: Size, Shape, Capping Ligand, and Biological Corona. ACS Omega 2019, 4, 242–256. [Google Scholar] [CrossRef]
- Raliya, R.; Franke, C.; Chavalmane, S.; Nair, R.; Reed, N.; Biswas, P. Quantitative understanding of nanoparticle uptake in watermelon plants. Front. Plant Sci. 2016, 7, 1288. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.-J.; Wang, H.; Yan, B.; Zheng, H.; Jiang, Y.; Miranda, O.R.; Rotello, V.M.; Xing, B.; Vachet, R.W. Effect of surface charge on the uptake and distribution of gold nanoparticles in four plant species. Environ. Sci. Technol. 2012, 46, 12391–12398. [Google Scholar] [CrossRef]
- Spielman-Sun, E.; Avellan, A.; Bland, G.D.; Tappero, R.V.; Acerbo, A.S.; Unrine, J.M.; Giraldo, J.P.; Lowry, G.V. Nanoparticle surface charge influences translocation and leaf distribution in vascular plants with contrasting anatomy. Environ. Sci. Nano 2019, 6, 2508–2519. [Google Scholar] [CrossRef]
- Wang, D.; Jin, Y.; Jaisi, D.P. Effect of size-selective retention on the cotransport of hydroxyapatite and goethite nanoparticles in saturated porous media. Environ. Sci. Technol. 2015, 49, 8461–8470. [Google Scholar] [CrossRef]
- Ditta, A. How helpful is nanotechnology in agriculture? Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 033002. [Google Scholar] [CrossRef]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, B.; Iafisco, M.; Laforgia, M.; Margiotta, N.; Natile, G.; Bianchi, C.L.; Walsh, D.; Mann, S.; Roveri, N. Biomimetic hydroxyapatite–drug nanocrystals as potential bone substitutes with antitumor drug delivery properties. Adv. Funct. Mater. 2007, 17, 2180–2188. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, P.; Kopittke, P.M. Tailoring hydroxyapatite nanoparticles to increase their efficiency as phosphorus fertilisers in soils. Geoderma 2018, 323, 116–125. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.-T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef]
- Loo, S.C.J.; Siew, Y.E.; Ho, S.; Boey, F.Y.C.; Ma, J. Synthesis and hydrothermal treatment of nanostructured hydroxyapatite of controllable sizes. J. Mater. Sci. Mater. Med. 2008, 19, 1389–1397. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, P.; Hunter, M.N.; Kopittke, P.M. Bioavailability and movement of hydroxyapatite nanoparticles (HA-NPs) applied as a phosphorus fertiliser in soils. Environ. Sci. Nano 2018, 5, 2888–2898. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.B.; Dal Sasso, G.; Carmona, F.J.; Miguel-Rojas, C.; Pérez-de-Luque, A.; Masciocchi, N.; Guagliardi, A.; Delgado-López, J.M. Engineering Biomimetic Calcium Phosphate Nanoparticles: A Green Synthesis of Slow-Release Multinutrient (NPK) Nanofertilizers. ACS Appl. Bio Mater. 2020, 3, 1344–1353. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Lee, J.G.; Esposti, L.D.; Iafisco, M.; Kim, P.J.; Shin, S.G.; Jeon, J.-R.; Adamiano, A. Synergistic release of crop nutrients and stimulants from hydroxyapatite nanoparticles functionalized with humic substances: Toward a multifunctional nanofertilizer. ACS Omega 2020, 5, 6598–6610. [Google Scholar] [CrossRef] [PubMed]
- Rico, C.M.; Hong, J.; Morales, M.I.; Zhao, L.; Barrios, A.C.; Zhang, J.-Y.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effect of cerium oxide nanoparticles on rice: A study involving the antioxidant defense system and in vivo fluorescence imaging. Environ. Sci. Technol. 2013, 47, 5635–5642. [Google Scholar] [CrossRef] [PubMed]
- Navarro, D.A.; Bisson, M.A.; Aga, D.S. Investigating uptake of water-dispersible CdSe/ZnS quantum dot nanoparticles by Arabidopsis thaliana plants. J. Hazard. Mater. 2012, 211, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ma, C.; Mukherjee, A.; Musante, C.; Zhang, J.; White, J.C.; Dhankher, O.P.; Xing, B. Tannic acid alleviates bulk and nanoparticle Nd2O3 toxicity in pumpkin: A physiological and molecular response. Nanotoxicology 2016, 10, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Luna Zaragoza, D.; Romero Guzmán, E.T.; Reyes Gutiérrez, L.R. Surface and physicochemical characterization of phosphates vivianite, Fe2(PO4)3 and hydroxyapatite, Ca5(PO4)3OH. J. Miner. Mater. Char. Eng. 2009, 8, 591–609. [Google Scholar]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.T.; Zhang, L.; He, S.Y. Plant-microbe interactions facing environmental challenge. Cell Host Microbe 2019, 26, 183–192. [Google Scholar] [CrossRef]
- Chen, T.; Nomura, K.; Wang, X.; Sohrabi, R.; Xu, J.; Yao, L.; Paasch, B.C.; Ma, L.; Kremer, J.; Cheng, Y. A plant genetic network for preventing dysbiosis in the phyllosphere. Nature 2020, 580, 653–657. [Google Scholar] [CrossRef]
- Xiang, Q.; Lott, A.A.; Assmann, S.M.; Chen, S. Advances and perspectives in the metabolomics of stomatal movement and the disease triangle. Plant Sci. 2020, 110697. [Google Scholar]
- Unger, P.W.; Kaspar, T.C. Soil compaction and root growth: A review. Agron. J. 1994, 86, 759–766. [Google Scholar] [CrossRef]
- Guidi, L.; Degl’Innocenti, E. Imaging of chlorophyll a fluorescence: A tool to study abiotic stress in plants. In Abiotic Stress Plants—Mechanisms and Adaptions; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, M.H.; Goltsev, V.N.; Żuk-Gołaszewska, K.; Zivcak, M.; Brestic, M. Chlorophyll Fluorescence: Understanding Crop Performance—Basics and Applications; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Carstensen, A.; Szameitat, A.E.; Frydenvang, J.; Husted, S. Chlorophyll a fluorescence analysis can detect phosphorus deficiency under field conditions and is an effective tool to prevent grain yield reductions in spring barley (Hordeum vulgare L.). Plant Soil 2019, 434, 79–91. [Google Scholar] [CrossRef]
- Hepler, P.K. Calcium: A central regulator of plant growth and development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef]
- de Bang, T.C.; Husted, S.; Laursen, K.H.; Persson, D.P.; Schjoerring, J.K. The molecular–physiological functions of mineral macronutrients and their consequences for deficiency symptoms in plants. New Phytol. 2021, 229, 2446–2469. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ma, Y.; Wei, R.; Chen, Y.; Weng, L.; Ouyang, X.; Li, Y. Phosphorus transport in different soil types and the contribution of control factors to phosphorus retardation. Chemosphere 2021, 276, 130012. [Google Scholar] [CrossRef]
- Gérard, F. Clay minerals, iron/aluminum oxides, and their contribution to phosphate sorption in soils—A myth revisited. Geoderma 2016, 262, 213–226. [Google Scholar] [CrossRef]
- Mayakaduwage, S.; Mosley, L.M.; Marschner, P. Threshold for labile phosphate in a sandy acid sulfate soil. Geoderma 2020, 371, 114359. [Google Scholar] [CrossRef]
- Fernández, V.; Eichert, T. Uptake of hydrophilic solutes through plant leaves: Current state of knowledge and perspectives of foliar fertilization. Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar] [CrossRef]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Droux, M. Sulfur assimilation and the role of sulfur in plant metabolism: A survey. Photosynth. Res. 2004, 79, 331–348. [Google Scholar] [CrossRef]
- Wilhelm Scherer, H. Sulfur in soils. J. Plant Nutr. Soil Sci. 2009, 172, 326–335. [Google Scholar] [CrossRef]
- Prodhan, M.A.; Finnegan, P.M.; Lambers, H. How does evolution in phosphorus-impoverished landscapes impact plant nitrogen and sulfur assimilation? Trends Plant Sci. 2019, 24, 69–82. [Google Scholar] [CrossRef]
- Veneklaas, E.J.; Lambers, H.; Bragg, J.; Finnegan, P.M.; Lovelock, C.E.; Plaxton, W.C.; Price, C.A.; Scheible, W.R.; Shane, M.W.; White, P.J. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol. 2012, 195, 306–320. [Google Scholar] [CrossRef]
- Singh, S.K.; Reddy, V.R.; Fleisher, D.H.; Timlin, D.J. Phosphorus nutrition affects temperature response of soybean growth and canopy photosynthesis. Front. Plant Sci. 2018, 9, 1116. [Google Scholar] [CrossRef]
- Priester, J.H.; Ge, Y.; Mielke, R.E.; Horst, A.M.; Moritz, S.C.; Espinosa, K.; Gelb, J.; Walker, S.L.; Nisbet, R.M.; An, Y.-J. Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. Proc. Natl. Acad. Sci. USA 2012, 109, E2451–E2456. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, U.R.; Kaur, G.; Khan, M. Calcium, phosphorus, and harvest stages effects soybean seed production and quality. J. Plant Nutr. 2007, 30, 2119–2127. [Google Scholar] [CrossRef]

| Label | Treatment Details |
|---|---|
| Control (CK) | 4 mL water sprayed onto foliage |
| A (0%) | 4 mL nHA (0.7 mmol/L) sprayed onto foliage |
| B (30%) | 2.8 mL nHA (0.7 mmol/L) sprayed onto foliage, and 1.2 mL nHA (0.7 mmol/L) added into soil |
| C (60%) | 1.6 mL nHA (0.7 mmol/L) sprayed onto foliage, and 2.4 mL nHA (0.7 mmol/L) added into soil |
| D (100%) | 4 mL nHA (0.7 mmol/L) added into soil |
| A’ (0%) | 4 mL pi (0.7 mmol/L) sprayed onto foliage |
| B’ (30%) | 2.8 mL pi (0.7 mmol/L) sprayed onto foliage, and 1.2 mL pi (0.7 mmol/L) added into soil |
| C’ (60%) | 1.6 mL pi (0.7 mmol/L) sprayed onto foliage, and 2.4 mL pi (0.7 mmol/L) added into soil |
| D’ (100%) | 4 mL water sprayed onto foliage |
| Label | Treatment Details |
|---|---|
| Control (CK) | 4 mL water sprayed onto foliage |
| A (no wash-off) | 4 mL nHA (0.7 mmol/L) sprayed onto foliage |
| A’ (no wash-off) | 4 mL pi (0.7 mmol/L) sprayed onto foliage |
| E (20 cm) | A with precipitation from 20 cm above seedlings |
| F (120 cm) | A with precipitation from 120 cm above seedlings |
| G (240 cm) | A with precipitation from 240 cm above seedlings |
| E’ (20 cm) | A’ with precipitation from 20 cm above seedlings |
| F’ (120 cm) | A’ with precipitation from 120 cm above seedlings |
| G’ (240 cm) | A’ with precipitation from 240 cm above seedlings |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Ma, C.; White, J.C.; Xing, B. Effects of Phosphorus Ensembled Nanomaterials on Nutrient Uptake and Distribution in Glycine max L. under Simulated Precipitation. Agronomy 2021, 11, 1086. https://doi.org/10.3390/agronomy11061086
Li Q, Ma C, White JC, Xing B. Effects of Phosphorus Ensembled Nanomaterials on Nutrient Uptake and Distribution in Glycine max L. under Simulated Precipitation. Agronomy. 2021; 11(6):1086. https://doi.org/10.3390/agronomy11061086
Chicago/Turabian StyleLi, Qingqing, Chuanxin Ma, Jason C. White, and Baoshan Xing. 2021. "Effects of Phosphorus Ensembled Nanomaterials on Nutrient Uptake and Distribution in Glycine max L. under Simulated Precipitation" Agronomy 11, no. 6: 1086. https://doi.org/10.3390/agronomy11061086
APA StyleLi, Q., Ma, C., White, J. C., & Xing, B. (2021). Effects of Phosphorus Ensembled Nanomaterials on Nutrient Uptake and Distribution in Glycine max L. under Simulated Precipitation. Agronomy, 11(6), 1086. https://doi.org/10.3390/agronomy11061086








