Chemical Composition and Allelopathic Effect of Essential Oil of Litsea pungens
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Isolation of the Essential Oil
2.3. Gas Chromatography–Mass Spectrometry Analysis
2.4. Allelopathic Activity Assays
2.4.1. Seed Germination Experiment (Petri Dish Experiment)
2.4.2. Pot Experiment
2.4.3. Determination of Photosynthetic Pigments
2.4.4. Determination of Antioxidant Enzyme Activity
2.4.5. Determination of the Content of Malondialdehyde (MDA) and Electrolyte Leakage (EL)
2.5. Statistical Analysis
3. Results
3.1. Chemical Characterization Analysis of L. Pungens Essential Oil
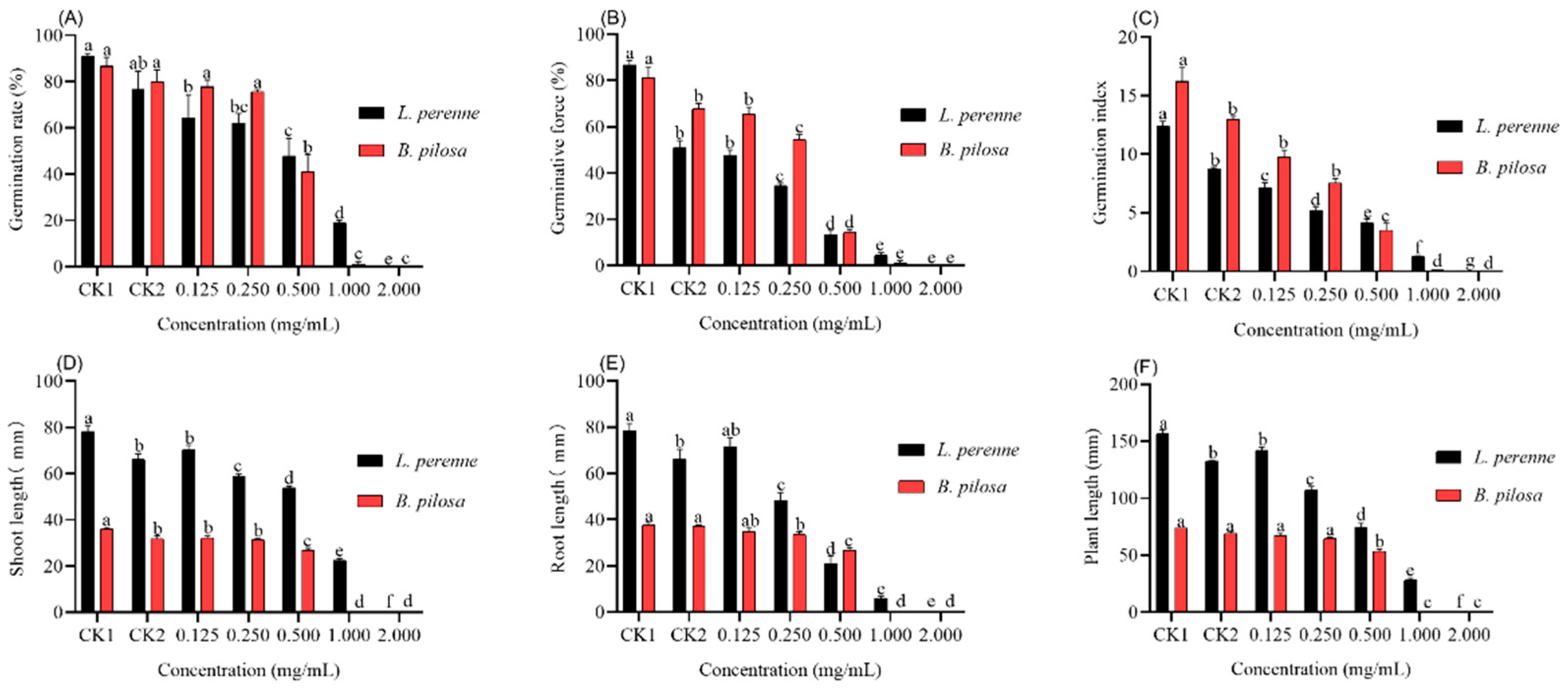
3.2. Effect of L. Pungens Essential Oil on Germination and Seedling Growth (Petri Dish Experiment)
3.3. Effect of L. Pungens Essential Oil on Photosynthetic Pigments Content (Petri Dish Experiment)
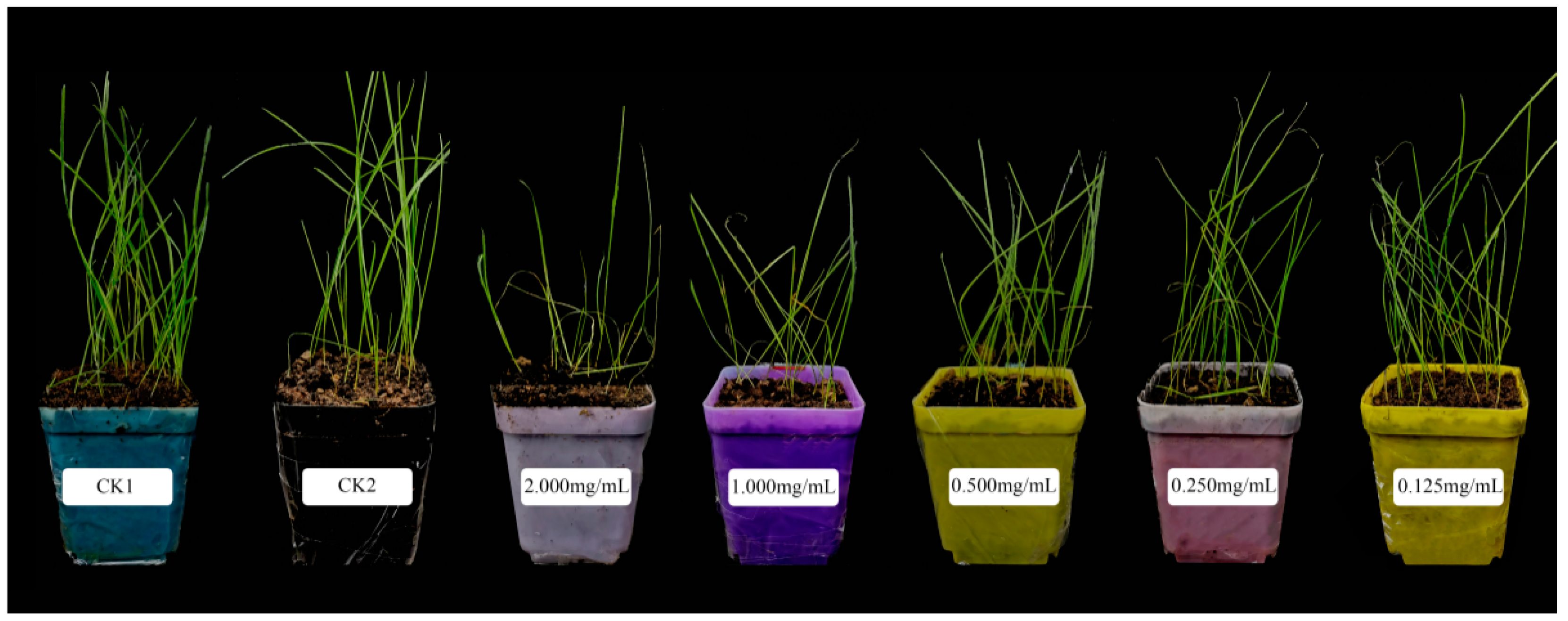
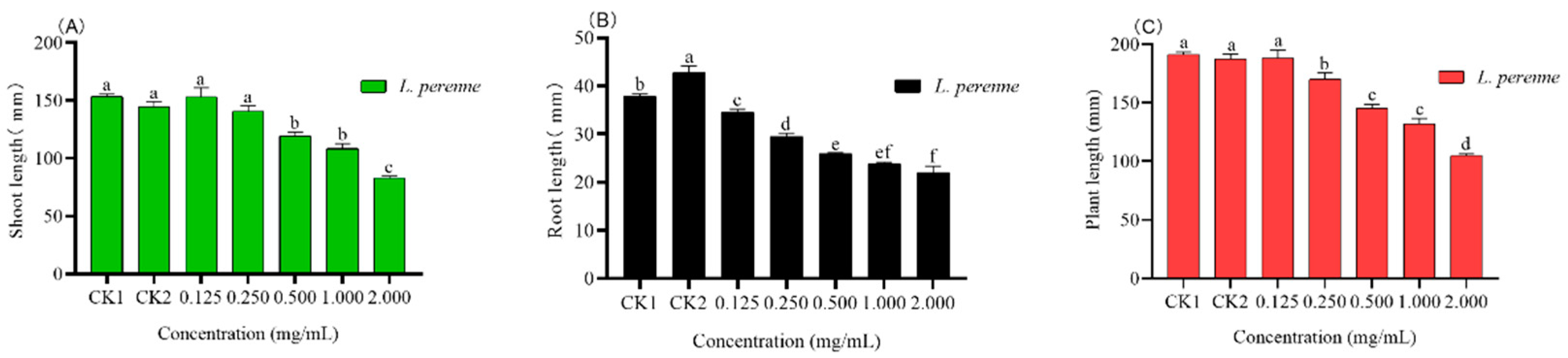
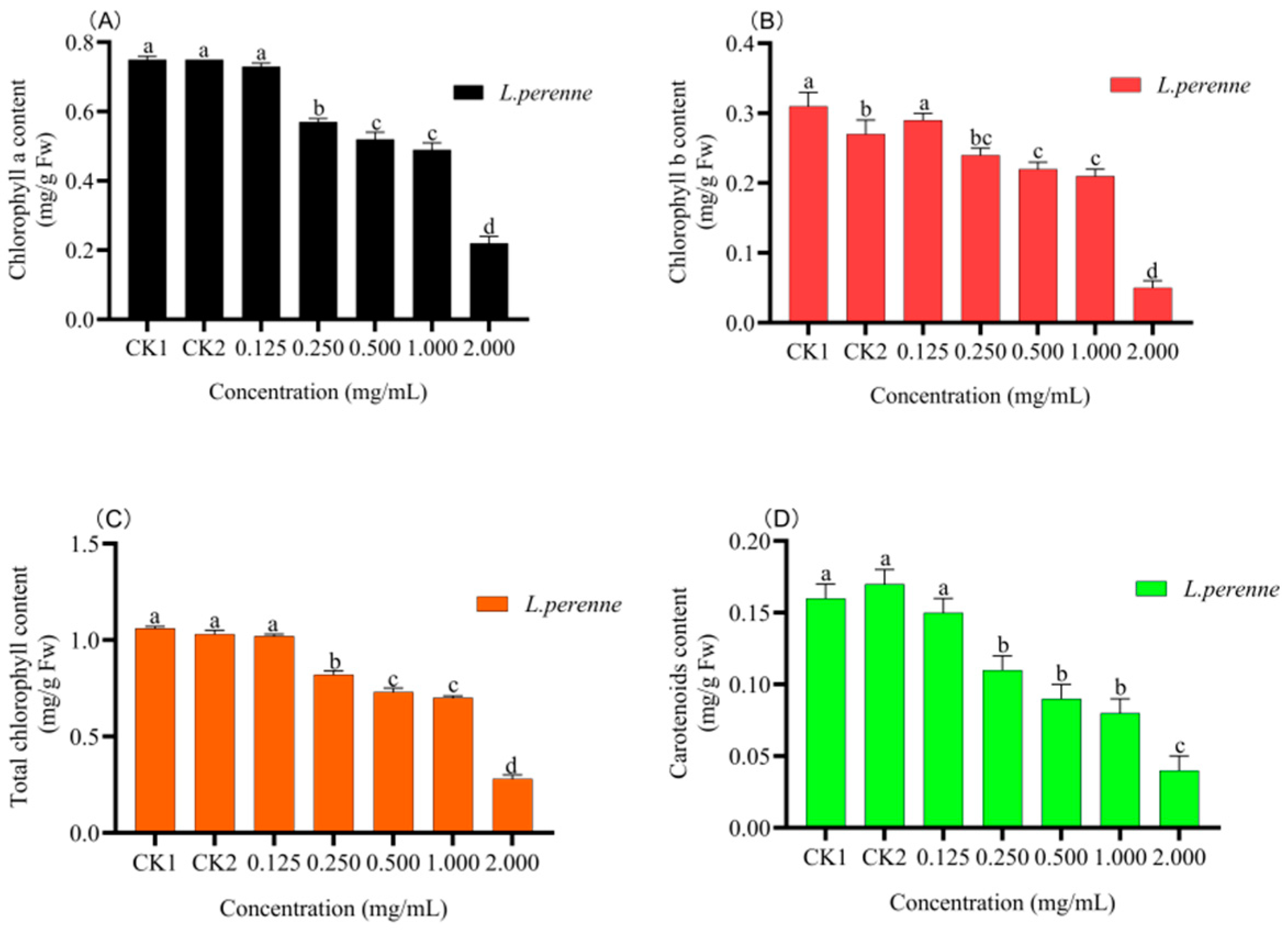
3.4. Pot Experiment
3.4.1. Seedling Growth Parameters
3.4.2. Photosynthetic Pigments Content
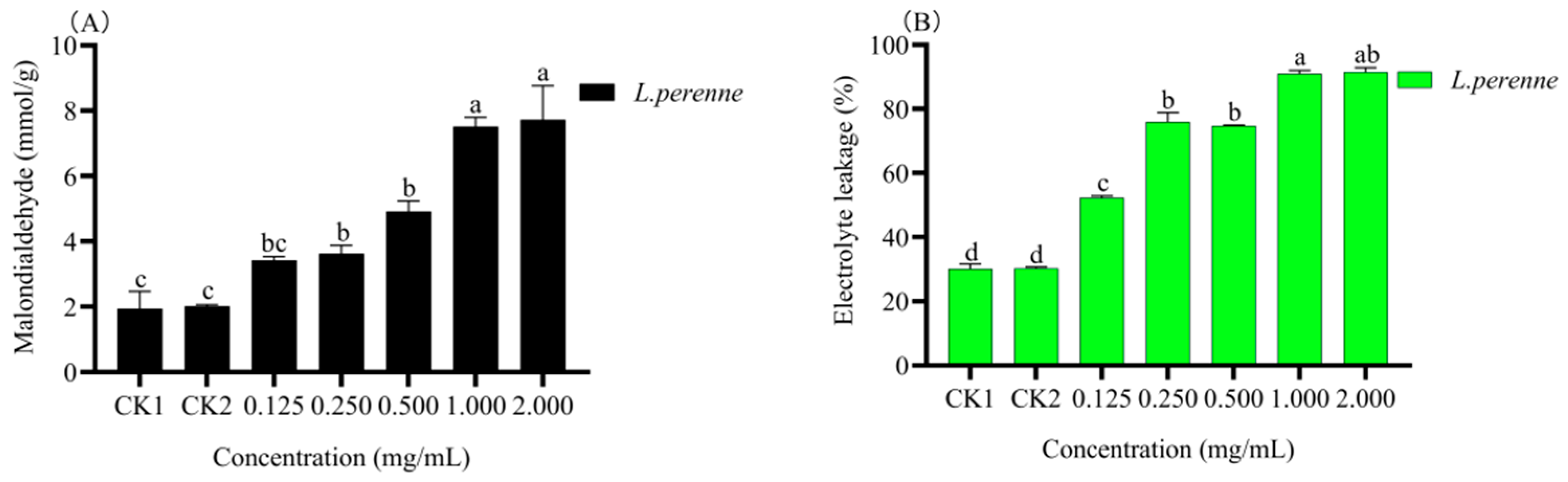
3.4.3. Malondialdehyde (MDA) Content and Electrolyte Leakage (EL)
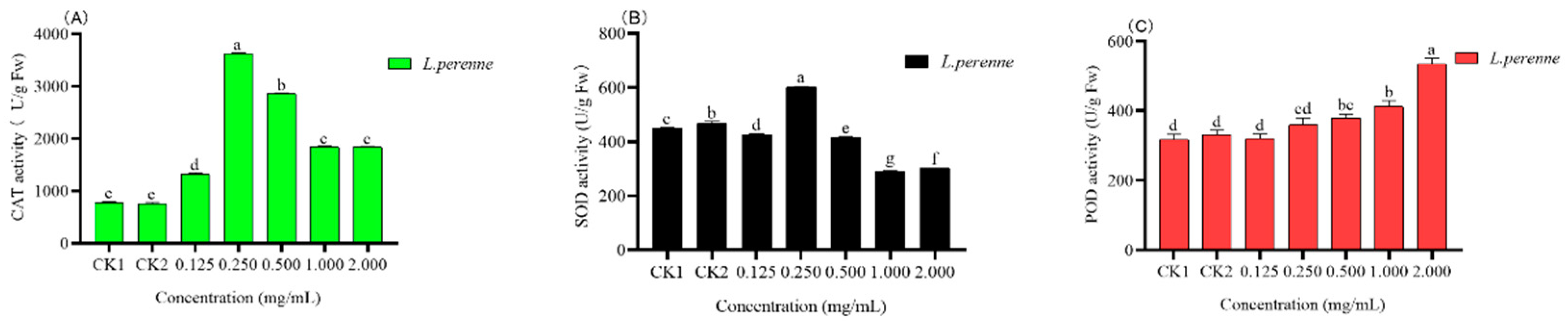
3.4.4. Antioxidant Enzymes Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angelini, L.G.; Carpanese, G.; Cioni, P.L.; Morelli, I.; Macchia, M.; Flamini, G. Essential oils from Mediterranean lamiaceae as weed germination inhibitors. J. Agr. Food Chem. 2003, 51, 6158–6164. [Google Scholar] [CrossRef]
- Batish, D.R.; Setia, N.; Singh, H.P.; Kohlia, R.K. Phytotoxicity of lemon-scented eucalypt oil and its potential use as a bioherbicide. Crop Prot. 2004, 23, 1209–1214. [Google Scholar] [CrossRef]
- Beckie, H.J.; Tardif, F.J. Herbicide cross resistance in weeds. Crop Prot. 2012, 35, 15–28. [Google Scholar] [CrossRef]
- Ercoli, L.; Masoni, A.; Pampana, S.; Arduini, I. Allelopathic effects of rye, brown mustard and hairy vetch on redroot pigweed, common lambsquarter and knotweed. Allelopathy J. 2007, 19, 249–256. [Google Scholar]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural products in crop protection. Bioorgan. Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, H.P.; Mittal, S. Phytotoxic effects of volatile oil from Artemisia scoparia against weeds and its possible use as a bioherbicide. Ind. Crops Prod. 2010, 32, 54–61. [Google Scholar] [CrossRef]
- Mekky, M.S.; Hassanien, A.M.; Kamel, E.M.; Ismail, A.E.A. Allelopathic effect of Ocimum basilicum L. extracts on weeds and some crops and its possible use as new crude bio-herbicide. Ann. Agric. Sci. 2019, 64, 211–221. [Google Scholar] [CrossRef]
- Ahuja, N.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Herbicidal activity of eugenol towards some grassy and broad-leaved weeds. J. Pest Sci. 2015, 88, 209–218. [Google Scholar] [CrossRef]
- Feldlaufer, M.F.; Ulrich, K.R. Essential Oils as Fumigants for Bed Bugs (Hemiptera: Cimicidae). J. Entomol. Sci. 2015, 50, 129–137. [Google Scholar] [CrossRef]
- Laosinwattana, C.; Wichittrakarn, P.; Teerarak, M. Chemical composition and herbicidal action of essential oil from Tagetes erecta L. leaves. Ind. Crops Prod. 2018, 126, 129–134. [Google Scholar] [CrossRef]
- Aghbash, B.N.; Pouresmaeil, M.; Dehghan, G.; Nojadeh, M.S.; Mobaiyen, H.; Maggi, F. Chemical composition, antibacterial and radical scavenging activity of essential oils from Satureja macrantha C.A.Mey. At different growth stages. Foods 2020, 9, 494. [Google Scholar] [CrossRef]
- Saric-Krsmanovic, M.; Umiljendic, J.G.; Radivojevic, L.; Rajkovic, M.; Santric, L.; Durovic-Pejcev, R. Chemical composition of Ambrosia trifida essential oil and phytotoxic effect on other plants. Chem. Biodivers. 2020, 17, e1900508. [Google Scholar] [CrossRef] [PubMed]
- Heap, I. Global perspective of herbicide-resistant weeds. Pest. Manag. Sci. 2014, 70, 1306–1315. [Google Scholar] [CrossRef]
- Soltani, N.; Dille, J.A.; Burke, I.C.; Everman, W.J.; Vangessel, M.J.; Davis, V.M.; Sikkema, P.H. Potential corn yield losses from weeds in North America. Weed Technol. 2016, 30, 979–984. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.K.; Lee, K.E.; Bajpai, V.K.; Gajurel, P.R.; Gu, K.S.; Kumar, P. Ethnopharmacological properties and medicinal uses of Litsea cubeba. Plants-Basel 2019, 8, 150. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y. Chemical composition and antibacterial activity of essential oils from different parts of Litsea cubeba. Chem. Biodivers. 2010, 7, 229–235. [Google Scholar] [CrossRef]
- Hu, W.; Li, C.; Dai, J.; Lin, L. Antibacterial activity and mechanism of Litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA). Ind. Crops Prod. 2019, 130, 34–41. [Google Scholar] [CrossRef]
- Yang, K.; Wang, C.F.; You, C.X.; Geng, Z.F.; Sun, R.Q.; Guo, S.S.; Du, S.S.; Liu, Z.L.; Deng, Z.W. Bioactivity of essential oil of Litsea cubeba from China and its main compounds against two stored product insects. J. Asia-Pac. Entomol. 2014, 17, 459–466. [Google Scholar] [CrossRef]
- Zheng, K.W.; Li, W.; Fu, B.Q.; Ren, Q.L.; Yang, F.; Qin, C.Q. Physical, antibacterial and antioxidant properties of chitosan films containing hardleaf oatchestnut starch and Litsea cubeba oil. Int. J. Biol. Macromol. 2018, 118, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Akhtar, Y.; Bradbury, R.; Zhang, X.; Isman, M.B. Comparative toxicity of essential oils of L. pungens and Litsea cubeba and blends of their major constituents against the cabbage looper, Trichoplusia ni. J. Agric. Food Chem. 2009, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Fahim, M.; Ibrahim, M.; Zahiruddin, S.; Parveen, R.; Khan, W.; Ahmad, S.; Shrivastava, B.; Shrivastava, A.K. TLC-bioautography identification and GC-MS analysis of antimicrobial and antioxidant active compounds in Musa × paradisiaca L. fruit pulp essential oil. Phytochem. Anal. 2019, 30, 332–345. [Google Scholar] [CrossRef]
- Benchaa, S.; Hazzit, M.; Abdelkrim, H. Allelopathic effect of Eucalyptus citriodora essential oil and its potential use as bioherbicide. Chem. Biodivers. 2018, 15, e1800202. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Chemical profiling, cytotoxicity and phytotoxicity of foliar volatiles of Hyptis suaveolens. Ecotox. Environ. Safe 2019, 171, 863–870. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Method. Enzymol. 1987, 148, 350–382. [Google Scholar]
- Nayyar, H.; Gupta, D. Differential sensitivity of C3 and C4 plants to water deficit stress: Association with oxidative stress and antioxidants. Environ. Exp. Bot. 2006, 58, 106–113. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.L.; Wang, Y. Study on the chemical constituents of the essential oil from the leaves of L. pungens Hemsl. Nat. Prod. Res. 1992, 4, 20–23. [Google Scholar]
- Si, L.; Chen, Y.; Han, X.; Zhan, Z.; Tian, S.; Cui, Q.; Wang, Y. Chemical composition of essential oils of Litsea cubeba harvested from its distribution areas in China. Molecules 2012, 8, 7057. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Ho, C.L. Essential oil compositions and antimicrobial activities of various parts of Litsea cubeba from Taiwan. Nat. Prod. Commun. 2016, 11, 515–518. [Google Scholar] [CrossRef]
- Ben, K.S.; Lins, L.; Hanafi, M.; Bettaieb, R.I.; Deleu, M.; Fauconnier, M.L.; Ksouri, R.; Jijakli, M.H.; Clerck, C. Cynara cardunculus crude extract as a powerful natural herbicide and insight into the mode of action of its bioactive molecules. Biomolecules 2020, 10, 209. [Google Scholar]
- Verdeguer, M.; García-Rellán, D.; Boira, H.; Pérez, E.; Gandolfo, S.; Blazquez, M.A. Herbicidal activity of Peumus boldus and Drimys winterii essential oils from Chile. Molecules 2011, 16, 403–411. [Google Scholar] [CrossRef] [PubMed]
- El-Shora, H.M.; Abd El-Gawad, A.M. Evaluation of allelopathic potential of Rumex dentatus root extract and allelochemicals on Cicer arietinum. J. Physiol. Biochem. 2014, 10, 167–180. [Google Scholar]
- Marco, C.A.; Teixeira, E.; Simplício, A.; Oliverira, C.; Costa, J.; Feitosa, J. Chemical composition and allelopathyc activity of essential oil of Lippia sidoides cham. Chil. J. Agr. Res. 2012, 72, 157–160. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Setia, N.; Kohli, R.K.; Kaur, S.; Yaday, S.S. Alternative control of little seed canary grass using eucalypt oil. Agron Sustain. Dev. 2007, 27, 171–177. [Google Scholar] [CrossRef]
- Mohammad, P.; Mohsen, S.N.; Ali, M.; Filippo, M. Exploring the bio-control efficacy of Artemisia fragrans essential oil on the perennial weed Convolvulus arvensis: Inhibitory effects on the photosynthetic machinery and induction of oxidative stress. Ind. Crops Prod. 2020, 155, 112785. [Google Scholar]
- Poonpaiboonpipat, T.; Pangnakorn, U.; Suvunnamek, U.; Teerarak, M.; Charoenying, P.; Laosinwattana, C. Phytotoxic effects of essential oil from Cymbopogon citratus and its physiological mechanisms on barnyardgrass (Echinochloa crus-galli). Ind. Crops Prod. 2013, 41, 403–407. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kaur, S.; Ramezani, H.; Kohli, R.K. Comparative phytotoxicity of four monoterpenes against Cassia occidentalis. Ann. Appl. Biol. 2002, 141, 111–116. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Bioch. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Meloni, D.A.; Oliva, M.A.; Martinez, C.A.; Cambraia, J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot. 2003, 49, 69–76. [Google Scholar] [CrossRef]
- Mutlu, S.; Atici, O.; Nalbantoglu, B. Effects of salicylic acid and salinity on apoplastic antioxidant enzymes in two wheat cultivars differing in salt tolerance. Bio. Plantarum. 2009, 53, 334–338. [Google Scholar] [CrossRef]
- Mutlu, S.; Atici, O.; Kaya, Y. Effect of cement dust on the diversity and the antioxidant enzyme activities of plants growing around a cement factory. Fresen. Environ Bull. 2009, 18, 1823–1827. [Google Scholar]
- Mutlu, S.; Atici, O. Allelopathic effect of Nepeta meyeri Benth. extracts on seed germination and seedling growth of some crop plants. Acta Physiol. Plant. 2009, 31, 89–93. [Google Scholar] [CrossRef]
- Amiri, H. Volatile constituents and antioxidant activity of flowers, stems and leaves of Nasturtium officinale R. Br. Nat. Prod. Res. 2012, 26, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Oracz, K.; Bailly, C.; Gniazdowska, A.; Come, D.; Corbineau, F.; Bogatek, R. Induction of oxidative stress by sunflflower phytotoxins in germinating mustard seeds. J. Chem. Ecol. 2007, 33, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Turina, A.V.; Perillo, M.A. Monoterpenes affect chlorodiazepoxide–micelle interaction through micellar dipole potential modififications. BBA-Biomembranes 2003, 1616, 112–120. [Google Scholar] [CrossRef]

| NO. | Retention Time (min) | Area (%) | Identified Compounds | Type of Compounds | Retention Indices |
|---|---|---|---|---|---|
| 1 | 6.41 | 4.32 | α-Pinene | DM | 922 |
| 2 | 6.76 | 1.50 | Camphene | DM | 943 |
| 3 | 7.37 | 1.10 | Bicyclo(3,1,0)hexane,4-methylene-1-(1-methylethyl)- | DM | 964 |
| 4 | 7.44 | 3.23 | β-Pinene | DM | 967 |
| 5 | 7.69 | 4.43 | 6-methyl-5-Hepten-2-one | Ketone | 958 |
| 6 | 7.80 | 3.73 | Myrcence | CM | 979 |
| 7 | 8.75 | 30.29 | Limonene | MM | 1018 |
| 8 | 8.81 | 5.93 | Eucalyptol | DLM | 1023 |
| 9 | 10.52 | 6.38 | Linalool | CM | 1081 |
| 10 | 11.83 | 1.09 | Citronellal | CM | 1132 |
| 11 | 12.44 | 0.37 | 4-methyl-1-(1-methylethyl)-3-Cyclohexen-1-ol | MA | 1160 |
| 12 | 12.55 | 1.41 | Cis-verbenol | DLM | 1127 |
| 13 | 12.76 | 1.64 | α-Terpineol | MA | 1172 |
| 14 | 14.73 | 29.44 | neral | Cm | 1241 |
| 15 | 18.57 | 3.74 | β-caryophyllene | DS | 1424 |
| 16 | 19.23 | 0.34 | Humulene | SS | 1456 |
| 17 | 21.77 | 0.60 | Caryophyllene Oxide | BS | 1576 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, Q.; Zhou, L.; Wang, X.; Luo, S.; Li, J.; Xiao, H.; Zhang, X.; Xiang, T.; Feng, S.; Chen, T.; et al. Chemical Composition and Allelopathic Effect of Essential Oil of Litsea pungens. Agronomy 2021, 11, 1115. https://doi.org/10.3390/agronomy11061115
Kong Q, Zhou L, Wang X, Luo S, Li J, Xiao H, Zhang X, Xiang T, Feng S, Chen T, et al. Chemical Composition and Allelopathic Effect of Essential Oil of Litsea pungens. Agronomy. 2021; 11(6):1115. https://doi.org/10.3390/agronomy11061115
Chicago/Turabian StyleKong, Qingbo, Lijun Zhou, Xiaoju Wang, Siyuan Luo, Jiajia Li, Hanyong Xiao, Xinyao Zhang, Tingting Xiang, Shiling Feng, Tao Chen, and et al. 2021. "Chemical Composition and Allelopathic Effect of Essential Oil of Litsea pungens" Agronomy 11, no. 6: 1115. https://doi.org/10.3390/agronomy11061115
APA StyleKong, Q., Zhou, L., Wang, X., Luo, S., Li, J., Xiao, H., Zhang, X., Xiang, T., Feng, S., Chen, T., Yuan, M., & Ding, C. (2021). Chemical Composition and Allelopathic Effect of Essential Oil of Litsea pungens. Agronomy, 11(6), 1115. https://doi.org/10.3390/agronomy11061115









