Citrus Varieties with Different Tolerance Grades to Tristeza Virus Show Dissimilar Volatile Terpene Profiles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants
2.2. VOCs Collection and Analysis
2.2.1. VOCs Emission
2.2.2. VOCs Sequestered in Leaf Tissues
2.3. Statistical Analysis
3. Results
3.1. VOCs Profiles Emitted
3.2. VOCs Sequestered in Leaf Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kessler, A.; Baldwin, I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Llusià, J. Plant VOC emissions: Making use of the unavoidable. Trends Ecol. Evol. 2004, 19, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef]
- Bruce, T.J.; Pickett, J.A. Perception of plant volatile blends by herbivorous insects–finding the right mix. Phytochemistry 2011, 72, 1605–1611. [Google Scholar] [CrossRef]
- Krieger, J.; Breer, H. Olfactory reception in invertebrates. Science 1999, 286, 720–723. [Google Scholar] [CrossRef]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponzio, C.; Gols, R.; Pieterse, C.M.; Dicke, M. Ecological and phytohormonal aspects of plant volatile emission in response to single and dual infestations with herbivores and phytopathogens. Funct. Ecol. 2013, 27, 587–598. [Google Scholar] [CrossRef]
- Blackmer, J.L.; Rodriguez-Saona, C.; Byers, J.A.; Shope, K.L.; Smith, J.P. Behavioral response of Lygus hesperus to conspecifics and headspace volatiles of alfalfa in a Y-tube olfactometer. J. Chem. Ecol. 2004, 30, 1547–1564. [Google Scholar] [CrossRef] [Green Version]
- Guarino, S.; Peri, E.; Colazza, S. Plant and stink bug interactions at different trophic levels. In Stinkbugs: Biorational Control Based on Communication Processes; Borges, M., Cokl, A., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 180–199. [Google Scholar]
- Guarino, S.; Arif, M.A.; Millar, J.G.; Colazza, S.; Peri, E. Volatile unsaturated hydrocarbons emitted by seedlings of Brassica species provide host location cues to Bagrada hilaris. PLoS ONE 2018, 13, e0209870. [Google Scholar] [CrossRef]
- Fraenkel, G.S. The raison d’etre of secondary plant substances. Science 1959, 129, 1466–1470. [Google Scholar] [CrossRef]
- Birkett, M.A.; Campbell, C.A.; Chamberlain, K.; Guerrieri, E.; Hick, A.J.; Martin, J.L.; Matthes, M.; Napier, J.A.; Pettersson, J.; Pickett, J.A.; et al. New roles for cis-jasmone as an insect semiochemical and in plant defense. Proc. Natl. Acad. Sci. USA 2000, 97, 9329–9334. [Google Scholar] [CrossRef] [Green Version]
- Heil, M. Direct defense or ecological costs: Responses of herbivorous beetles to volatiles released by wild lima bean (Phaseolus lunatus). J. Chem. Ecol. 2004, 30, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A.; Schaller, A. Direct defenses in plants and their induction by wounding and insect herbivores. In Induced Plant Resistance to Herbivory; Schaller, A., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 7–29. [Google Scholar]
- Kogan, M.; Ortman, E.F. Antixenosis–a new term proposed to define Painter’s “nonpreference” modality of resistance. ESA Bull. 1978, 24, 175–176. [Google Scholar] [CrossRef] [Green Version]
- Core, R.J.; Henning, J.A.; Gardea-Torresdey, J. Volatile compounds from Medicago spp. as potential signals for alfalfa weevil response. J. Agric. Food Chem. 1994, 42, 2932–2936. [Google Scholar] [CrossRef]
- Colazza, S.; Fucarino, A.; Peri, E.; Salerno, G.; Conti, E.; Bin, F. Insect oviposition induces volatile emission in herbaceous plants that attracts egg parasitoids. J. Exp. Biol. 2004, 207, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Degenhardt, J. Indirect defense responses to herbivory in grasses. Plant Physiol. 2009, 149, 96–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frati, F.; Cusumano, A.; Conti, E.; Colazza, S.; Peri, E.; Guarino, S.; Martorana, L.; Romani, R.; Salerno, G. Foraging behaviour of an egg parasitoid exploiting plant volatiles induced by pentatomids: The role of adaxial and abaxial leaf surfaces. PeerJ 2017, 5, e3326. [Google Scholar] [CrossRef]
- Rodríguez, A.; San Andrés, V.; Cervera, M.; Redondo, A.; Alquézar, B.; Shimada, T.; Gadea, J.; Rodrigo, M.J.; Zacarías, L.; Palou, L.; et al. Terpene down-regulation in orange reveals the role of fruit aromas in mediating interactions with insect herbivores and pathogens. Plant Physiol. 2011, 156, 793–802. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, A.; Shimada, T.; Cervera, M.; Alquézar, B.; Gadea, J.; Gómez-Cadenas, A.; De Ollas, C.J.; Rodrigo, M.J.; Zacarías, L.; Peña, L. Terpene down-regulation triggers defense responses in transgenic orange leading to resistance against fungal pathogens. Plant Physiol. 2014, 164, 321–339. [Google Scholar] [CrossRef] [Green Version]
- Jan, P.; Andres, Q.; Elham, F.A. Aphid antixenosis mediated by volatiles in cereals. Acta Agric. Scand. B Soil Plant Sci. 1996, 46, 135–140. [Google Scholar] [CrossRef]
- Le Roux, V.; Dugravot, S.; Brunissen, L.; Vincent, C.; Pelletier, Y.; Giordanengo, P. Antixenosis phloem-based resistance to aphids: Is it the rule? Ecol. Entomol. 2010, 35, 407–416. [Google Scholar] [CrossRef]
- Mohammed, K.; Agarwal, M.; Li, B.; Newman, J.; Liu, T.; Ren, Y. Evaluation of d-limonene and β-ocimene as attractants of Aphytis melinus (Hymenoptera: Aphelinidae), a parasitoid of Aonidiella aurantii (Hemiptera: Diaspididae) on Citrus spp. Insects 2020, 11, 44. [Google Scholar] [CrossRef] [Green Version]
- Hijaz, F.; Nehela, Y.; Killiny, N. Possible role of plant volatiles in tolerance against huanglongbing in citrus. Plant Signal. Behav. 2016, 11, e1138193. [Google Scholar] [CrossRef] [Green Version]
- Sylvester, E.S. Circulative and propagative virus transmission by aphids. Ann. Rev. Entomol. 1980, 25, 257–286. [Google Scholar] [CrossRef]
- Ragsdale, D.W.; McCornack, B.P.; Venette, R.C.; Potter, B.D.; MacRae, I.V.; Hodgson, E.W.; O’Neal, M.E.; Johnson, K.D.; O’neil, R.J.; DiFonzo, C.D.; et al. Economic threshold for soybean aphid (Hemiptera: Aphididae). J. Econ. Entomol. 2007, 100, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.; Ambrós, S.; Albiach-Martí, M.R.; Guerri, J.; Pena, L. Citrus tristeza virus: A pathogen that changed the course of the citrus industry. Mol. Plant Pathol. 2008, 9, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Castle, W.S.; Nunnallee, J.; Manthey, J.A. Screening citrus rootstocks and related selections in soil and solution culture for tolerance to low-iron stress. Hort. Sci. 2009, 44, 638–645. [Google Scholar] [CrossRef] [Green Version]
- Forner-Giner, M.A.; Continella, A.; Grosser, J.W. Citrus Rootstock Breeding and Selection. In The Citrus Genome. Compendium of Plant Genomes; Gentile, A., La Malfa, S., Deng, Z., Eds.; Springer: Cham, Switzerland, 2020; pp. 49–74. [Google Scholar]
- Bar-Joseph, M.; Lee, R. Citrus tristeza virus. AAB Descr. Plant Viruses 1989, 353, 1–7. [Google Scholar]
- Laino, P.; Russo, M.P.; Guardo, M.; Reforgiato-Recupero, G.; Valè, G.; Cattivelli, L.; Moliterni, V.M. Rootstock–scion interaction affecting citrus response to CTV infection: A proteomic view. Physiol. Plant. 2016, 156, 444–467. [Google Scholar] [CrossRef] [PubMed]
- Dambier, D.; Benyahia, H.; Pensabene-Bellavia, G. Somatic hybridization for citrus rootstock breeding: An effective tool to solve some important issues of the Mediterranean citrus industry. Plant Cell Rep. 2011, 30, 883–900. [Google Scholar] [CrossRef]
- Abbate, L.; Panno, S.; Mercati, F.; Davino, S.; Fatta Del Bosco, S. Citrus rootstock breeding: Response of four allotetraploid somatic hybrids to Citrus tristeza virus induced infections. Eur. J. Plant Pathol. 2019, 153, 837–847. [Google Scholar] [CrossRef]
- Roose, M.L. Choosing a Rootstock. In Citrus Production Manual; Ferguson, L., Grafton-Carwell, E.E., Eds.; University of California: California, CA, USA, 2014; Volume 3539, p. 95. [Google Scholar]
- Salibe, A.A.; Cereda, E. Limitations on the use of Volkamer Lemon as rootstock for Citrus. In Proceedings of the International Organization of Citrus Virologists Conference Proceedings, (1957–2010); University of California: California, CA, USA, 1984; Volume 9. [Google Scholar]
- Emmanouilidou, M.G.; Kyriacou, M.C. Rootstock-modulated yield performance, fruit maturation and phytochemical quality of ‘Lane Late’and ‘Delta’sweet orange. Sci. Hortic. 2017, 225, 112–121. [Google Scholar] [CrossRef]
- Castle, W.S. Rootstock as a fruit quality factor in citrus and deciduous tree crops. N. Z. J. Crop Hort. Sci. 1995, 23, 383–394. [Google Scholar] [CrossRef]
- Castle, W.S. A career perspective on citrus rootstocks, their development, and commercialization. Hort. Sci. 2010, 45, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Forner, J.B.; Forner-Giner, M.A.; Alcaide, A. Forner-Alcaide 5 and Forner-Alcaide 13: Two new citrus rootstocks released in Spain. Hort. Sci. 2003, 38, 629–630. [Google Scholar] [CrossRef] [Green Version]
- Forner-Giner, M.A.; Primo-Millo, E.; Forner, J.B. Performance of Forner-Alcaide 5 and Forner-Alcaide 13, hybrids of Cleopatra mandarin x Poncirus trifoliate, as salinity-tolerant citrus rootstocks. J. Am. Pomol. Soc. 2009, 63, 72. [Google Scholar]
- Niederbacher, B.; Winkler, J.B.; Schnitzler, J.P. Volatile organic compounds as non-invasive markers for plant phenotyping. J. Exp. Bot. 2015, 66, 5403–5416. [Google Scholar] [CrossRef] [PubMed]
- Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M.; Hardie, J. The chemical ecology of aphids. Ann. Rev. Entomol. 1992, 37, 67–90. [Google Scholar] [CrossRef]
- Le Roux, V.; Dugravot, S.; Campan, E.; Dubois, F.; Vincent, C.; Giordanengo, P. Wild Solanum resistance to aphids: Antixenosis or antibiosis? J. Econ. Entomol. 2014, 101, 584–591. [Google Scholar] [CrossRef]
- Da Costa, J.G.; Pires, E.V.; Riffel, A.; Birkett, M.A.; Bleicher, E.; Sant’Ana, A.E.G. Differential preference of Capsicum spp. cultivars by Aphis gossypii is conferred by variation in volatile semiochemistry. Euphytica 2011, 177, 299–307. [Google Scholar] [CrossRef]
- Huang, M.; Sanchez-Moreiras, A.M.; Abel, C.; Sohrabi, R.; Lee, S.; Gershenzon, J.; Tholl, D. The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-β-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 2012, 193, 997–1008. [Google Scholar] [CrossRef]
- Boncan, D.A.T.; Tsang, S.S.; Li, C.; Lee, I.H.; Lam, H.M.; Chan, T.F.; Hui, J.H. Terpenes and terpenoids in plants: Interactions with environment and insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef]
- Bansal, A.; Jan, I.; Sharma, N.R. Anti-phytoviral activity of carvacrol vis-a-vis cauliflower mosaic virus (CaMV). Proc. Natl. Acad. Sci. India Sect. B Boil. Sci. 2020, 90, 981–988. [Google Scholar] [CrossRef]
- Isman, M.B.; Tak, J.H. Commercialization of insecticides based on plant essential oils: Past, present and future. In Green Pesticides Handbook: Essential Oils for Pest Control; CRC Press: Boca Raton, FL, USA, 2017; pp. 27–39. [Google Scholar]
- Zhou, F.; Pichersky, E. More is better: The diversity of terpene metabolism in plants. Curr. Opin. Plant Biol. 2020, 55, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pawliszyn, J. Solid Phase Microextraction: Theory and Practice; John Wiley & Sons: New York, NY, USA, 1997. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Avé, D.A.; Gregory, P.; Tingey, W.M. Aphid repellent sesquiterpenes in glandular trichomes of Solanum berthaultii and S. tuberosum. Entomol. Exp. Appl. 1987, 44, 131–138. [Google Scholar] [CrossRef]
- Wang, F.; Park, Y.L.; Gutensohn, M. Glandular trichome-derived sesquiterpenes of wild tomato accessions (Solanum habrochaites) affect aphid performance and feeding behavior. Phytochemistry 2020, 180, 112532. [Google Scholar] [CrossRef]
- Alquézar, B.; Volpe, H.X.L.; Magnani, R.F.; de Miranda, M.P.; Santos, M.A.; Wulff, N.A.; Bento, J.M.S.; Parra, J.R.P.; Peña, L. β-caryophyllene emitted from a transgenic Arabidopsis or chemical dispenser repels Diaphorina citri, vector of Candidatus Liberibacters. Sci. Rep. 2017, 7, 5639. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xue, M.; Zhang, Q.; Zhou, F.; Wei, J. Toxicity of β-caryophyllene from Vitex negundo (Lamiales: Verbenaceae) to Aphis gossypii Glover (Homoptera: Aphididae) and its action mechanism. Acta Entomol. Sin. 2010, 53, 396–404. [Google Scholar]
- Bruce, T.J.; Birkett, M.A.; Blande, J.; Hooper, A.M.; Martin, J.L.; Khambay, B.; Prosser, I.; Smart, L.E.; Wadhams, L.J. Response of economically important aphids to components of Hemizygia petiolata essential oil. Pest Manag. Sci. 2005, 61, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Park, Y.L.; Gutensohn, M. Glandular trichome-derived mono-and sesquiterpenes of tomato have contrasting roles in the interaction with the potato aphid Macrosiphum euphorbiae. J. Chem. Ecol. 2021, 47, 204–214. [Google Scholar] [CrossRef]
- Kasal-Slavik, T.; Eschweiler, J.; Kleist, E.; Mumm, R.; Goldbach, H.E.; Schouten, A.; Wildt, J. Early biotic stress detection in tomato (Solanum lycopersicum) by BVOC emissions. Phytochemistry 2017, 144, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Kivimäenpää, M.; Babalola, A.B.; Joutsensaari, J.; Holopainen, J.K. Methyl salicylate and sesquiterpene emissions are indicative for aphid infestation on scots pine. Forests 2020, 11, 573. [Google Scholar] [CrossRef]
- Killiny, N.; Valim, M.F.; Jones, S.E.; Omar, A.A.; Hijaz, F.; Gmitter, F.G.; Grosser, J.W. Metabolically speaking: Possible reasons behind the tolerance of ‘Sugar Belle’ mandarin hybrid to huanglongbing. Plant Physiol. Bioch. 2017, 116, 36–47. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Tee, C.S.; Jiang, Y.L.; Jiang, X.Y.; Venkatesh, P.N.; Sarojam, R.; Ye, J. A terpenoid phytoalexin plays a role in basal defense of Nicotiana benthamiana against Potato virus X. Sci. Rep. 2015, 5, 9682. [Google Scholar] [CrossRef] [Green Version]
- Bezić, N.; Vuko, E.; Dunkić, V.; Ruščić, M.; Blažević, I.; Burčul, F. Antiphytoviral activity of sesquiterpene-rich essential oils from four Croatian Teucrium species. Molecules 2011, 16, 8119–8129. [Google Scholar] [CrossRef] [Green Version]
- Meldau, S.; Wu, J.; Baldwin, I.T. Silencing two herbivory-activated MAP kinases, SIPK and WIPK, does not increase Nicotiana attenuata’s susceptibility to herbivores in the glasshouse and in nature. New Phytol. 2009, 181, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, S.; Miyoshi, S.; Tamaoki, D.; Yamada, S.; Tanaka, K.; Uji, Y.; Tanaka, S.; Akimitsu, K.; Gomi, K. Isolation of jasmonate-induced sesquiterpene synthase of rice: Product of which has an antifungal activity against Magnaporthe oryzae. J. Plant Physiol. 2014, 171, 625–632. [Google Scholar] [CrossRef]
- Asano, T.; Tamura, Y.; Yasui, H.; Satoh, K.; Hattori, M.; Yasui, H.; Kikuchi, S. The rice GRH2 and GRH4 activate various defense responses to the green rice leafhopper and confer strong insect resistance. Plant Biotechnol. 2018, 32, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Huffaker, A.; Kaplan, F.; Vaughan, M.M.; Dafoe, N.J.; Ni, X.; Rocca, J.R.; Alborn, H.T.; Teal, P.E.; Schmelz, E.A. Novel acidic sesquiterpenoids constitute a dominant class of pathogen- induced phytoalexins in maize. Plant Physiol. 2011, 156, 2082–2097. [Google Scholar] [CrossRef] [Green Version]
- van der Linde, K.; Kastner, C.; Kumlehn, J.; Kahmann, R.; Doehlemann, G. Systemic virus-induced gene silencing allows functional characterization of maize genes during biotrophic interaction with Ustilago maydis. New Phytol. 2011, 189, 471–483. [Google Scholar] [CrossRef]
- Hare, J.D. Ecological role of volatiles produced by plants in response to damage by herbivorous insects. Annu. Rev. Entomol. 2010, 56, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Enan, E. Insecticidal activity of essential oils: Octopaminergic sites of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 130, 325–337. [Google Scholar] [CrossRef]
- Jung, W.C.; Jang, Y.S.; Hieu, T.T.; Lee, C.K.; Ahn, Y.J. Toxicity of Myristica fragrans seed compounds against Blattella germanica (Dictyoptera: Blattellidae). J. Med. Entomol. 2007, 44, 524–529. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Li, J.; Dong, Y.; Hao, H.; Ling, Z.; Bai, H.; Wang, H.; Cui, H.; Shi, L. Time-series transcriptome provides insights into the gene regulation network involved in the volatile terpenoid metabolism during the flower development of lavender. BMC Plant Biol. 2019, 19, 313. [Google Scholar] [CrossRef] [Green Version]
- Ebadollahi, A.; Ziaee, M.; Palla, F. Essential Oils Extracted from Different Species of the Lamiaceae Plant Family as Prospective Bioagents against Several Detrimental Pests. Molecules 2020, 25, 1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelgaleil, S.A.M.; Al-Nagar, N.M.A.; Abou-Taleb, H.K.; Shawir, M.S. Effect of monoterpenes, phenylpropenes and sesquiterpenes on development, fecundity and fertility of Spodoptera littoralis (Boisduval). Int. J. Trop. Insect. Sci. 2021, 1–9. [Google Scholar] [CrossRef]
- Fouad, A.H.; de Souza Tavares, W.; Zanuncio, C.J. Toxicity and repellent activity of monoterpene enantiomers to rice weevils (Sitophilus oryzae). Pest Manag. Sci. 2021, 1–8. [Google Scholar] [CrossRef]
- Hori, M. Repellency of rosemary oil against Myzus persicae in a laboratory and in a screenhouse. J. Chem. Ecol. 1998, 24, 1425–1432. [Google Scholar] [CrossRef]
- Mitra, S.; Karmakar, A.; Mukherjee, A.; Barik, A. The Role of Leaf Volatiles of Ludwigia octovalvis (Jacq.) Raven in the Attraction of Altica cyanea (Weber) (Coleoptera: Chrysomelidae). J. Chem. Ecol. 2017, 43, 679–692. [Google Scholar] [CrossRef]
- Naidoo, S.; Christie, N.; Acosta, J.J.; Mphahlele, M.M.; Payn, K.G.; Myburg, A.A.; Külheim, C. Terpenes associated with resistance against the gall wasp, Leptocybe invasa, in Eucalyptus grandis. Plant Cell Environ. 2018, 41, 1840–1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.; Han, Z.; Xu, Y.; Yao, L. In vitro and in vivo anti-tobacco mosaic virus activities of essential oils and individual compounds. J. Microbiol. Biotechnol. 2013, 23, 771–778. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.S.; Song, H.S.; Ukeda, H.; Sawamura, M. Radical-scavenging activities of citrus essential oils and their components: Detection using 1, 1-diphenyl-2-picrylhydrazyl. J. Agric. Food Chem. 2000, 48, 4156–4161. [Google Scholar] [CrossRef]
- Sarrou, E.; Chatzopoulou, P.; Dimassi-Theriou, K.; Therios, I. Volatile constituents and antioxidant activity of peel, flowers and leaf oils of Citrus aurantium L. growing in Greece. Molecules 2013, 18, 10639–10647. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.S.; Yang, Y.C.; Choi, D.S.; Ahn, Y.J. Vapor phase toxicity of marjoram oil compounds and their related monoterpenoids to Blattella germanica (Orthoptera: Blattellidae). J. Agric. Food Chem. 2005, 53, 7892–7898. [Google Scholar] [CrossRef]
- Wang, X.X.; Zhang, Y.; Zhang, Z.F.; Tian, H.G.; Liu, T.X. Deciphering the function of octopaminergic signaling on wing polyphenism of the pea aphid Acyrthosiphon pisum. Front. Physiol. 2016, 7, 603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hijaz, F.; Killiny, N. Collection and chemical composition of phloem sap from Citrus sinensis L. Osbeck (sweet orange). PLoS ONE 2014, 9, e101830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino Acid biosynthesis in plants. Ann. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Shao, J.; Zhou, C.; Hartung, J.S. Transcriptome analysis of sweet orange trees infected with ‘Candidatus Liberibacter asiaticus’ and two strains of Citrus Tristeza Virus. BMC Genom. 2016, 17, 349. [Google Scholar] [CrossRef] [Green Version]
- Killiny, N.; Hijaz, F.; Harper, S.J.; Dawson, W.O. Effects of Citrus tristeza closterovirus infection on phloem sap and released volatile organic compounds in Citrus macrophylla. Physiol. Mol. Plant Pathol. 2017, 98, 25–36. [Google Scholar] [CrossRef]
- Albrecht, U.; Fiehn, O.; Bowman, K.D. Metabolic variations in different citrus rootstock cultivars associated with different responses to Huanglongbing. Plant Physiol. Biochem. 2016, 107, 33–44. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Coutinho, T.A.; Gershenzon, J. Roles of plant volatiles in defence against microbial pathogens and microbial exploitation of volatiles. Plant Cell Environ. 2019, 42, 2827–2843. [Google Scholar] [CrossRef] [Green Version]
- Peter, G.F. Breeding and Engineering Trees to Accumulate High Levels of Terpene Metabolites for Plant Defense and Renewable Chemicals. Front. Plant Sci. 2018, 9, 1672. [Google Scholar] [CrossRef]
- Langenheim, J.H. Higher plant terpenoids: A phytocentric overview of their ecological roles. J. Chem. Ecol. 1994, 20, 1223–1280. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Krokene, P.; Christiansen, E.; Krekling, T. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol. 2005, 167, 353–375. [Google Scholar] [CrossRef] [PubMed]
- Madmony, A.; Tognetti, R.; Zamponi, L.; Capretti, P.; Michelozzi, M. Monoterpene responses to interacting effects of drought stress and infection by the fungus Heterobasidion parviporum in two clones of Norway spruce (Picea abies). Environ. Exp. Bot. 2018, 152, 137–148. [Google Scholar] [CrossRef]
- Cascone, P.; Iodice, L.; Maffei, M.E.; Bossi, S.; Arimura, G.I.; Guerrieri, E. Tobacco overexpressing β-ocimene induces direct and indirect responses against aphids in receiver tomato plants. J. Plant Physiol. 2015, 173, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Guler, Z.; Karaca, F.; Yetisir, H. Volatile compounds in the peel and flesh of cucumber (Cucumis sativus L.) grafted onto bottle gourd (Lagenaria siceraria) rootstocks. J. Hortic. Sci. Biotechnol. 2013, 88, 123–128. [Google Scholar] [CrossRef]
- Guler, Z.; Candir, E.; Yetisir, H.; Karaca, F.; Solmaz, I. Volatile organic compounds in watermelon (Citrullus lanatus) grafted onto 21 local and two commercial bottle gourd (Lagenaria siceraria) rootstocks. J. Hortic. Sci. Biotechnol. 2014, 89, 448–452. [Google Scholar] [CrossRef]
- Albrecht, U.; Tripathi, I.; Bowman, K.D. Rootstock influences the metabolic response to Candidatus Liberibacter asiaticus in grafted sweet orange trees. Trees 2020, 34, 405–431. [Google Scholar] [CrossRef]

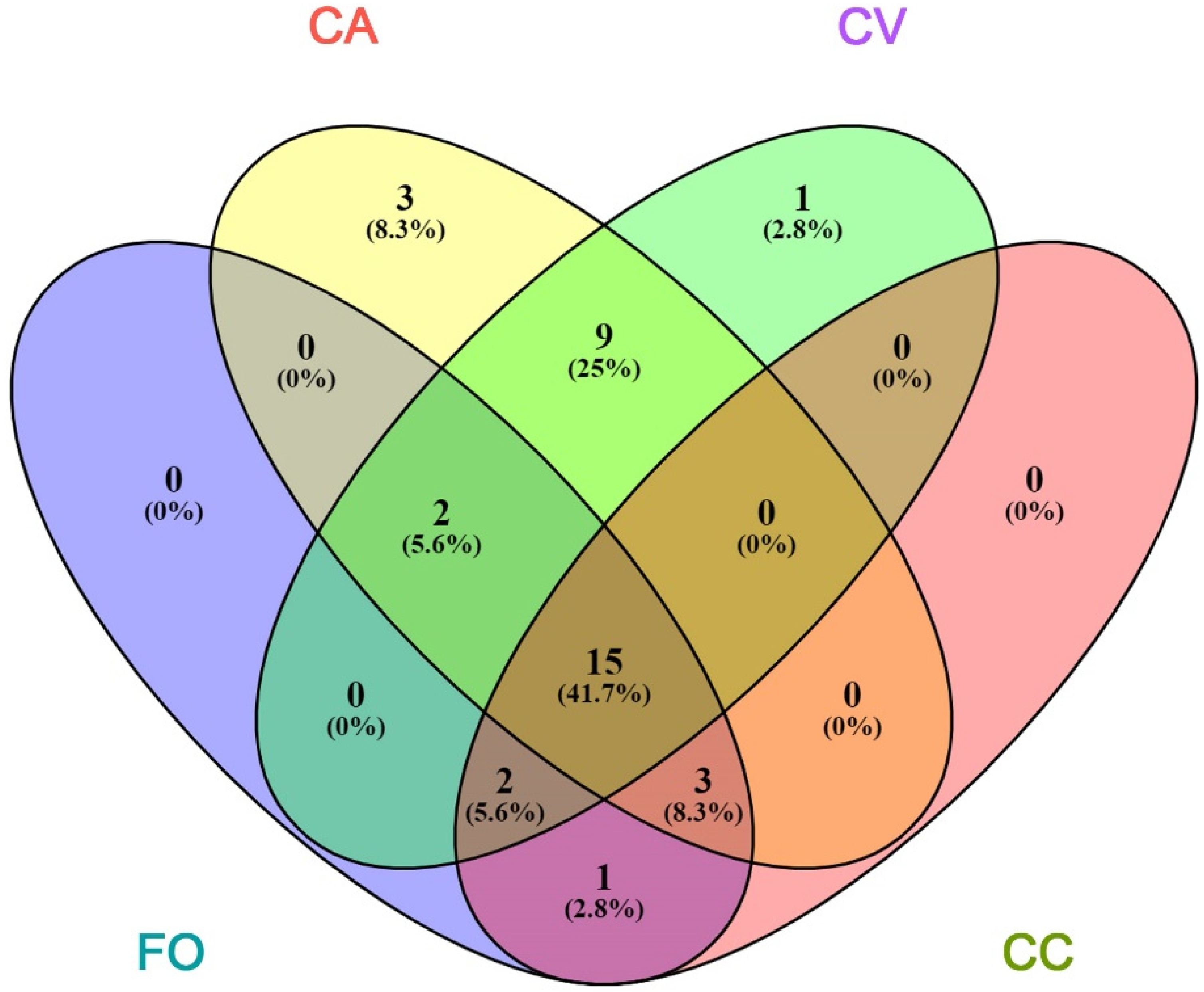
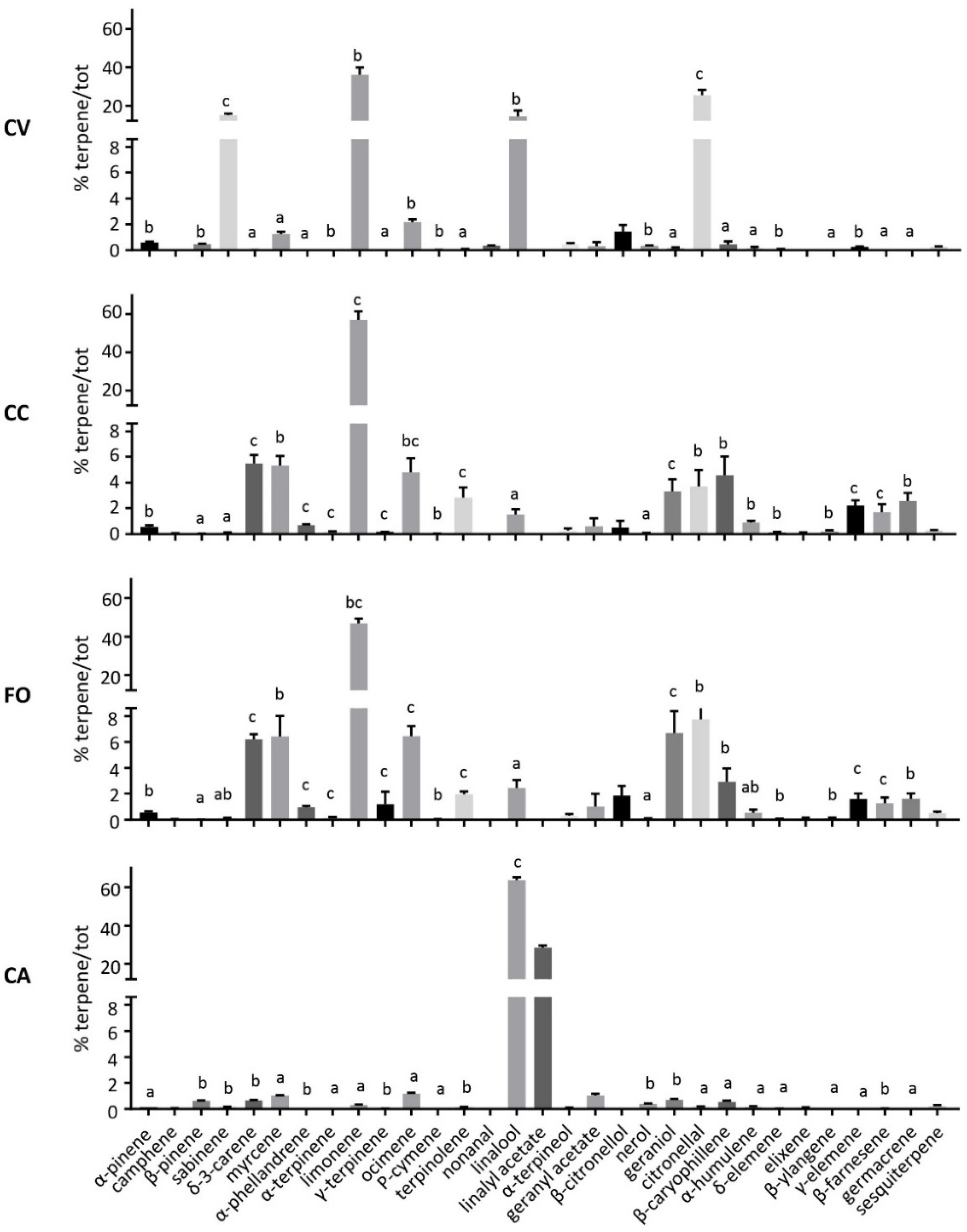
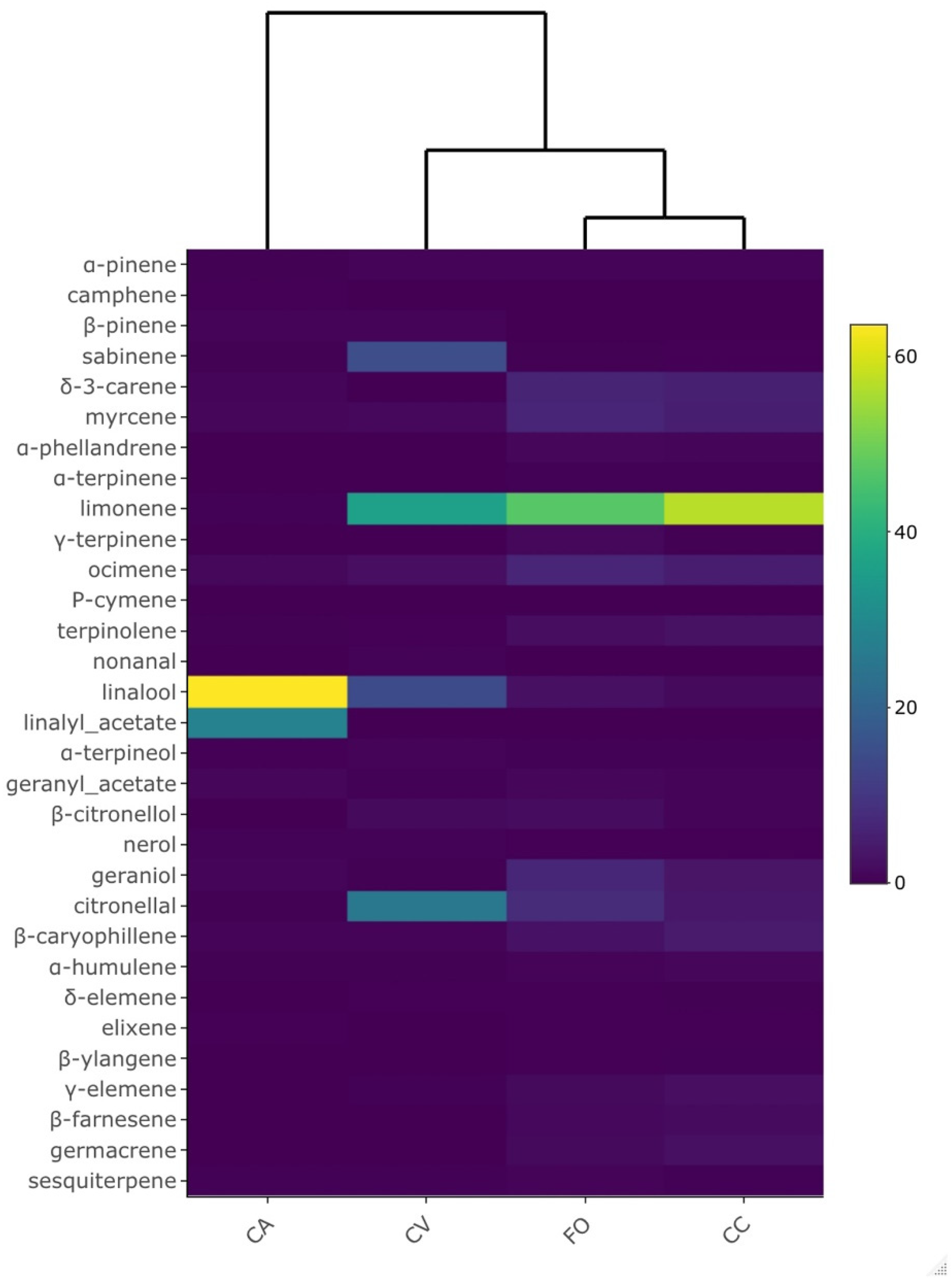
| Peak | RT | LRI | Compound | Group | FO | CA | CV | CC |
|---|---|---|---|---|---|---|---|---|
| 1 | 9.357 | 925 | α-pinene * | Mt. hd. | 0 | 0.11 ± 0.03 | 0.92 ± 0.16 | 0 |
| 2 | 10.311 | 968 | sabinene | Mt. hd. | 0 | 0.22 ± 0.09 | 19.99 ± 1.94 | 0 |
| 3 | 10.416 | 973 | β-pinene | Mt. hd. | 0 | 2.57 ± 1.03 | 0 | 0 |
| 4 | 10.681 | 985 | myrcene * | Mt. hd. | 1.97 ± 2.38 | 4.46 ± 2.38 | 2.93 ± 0.88 | 2.52 ± 0.91 |
| 5 | 11.073 | 1003 | δ-3-carene | Mt. hd. | 2.13 ± 0.33 | 1.68 ± 0.33 | 0 | 4.18 ± 0.75 |
| 6 | 11.499 | 1026 | limonene * | Mt. hd. | 16.42 ± 7.07 | 2.83 ± 0.97 | 49.21 ± 3.51 | 40.01 ± 5.88 |
| 7 | 11.613 | 1032 | cis-β-ocimene | Mt. hd. | 0 | 1.25 ± 0.09 | 0 | 0 |
| 8 | 11.824 | 1044 | trans-β-ocimene | Mt. hd. | 1.12 ± 0.60 | 3.82 ± 0.23 | 2.76 ± 0.24 | 1.79 ± 0.54 |
| 9 | 12.065 | 1057 | γ-terpinene | Mt. hd. | 0 | 0.02 ± 0.00 | 0.25 ± 0.08 | 0 |
| 10 | 12.291 | 1069 | sabinene hydrate | Mt. est. | 0 | 0.06 ± 0.01 | 0.50 ± 0.19 | 0 |
| 11 | 12.562 | 1084 | α-terpinolene * | Mt. hd. | 0.14 ± 0.16 | 0.33 ± 0.02 | 0 | 0.38 ± 0.55 |
| 12 | 12.876 | 1101 | linalool * | Mt. alc. | 0 | 30.16 ± 3.05 | 6.60 ± 1.77 | 0 |
| 13 | 13.281 | 1127 | allo-ocimene | Mt. hd. | 0 | 0.69 ± 0.12 | 0.27 ± 0.09 | 0 |
| 14 | 13.681 | 1151 | citronellal | Mt. ald. | 0.60 ± 0.93 | 0.18 ± 0.02 | 6.06 ± 1.06 | 0.94 v 0.88 |
| 15 | 14.124 | 1178 | terpin 4-ol | Mt. alc. | 0 | 0.30 ± 0.04 | 0.13 v 0.05 | 0 |
| 16 | 14.421 | 1197 | α-terpineol * | Mt. alc. | 0.04 ± 0.07 | 0.11 ± 0.01 | 0.53 ± 0.22 | 0 |
| 17 | 14.821 | 1224 | citronellol * | Mt. alc. | 0.08 ± 0.15 | 0.13 ± 0.05 | 0.13 ± 0.08 | 0.02 ± 0.02 |
| 18 | 15.047 | 1239 | neral | Mt. ald. | 0 | 2.33 ± 0.34 | 0.72 ± 0.41 | 0 |
| 19 | 15.225 | 1251 | linalyl acetate | Mt. est. | 0 | 38.05 ± 5.20 | 0 | 0 |
| 20 | 15.487 | 1268 | citral * | Mt. ald. | 0.12 ± 0.23 | 3.14 ± 0.64 | 0.53 ± 0.33 | 0 |
| 21 | 16.035 | 1306 | undecanal | Aldehyde | 0 | 0 | 0.05 ± 0.03 | 0 |
| 22 | 16.458 | 1336 | bicycloelemene | Sqt. hd. | 1.36 ± 0.43 | 0.16 ± 0.04 | 0.55 ± 0.21 | 0.64 ± 0.24 |
| 23 | 16.493 | 1339 | δ-elemene | Sqt. hd. | 1.99 ± 0.33 | 0.18 0.04 | 0.88 ± 0.27 | 0.99 ± 0.23 |
| 24 | 16.716 | 1355 | neryl acetate | Sqt. est. | 0 | 1.11 ± 0.37 | 0.11 ± 0.06 | 0 |
| 25 | 16.989 | 1375 | geranyl acetate | Mt. est. | 1.88 ± 1.57 | 2.63 ± 0.50 | 0.15 ± 0.14 | 0.71 ± 0.07 |
| 26 | 17.080 | 1382 | α-copaene | Sqt. hd. | 0.22 ± 0.14 | 1.25 ± 0.52 | 0 | 0.07 ± 0.06 |
| 27 | 17.241 | 1393 | β-elemene | Sqt. hd. | 8.37 ± 1.36 | 0 | 0 | 4.47 ± 0.83 |
| 28 | 17.716 | 1429 | trans-β-caryophyllene * | Sqt. hd. | 30.40 ± 4.73 | 1.40 ± 0.50 | 2.96 ± 0.84 | 23.65 ± 2.69 |
| 29 | 17.764 | 1433 | γ-elemene | Sqt. hd. | 6.55 ± 0.97 | 0.04 ± 0.01 | 0.23 ± 0.19 | 2.48 ± 1.07 |
| 30 | 17.802 | 1436 | trans-α-bergamotene | Sqt. hd. | 1.95 ± 0.189 | 0 | 0.88 ± 0.24 | 1.31 ± 0.21 |
| 31 | 17.988 | 1450 | cis-β-farnesene * | Sqt. hd. | 9.16 ± 0.93 | 0.29 ± 0.11 | 0.04 v 0.04 | 7.49 ± 1.69 |
| 32 | 18.177 | 1466 | humulene | Sqt. hd. | 2.42 ± 0.61 | 0.12 ± 0.02 | 0.17 v 0.10 | 2.89 ± 1.39 |
| 33 | 18.490 | 1490 | germacrene D | Sqt. hd. | 10.17 ± 2.56 | 0.11 ± 0.02 | 0.37 v 0.11 | 4.35 ± 1.34 |
| 34 | 18.672 | 1504 | bicyclogermacrene | Sqt. hd. | 2.02 ± 0.66 | 0.23 ± 0.03 | 0.63 ± 0.11 | 0.88 ± 0.23 |
| 35 | 18.736 | 1509 | β-bisabolene * | Sqt. hd. | 0.76 ± 0.25 | 0 | 0.61 v 0.16 | 0.19 ± 0.10 |
| 36 | 18.903 | 1523 | δ-cadinene | Sqt. hd. | 0.12 ± 0.08 | 0.01 ± 0.00 | 0.06 ± 0.00 | 0.03 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarino, S.; Abbate, L.; Mercati, F.; Fatta Del Bosco, S.; Motisi, A.; Arif, M.A.; Cencetti, G.; Palagano, E.; Michelozzi, M. Citrus Varieties with Different Tolerance Grades to Tristeza Virus Show Dissimilar Volatile Terpene Profiles. Agronomy 2021, 11, 1120. https://doi.org/10.3390/agronomy11061120
Guarino S, Abbate L, Mercati F, Fatta Del Bosco S, Motisi A, Arif MA, Cencetti G, Palagano E, Michelozzi M. Citrus Varieties with Different Tolerance Grades to Tristeza Virus Show Dissimilar Volatile Terpene Profiles. Agronomy. 2021; 11(6):1120. https://doi.org/10.3390/agronomy11061120
Chicago/Turabian StyleGuarino, Salvatore, Loredana Abbate, Francesco Mercati, Sergio Fatta Del Bosco, Antonio Motisi, Mokhtar Abdulsattar Arif, Gabriele Cencetti, Eleonora Palagano, and Marco Michelozzi. 2021. "Citrus Varieties with Different Tolerance Grades to Tristeza Virus Show Dissimilar Volatile Terpene Profiles" Agronomy 11, no. 6: 1120. https://doi.org/10.3390/agronomy11061120
APA StyleGuarino, S., Abbate, L., Mercati, F., Fatta Del Bosco, S., Motisi, A., Arif, M. A., Cencetti, G., Palagano, E., & Michelozzi, M. (2021). Citrus Varieties with Different Tolerance Grades to Tristeza Virus Show Dissimilar Volatile Terpene Profiles. Agronomy, 11(6), 1120. https://doi.org/10.3390/agronomy11061120










