Diversity of Leaf Stomatal Traits among Coffea canephora Pierre ex A. Froehner Genotypes
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material and Field Characterization
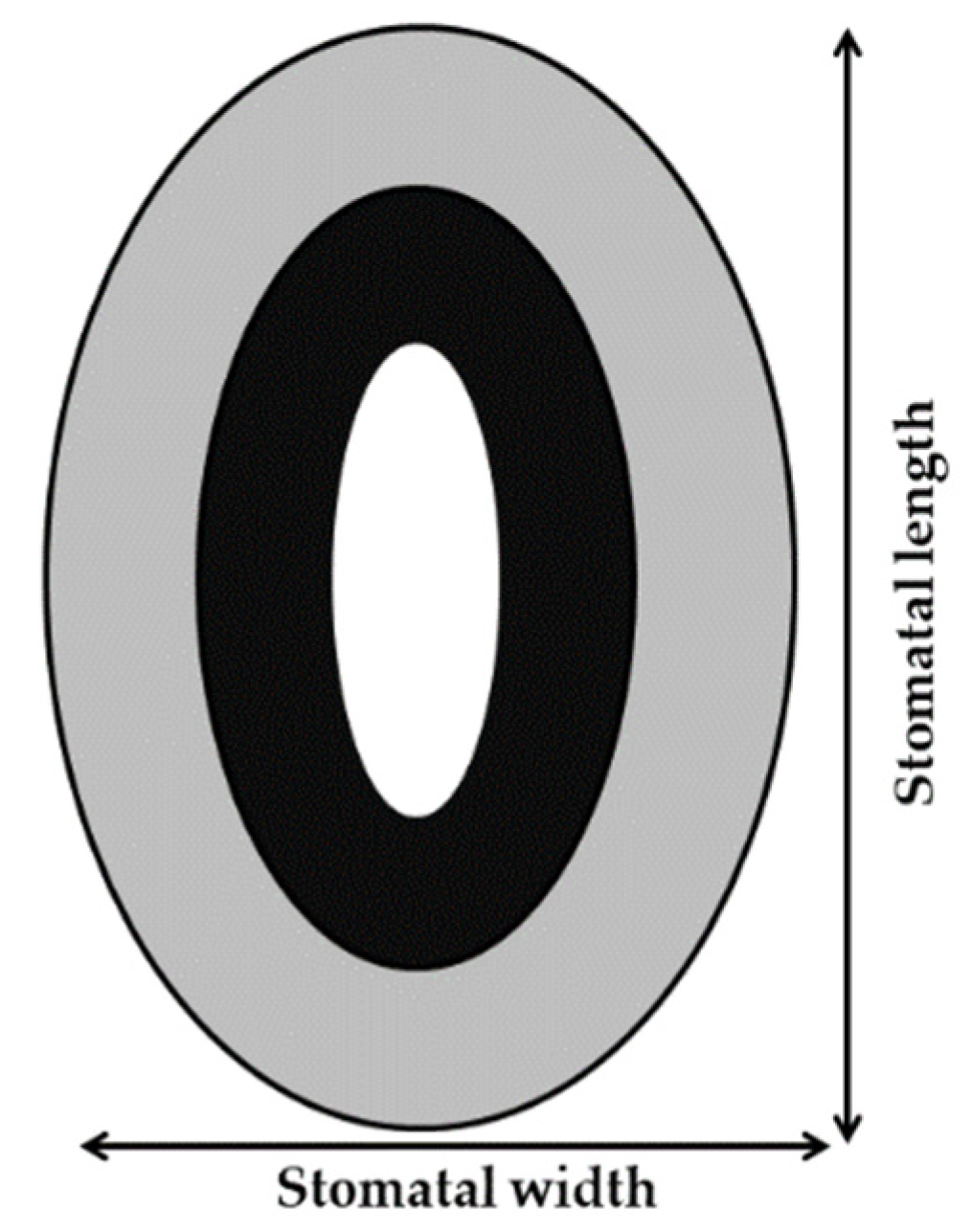
2.2. Leaf Morphological and Anatomical Traits Determination
2.3. Statistical Data Analysis
3. Results
3.1. Morpho-Anatomical Characterization
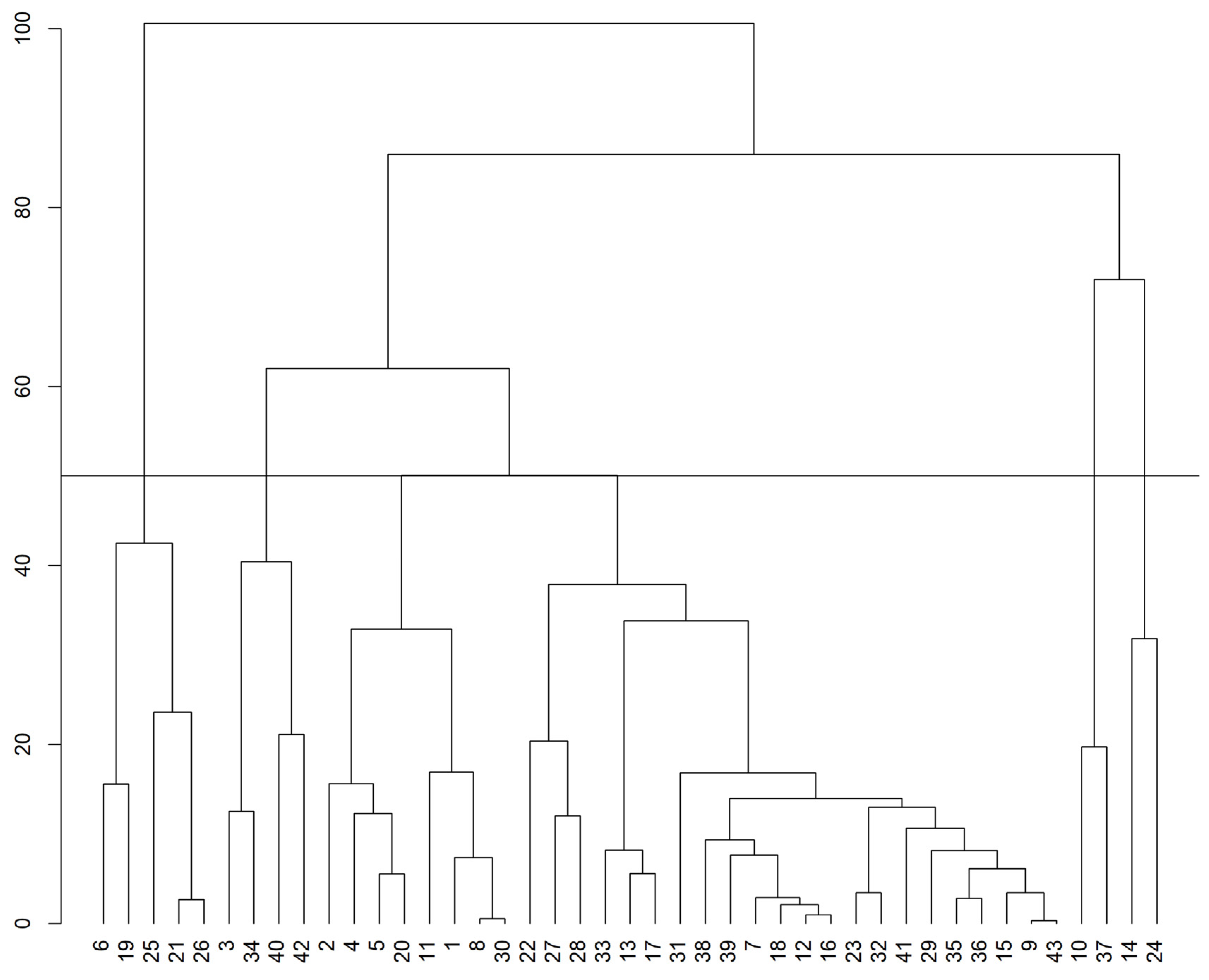
3.2. Dissimilarity among Genotypes
3.3. Correlation Studies of Biometric Variables
4. Discussion
4.1. Morpho-Anatomical Characterization
4.2. Dissimilarity between Genotypes
4.3. Correlation Studies of Biometric Variables
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, R.; Chen, J.M.; Liu, Z.; Arain, A. Evaluation of seasonal variations of remotely sensed leaf area index over five evergreen coniferous forests. ISPRS J. Photogramm. Remote Sens. 2017, 130, 187–201. [Google Scholar] [CrossRef]
- Liu, H.; Taylor, S.H.; Xu, Q.; Lin, Y.; Hou, H.; Wu, G.; Ye, Q. Life history is a key factor explaining functional trait diversity among subtropical grasses, and its influence differs between C3 and C4 species. J. Exp. Bot. 2019, 70, 1581–1595. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, R.; Arena, C.; De Micco, V.; Giordano, M.; Aronne, G.; De Pascale, S. Changes in leaf anatomical traits enhanced photosynthetic activity of soybean grown in hydroponics with plant growth-promoting microorganisms. Front. Plant Sci. 2017, 8, 674. [Google Scholar] [CrossRef] [PubMed]
- Partelli, F.L.; Oliosi, G.; Dalazen, J.R.; da Silva, C.A.; Vieira, H.D.; Espindula, M.C. Proportion of ripe fruit weight and volume to green coffee: Differences in 43 genotypes of Coffea canephora. Agron. J. 2021, 113, 1050–1057. [Google Scholar] [CrossRef]
- Flexas, J. Genetic improvement of leaf photosynthesis and intrinsic water use efficiency in C3 plants: Why so much little success? Plant Sci. 2016, 251, 155–161. [Google Scholar] [CrossRef]
- Bhusal, N.; Bhusal, S.J.; Yoon, T.M. Comparisons of physiological and anatomical characteristics between two cultivars in bi-leader apple trees (Malus × domestica Borkh.). Sci. Hortic. 2018, 231, 73–81. [Google Scholar] [CrossRef]
- Denoeud, F.; Carretero-Paulet, L.; Dereeper, A.; Droc, G.; Guyot, R.; Pietrella, M.; Zheng, C.; Alberti, A.; Anthony, F.; Aprea, G.; et al. The coffee genome provides insight into the convergent evolution of caffeine biosynthesis. Science 2014, 345, 1181–1184. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Rahn, E.; Läderach, P.; Ghini, R.; Ramalho, J.C. Why could the coffee crop endure climate change and global warming to a greater extent than previously estimated? Clim. Chang. 2019, 152, 167–178. [Google Scholar] [CrossRef]
- Conab. Conab—Safras. Available online: https://www.conab.gov.br/info-agro/safras (accessed on 19 October 2020).
- Agriculture, U.-U.S.D. Coffee: World Markets and Trade. Available online: https://www.fas.usda.gov/data/coffee-world-markets-and-trade (accessed on 19 October 2020).
- Conagin, C.H.T.M.; Mendes, A.J.T. Cyto-genetical investigations on three species of Coffea: Self incompatibility in Coffea canephora. Bragantia 1961, 20, 788–804. [Google Scholar] [CrossRef]
- Moraes, M.S.; Teixeira, A.L.; Ramalho, A.R.; EspAA ndula, M.C.; FerrAAo, M.A.G.; Rocha, R.B. Characterization of gametophytic self-incompatibility of superior clones of Coffea canephora. Genet. Mol. Res. 2018, 17. [Google Scholar] [CrossRef]
- Bragança, S.M.; De Carvalho, C.H.S.; Da Fonseca, A.F.A.; Ferrão, R.G. Clonal varieties of Conilon coffee for the Espirito Santo State, Brazil. Pesqui. Agropecuária Bras. 2001, 36, 765–770. [Google Scholar] [CrossRef]
- Ferrão, R.G.; Cruz, C.D.; Ferreira, A.; Cecon, P.R.; Ferrão, M.A.G.; da Fonseca, A.F.A.; Carneiro, P.C.d.S.; da Silva, M.F. Genetic parameters in Conilon coffee. Pesqui. Agropecuária Bras. 2008, 43, 61–69. [Google Scholar] [CrossRef]
- Ivoglo, M.G.; Fazuoli, L.C.; De Oliveira, A.C.B.; Gallo, P.B.; Mistro, J.C.; Varolla, M.B.S.; Toma-Braghini, M. Genetic divergence among robusta coffe progenies. Bragantia 2008, 67, 823–831. [Google Scholar] [CrossRef][Green Version]
- Partelli, F.L.; Vieira, H.D.; Santiago, A.R.; Barroso, D.G. Produção e desenvolvimento radicular de plantas de café “Conilon” propagadas por sementes e por estacas. Pesqui. Agropecuária Bras. 2006, 41, 949–954. [Google Scholar] [CrossRef]
- Partelli, F.L.; Covre, A.M.; Oliveira, M.G.; Alexandre, R.S.; da Vitória, E.L.; da Silva, M.B. Root system distribution and yield of “Conilon” coffee propagated by seeds or cuttings. Pesqui. Agropecuária Bras. 2014, 49, 349–355. [Google Scholar] [CrossRef][Green Version]
- Cheng, B.; Furtado, A.; Smyth, H.E.; Henry, R.J. Influence of genotype and environment on coffee quality. Trends Food Sci. Technol. 2016, 57, 20–30. [Google Scholar] [CrossRef]
- Semedo, J.N.; Rodrigues, A.P.; Lidon, F.C.; Pais, I.P.; Marques, I.; Gouveia, D.; Armengaud, J.; Silva, M.J.; Martins, S.; Semedo, M.C.; et al. Intrinsic non-stomatal resilience to drought of the photosynthetic apparatus in Coffea spp. is strengthened by elevated air [CO2]. Tree Physiol. 2020, 41, 708–727. [Google Scholar] [CrossRef] [PubMed]
- Dow, G.J.; Berry, J.A.; Bergmann, D.C. The physiological importance of developmental mechanisms that enforce proper stomatal spacing in Arabidopsis thaliana. New Phytol. 2014, 201, 1205–1217. [Google Scholar] [CrossRef]
- Rodrigues, W.P.; Martins, M.Q.; Fortunato, A.S.; Rodrigues, A.P.; Semedo, J.N.; Simões-Costa, M.C.; Pais, I.P.; Leitão, A.E.; Colwell, F.; Goulao, L.; et al. Long-term elevated air [CO2] strengthens photosynthetic functioning and mitigates the impact of supra-optimal temperatures in tropical Coffea arabica and C. canephora species. Glob. Chang. Biol. 2016, 22, 415–431. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.D.M.; Sparovek, G. Gas exchange and adaptive structural characteristics of coffee plants grown in different levels of radiation. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Dittberner, H.; Korte, A.; Mettler-Altmann, T.; Weber, A.P.M.; Monroe, G.; de Meaux, J. Natural variation in stomata size contributes to the local adaptation of water-use efficiency in Arabidopsis thaliana. Mol. Ecol. 2018, 27, 4052–4065. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Leonardo, J.; Gonçalves, M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2014, 22, 711–728. [Google Scholar] [CrossRef]
- dos Santos, H.; Jacomine, P.; Dos Anjos, L. Sistema Brasileiro de Classificação de Solos; Embrapa: Brasília, Brazil, 2018; ISBN 978-85-7035-817-2. [Google Scholar]
- Giles, J.A.D.; Ferreira, A.D.; Partelli, F.L.; Aoyama, E.M.; Ramalho, J.; Ferreira, A.; Falqueto, A.R. Divergence and genetic parameters between coffea sp. genotypes based in foliar morpho-anatomical traits. Sci. Hortic. 2019, 245, 231–236. [Google Scholar] [CrossRef]
- Dubberstein, D.; Lidon, F.C.; Rodrigues, A.P.; Semedo, J.N.; Marques, I.; Rodrigues, W.P.; Gouveia, D.; Armengaud, J.; Semedo, M.C.; Martins, S.; et al. Resilient and Sensitive Key Points of the Photosynthetic Machinery of Coffea spp. to the Single and Superimposed Exposure to Severe Drought and Heat Stresses. Front. Plant Sci. 2020, 11, 1049. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.Q.; Partelli, F.L.; Golynski, A.; de Sousa Pimentel, N.; Ferreira, A.; de Oliveira Bernardes, C.; Ribeiro-Barros, A.I.; Ramalho, J.C. Adaptability and stability of Coffea canephora genotypes cultivated at high altitude and subjected to low temperature during the winter. Sci. Hortic. 2019, 252, 238–242. [Google Scholar] [CrossRef]
- Partelli, F.L.; Golynski, A.; Ferreira, A.; Martins, M.Q.; Mauri, A.L.; Ramalho, J.C.; Vieira, H.D. Andina-first clonal cultivar of high-altitude conilon coffee. Crop Breed. Appl. Biotechnol. 2019, 19, 476–480. [Google Scholar] [CrossRef]
- De Melo, H.C.; de Castro, E.M.; Soares, Â.M.; de Melo, L.A.; Alves, J.D. Alterações anatômicas e fisiológicas em Setaria anceps Stapf ex Massey e Paspalum paniculatum L. sob condições de déficit hídrico. Hoehnea 2007, 34, 145–153. [Google Scholar] [CrossRef]
- Silva, L.O.E.; Schmidt, R.; Valani, G.P.; Ferreira, A.; Ribeiro-Barros, A.I.; Partelli, F.L. Root trait variability in coffea canephora genotypes and its relation to plant height and crop yield. Agronomy 2020, 10, 1394. [Google Scholar] [CrossRef]
- Aguiar, T.V.; Sant’Anna-Santos, B.F.; Azevedo, A.A.; Ferreira, R.S. ANATI QUANTI: Quantitative analysis software for plant anatomy studies. Planta Daninha 2007, 25, 649–659. [Google Scholar] [CrossRef]
- Cass, J.M.; Montgomery, D.C.; Peck, E.A. Introduction to Linear Regression Analysis. Appl. Stat. 1983, 32, 94. [Google Scholar] [CrossRef]
- Bhusal, N.; Kim, H.; Han, S.; Experimental, T.Y.-E. Photosynthetic traits and plant–water relations of two apple cultivars grown as bi-leader trees under long-term waterlogging conditions. Environ. Exp. Bot. 2020, 176, 104111. [Google Scholar] [CrossRef]
- Savvides, A.; Fanourakis, D.; Van Ieperen, W. Co-ordination of hydraulic and stomatal conductances across light qualities in cucumber leaves. J. Exp. Bot. 2012, 63, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Sack, L.; Buckley, T.N. The developmental basis of stomatal density and flux. Plant Physiol. 2016, 171, 2358–2363. [Google Scholar] [CrossRef]
- R Foundation for Statistical Computing. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012; Available online: https//www.R-project.org (accessed on 20 September 2020).
- Ramalho, J.C.; Rodrigues, A.P.; Semedo, J.N.; Pais, I.P.; Martins, L.D.; Simões-Costa, M.C.; Leitão, A.E.; Fortunato, A.S.; Batista-Santos, P.; Palos, I.M.; et al. Sustained photosynthetic performance of Coffea spp. under long-term enhanced [CO2]. PLoS ONE 2013, 8, e82712. [Google Scholar] [CrossRef]
- Guedes, J.M.; Vilela, D.J.M.; Rezende, J.C.; Silva, F.L.; Botelho, C.E.; Carvalho, S.P. Genetic divergence between coffee trees of the Maragogipe germplasm. Bragantia 2013, 72, 127–132. [Google Scholar] [CrossRef]
- Dalcomo, J.; Vieira, H.; Ferreira, A.; Lima, W.; Ferrão, R.; Fonseca, A.; Ferrão, M.; Partelli, F. Evaluation of genetic divergence among clones of conilon coffee after scheduled cycle pruning. Genet. Mol. Res. 2015, 14, 15417–15426. [Google Scholar] [CrossRef]
- Giles, J.A.D.; Partelli, F.L.; Ferreira, A.; Rodrigues, J.P.; Oliosi, G.; Silva, F.H. Genetic diversity of promising ‘conilon’ coffee clones based on morpho-agronomic variables. An. Acad. Bras. Cienc. 2018, 90, 2437–2446. [Google Scholar] [CrossRef] [PubMed]
- César, F.R.C.F.; Matsumoto, S.N.; Viana, A.E.S.; Santos, M.A.F.; Bonfim, J.A. Leaf morphophysiology of coffee plants under different levels of light restriction. Coffee Sci. 2010, 5, 262–271. [Google Scholar] [CrossRef]
- Oliveira, N.K.; de Castro, E.M.; Guimarães, R.J.; Pieve, L.M.; Baliza, D.P.; Machado, J.L.; Freitas, T. Foliar anatomy of coffee plants implanted using hydro retainer polymers. Coffee Sci. 2014, 9, 258–265. [Google Scholar] [CrossRef]
- Franks, P.J.; Beerling, D.J. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl. Acad. Sci. USA 2009, 106, 10343–10347. [Google Scholar] [CrossRef]
- Camargo, M.A.B.; Marenco, R.A. Density, size and distribution of stomata in 35 rainforest tree species in Central Amazônia. Acta Amaz. 2011, 41, 205–212. [Google Scholar] [CrossRef]
- Campa, C.; Urban, L.; Mondolot, L.; Fabre, D.; Roques, S.; Lizzi, Y.; Aarrouf, J.; Doulbeau, S.; Breitler, J.-C.; Letrez, C.; et al. Juvenile Coffee Leaves Acclimated to Low Light Are Unable to Cope with a Moderate Light Increase. Front. Plant Sci. 2017, 8, 1126. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, S.I.; Livingston, N.J.; Turpin, D.H. Stomatal development in new leaves is related to the stomatal conductance of mature leaves in poplar (Populus trichocarpax P. deltoides). J. Exp. Bot. 2006, 57, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Torii, K.U. Hormonal and environmental signals guiding stomatal development. BMC Biol. 2018, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Chater, C.C.C.; Oliver, J.; Casson, S.; Gray, J.E. Putting the brakes on: Abscisic acid as a central environmental regulator of stomatal development. New Phytol. 2014, 202, 376–391. [Google Scholar] [CrossRef]
- Papanatsiou, M.; Petersen, J.; Henderson, L.; Wang, Y.; Christie, J.M.; Blatt, M.R. Optogenetic manipulation of stomatal kinetics improves carbon assimilation, water use, and growth. Science 2019, 363, 1456–1459. [Google Scholar] [CrossRef]
- Cargnelutti Filho, A.; Storck, L. Measures of experimental precision degree in corn cultivar competition trials. Pesqui. Agropecuária Bras. 2009, 44, 111–117. [Google Scholar] [CrossRef]
- Fritsche-Neto, R.; Vieira, R.A.; Scapim, C.A.; Miranda, G.V.; Rezende, L.M. Updating the ranking of the coefficients of variation from maize experiments. Acta Sci. Agron. 2012, 34, 99–101. [Google Scholar] [CrossRef]
- Gomes, P. Curso de Estatística Experimental; POTAFOS: Piracicaba, Brazil, 1982; p. 476. [Google Scholar]
- Sebbenn, A.M.; Siqueira AD, F.; Kageyama, P.Y.; Machado, J.A.R. Genetic parameters for the conservation of cabreuva Myroxylon peruiferum LF Allemão. Sci. For. 1998, 53, 31–38. [Google Scholar]
- Rodrigues, W.N.; Tomaz, M.A.; Ferrão, R.G.; Ferrão, M.A.G.; da Fonseca, A.F.A.; de Miranda, F.D. Genetic parameters estimation in groups of conilon coffee clones. Coffee Sci. 2012, 7, 177–186. [Google Scholar]
- Valadares, R.N.; Melo, R.A.; Silva, J.A.S.; Araújo, A.L.R.; Silva, F.S.; Carvalho Filho, J.L.S.; Menezes, D. Estimativas de parâmetros genéticos e correlações em acessos de melão do grupo momordica. Hortic. Bras. 2017, 35, 557–563. [Google Scholar] [CrossRef]
- Vencovsky, R. Herança quantitativa. In Melhoramento e Produção do Milho no Brasil; Fundacao Cargil: Sao Paulo, Brazil, 1978; pp. 122–201. [Google Scholar]
- Fehr, W. Principles of Cultivar Development: Crop Species; Macmillan Publishing Company: New York, NY, USA, 1987; ISBN 0029491819. [Google Scholar]
- Cruz, C.D.; Carneiro, P.C.S. Modelos Biométricos Aplicados ao Melhoramento de Plantas; UFV: Viçosa, Brazil, 2004; ISBN 85-7269-010-7. [Google Scholar]
- Jolliffe, I.T. Discarding Variables in a Principal Component Analysis. I: Artificial Data. Appl. Stat. 1972, 21, 160. [Google Scholar] [CrossRef]
- Mojena, R. Hierarchical grouping methods and stopping rules: An evaluation. Comput. J. 1977, 20, 359–363. [Google Scholar] [CrossRef]
- Grisi, F.A.; Alves, J.D.; de Castro, E.M.; de Oliveira, C.; Biagiotti, G.; de Melo, L.A. Leaf anatomical evaluations in ‘Catuaí’ and ‘Siriema’ coffee seedlings submitted to water stress. Ciência Agrotecnologia 2008, 32, 1730–1736. [Google Scholar] [CrossRef]
- Oliveira, E.C.D.; Miglioranza, E. Density and stomata distribution in cassava Manihot esculenta Crantz IAC 576-70. Sci. Agropecu. 2014, 5, 135–140. [Google Scholar] [CrossRef]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot. 2013, 64, 495–505. [Google Scholar] [CrossRef]
- Queiroz-Voltan, R.B.; Nardin, C.F.; Fazuoli, L.C.; Braghini, M.T. Leaf anatomy characterization of Coffea arabica plants at different seasonal periods. Biotemas 2014, 27, 1. [Google Scholar] [CrossRef]
- Devore, J.L. Probabilidade Estatística Para Engenharia e Ciências; Cengage Learning: Boston, MA, USA, 2006; ISBN 9788522109241. [Google Scholar]



| Identification | Name | Identification | Name | Identification | Name |
|---|---|---|---|---|---|
| 1 | Verdim R | 16 | Pirata | 31 | Cheique |
| 2 | B01 | 17 | Peneirão | 32 | P2 |
| 3 | Bicudo | 18 | Z39 | 33 | Emcapa 02 |
| 4 | Alecrim | 19 | Z35 | 34 | Emcapa 153 |
| 5 | 700 | 20 | Z40 | 35 | P1 |
| 6 | CH1 | 21 | Z29 | 36 | LB1 |
| 7 | Imbigudinho | 22 | Z38 | 37 | 122 |
| 8 | AD1 | 23 | Z18 | 38 | Verdim D |
| 9 | Graudão HP | 24 | Z37 | 39 | Seed |
| 10 | Valcir P | 25 | Z21 | 40 | Emcapa 143 |
| 11 | Beira Rio 8 | 26 | Z36 | 41 | Ouro Negro 1 |
| 12 | Tardio V | 27 | Ouro Negro | 42 | Ouro Negro 2 |
| 13 | AP | 28 | 18 | 43 | ClementinoT |
| 14 | L80 | 29 | Tardio C | - | - |
| 15 | Bamburral | 30 | A1 | - | - |
| Variables | MS | Mean | CVe (%) | CVg (%) | VI | h2 (%) | |
|---|---|---|---|---|---|---|---|
| Genotype | Residual | ||||||
| ECD | 116,920 ** | 82.2 | 102.0 | 8.84 | 8.29 | 0.93 | 93.0 |
| SI | 47.1 ** | 4.80 | 23.2 | 9.42 | 7.22 | 0.76 | 89.8 |
| SD | 21,576 ** | 1670 | 282.2 | 14.50 | 12.9 | 0.89 | 92.3 |
| SS | 21,322 ** | 1337 | 435.0 | 8.40 | 8.38 | 0.99 | 93.7 |
| SL | 24.5 ** | 1.55 | 25.7 | 4.83 | 4.80 | 0.99 | 93.7 |
| SW | 8.03 ** | 0.768 | 16.9 | 5.19 | 4.12 | 0.79 | 90.4 |
| SLW | 0.390 ** | 0.007 | 1.52 | 5.68 | 3.03 | 0.53 | 81.1 |
| Genotype | ECD | SL | SW | SD | SS | SI | SLW |
|---|---|---|---|---|---|---|---|
| 1 | 112.4 b | 24.8 d | 16.3 c | 336.7 a | 407.2 d | 24.7 b | 1.52 c |
| 2 | 118.2 a | 23.8 e | 16.0 d | 288.9 c | 382.7 e | 21.0 d | 1.48 d |
| 3 | 100.0 c | 23.6 e | 15.7 d | 253.5 d | 372.0 e | 21.6 d | 1.51 d |
| 4 | 108.6 b | 23.7 e | 15.7 d | 295.6 c | 375.2 e | 22.9 c | 1.52 c |
| 5 | 110.0 b | 24.4 d | 16.1 d | 262.9 c | 396.3 e | 20.0 d | 1.51 d |
| 6 | 121.6 a | 24.5 d | 16.4 c | 344.5 a | 405.1 d | 23.6 b | 1.49 d |
| 7 | 102.0 c | 25.2 d | 17.1 b | 266.9 c | 433.4 c | 22.3 c | 1.47 d |
| 8 | 106.3 b | 25.3 d | 16.6 c | 320.7 b | 421.2 d | 24.7 b | 1.53 c |
| 9 | 98.4 c | 26.0 c | 16.8 c | 244.9 d | 439.3 c | 21.3 d | 1.55 c |
| 10 | 87.8 e | 28.4 a | 16.6 c | 254.7 d | 475.4 b | 23.9 b | 1.71 a |
| 11 | 102.7 c | 26.1 c | 16.0 d | 304.8 b | 421.1 d | 24.5 b | 1.63 b |
| 12 | 101.1 c | 25.1 d | 16.7 c | 276.2 c | 420.1 d | 22.9 c | 1.51 d |
| 13 | 92.9 d | 27.8 a | 18.2 a | 227.8 d | 507.9 a | 21.1 d | 1.53 c |
| 14 | 96.4 d | 28.0 a | 18.2 a | 342.7 a | 512.0 a | 27.9 a | 1.54 c |
| 15 | 99.0 c | 26.0 c | 16.7 c | 268.9 c | 435.4 c | 23.9 b | 1.56 c |
| 16 | 102.3 c | 25.1 d | 16.8 c | 285.2 c | 424.6 d | 23.3 c | 1.49 d |
| 17 | 94.3 d | 27.0 b | 18.1 a | 241.3 d | 491.5 b | 21.8 d | 1.49 d |
| 18 | 98.9 c | 25.3 d | 16.8 c | 267.1 c | 429.0 d | 24.0 b | 1.51 d |
| 19 | 123.0 a | 23.5 e | 15.4 d | 358.6 a | 364.3 e | 24.1 b | 1.52 c |
| 20 | 108.9 b | 24.9 d | 15.8 d | 270.6 c | 394.1 e | 21.3 d | 1.58 b |
| 21 | 110.8 b | 25.6 d | 17.0 b | 360.4 a | 436.1 c | 26.2 a | 1.51 d |
| 22 | 113.2 b | 25.9 c | 17.6 b | 269.4 c | 458.4 c | 20.7 d | 1.47 d |
| 23 | 95.4 d | 26.9 b | 17.2 b | 252.9 d | 464.2 c | 22.5 c | 1.56 c |
| 24 | 97.5 d | 28.0 a | 17.2 b | 293.8 c | 484.0 b | 24.7 b | 1.63 b |
| 25 | 122.0 a | 26.1 c | 17.3 b | 341.4 a | 454.5 c | 23.4 c | 1.51 d |
| 26 | 111.4 b | 25.8 c | 17.4 b | 357.3 a | 451.9 c | 25.9 a | 1.49 d |
| 27 | 105.0 c | 27.0 b | 18.2 a | 281.6 c | 493.9 b | 22.7 c | 1.48 d |
| 28 | 100.3 c | 26.2 c | 18.2 a | 262.6 c | 480.0 b | 22.3 c | 1.44 d |
| 29 | 104.0 c | 25.7 c | 17.2 b | 251.1 d | 442.5 c | 20.7 d | 1.50 d |
| 30 | 105.3 c | 25.5 d | 16.5 c | 315.2 b | 424.3 d | 24.7 b | 1.55 c |
| 31 | 93.4 d | 25.3 d | 16.4 c | 249.8 d | 418.4 d | 22.5 c | 1.54 c |
| 32 | 93.8 d | 26.5 c | 17.0 b | 270.6 c | 454.0 c | 23.9 b | 1.56 c |
| 33 | 91.2 d | 26.8 b | 17.7 a | 220.8 d | 476.2 b | 23.4 c | 1.52 c |
| 34 | 102.6 c | 22.7 f | 16.0 d | 268.1 c | 363.9 e | 22.1 c | 1.42 d |
| 35 | 104.0 c | 26.0 c | 16.7 c | 272.0 c | 435.6 c | 23.3 c | 1.56 c |
| 36 | 104.1 c | 26.2 c | 16.8 c | 251.8 d | 442.4 c | 22.0 c | 1.56 c |
| 37 | 84.2 e | 27.4 b | 17.0 b | 233.3 d | 466.5 c | 23.1 c | 1.62 b |
| 38 | 97.7 d | 25.9 c | 17.1 b | 299.9 b | 444.0 c | 25.1 b | 1.52 c |
| 39 | 96.7 d | 25.9 c | 17.5 b | 266.9 c | 453.8 c | 23.1 c | 1.48 d |
| 40 | 96.5 d | 24.9 d | 16.6 c | 326.8 b | 417.4 d | 27.1 a | 1.50 d |
| 41 | 102.0 c | 25.1 d | 16.3 c | 239.4 d | 413.0 d | 20.5 d | 1.54 c |
| 42 | 95.7 d | 24.1 e | 15.7 d | 292.0 c | 381.6 e | 25.0 b | 1.53 c |
| 43 | 97.6 d | 26.1 c | 16.9 b | 246.8 d | 444.2 c | 21.6 d | 1.54 c |
| Groups | Genotypes | ECD | SD | SL | SW |
|---|---|---|---|---|---|
| 1 | 6, 19, 25, 21, 26 | 118.0 | 352.4 | 25.1 | 16.7 |
| 2 | 3, 34, 40, 42 | 98.7 | 285.1 | 23.8 | 16.0 |
| 3 | 1, 2, 4, 5, 8, 11, 20, 30 | 109.6 | 299.4 | 24.8 | 16.1 |
| 4 | 7, 9, 12, 13, 15, 16, 17, 18, 22, 23, 27, 28, 29, 31, 32, 33, 35, 36, 38, 39, 41, 43 | 99.5 | 259.6 | 26.1 | 17.2 |
| 5 | 10, 37 | 86.0 | 244.0 | 27.9 | 16.8 |
| 6 | 14, 24 | 97.0 | 318.2 | 28.0 | 17.8 |
| Morpho-Anatomical Traits | |||||||
|---|---|---|---|---|---|---|---|
| Yield | ECD | SL | SW | SS | SI | SD | SLW |
| −0.13 | 0.31 * | 0.16 | 0.25 | 0.34 * | 0.13 | 0.24 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubberstein, D.; Oliveira, M.G.; Aoyama, E.M.; Guilhen, J.H.; Ferreira, A.; Marques, I.; Ramalho, J.C.; Partelli, F.L. Diversity of Leaf Stomatal Traits among Coffea canephora Pierre ex A. Froehner Genotypes. Agronomy 2021, 11, 1126. https://doi.org/10.3390/agronomy11061126
Dubberstein D, Oliveira MG, Aoyama EM, Guilhen JH, Ferreira A, Marques I, Ramalho JC, Partelli FL. Diversity of Leaf Stomatal Traits among Coffea canephora Pierre ex A. Froehner Genotypes. Agronomy. 2021; 11(6):1126. https://doi.org/10.3390/agronomy11061126
Chicago/Turabian StyleDubberstein, Danielly, Marcos Góes Oliveira, Elisa Mitsuko Aoyama, José Henrique Guilhen, Adésio Ferreira, Isabel Marques, José C. Ramalho, and Fábio Luiz Partelli. 2021. "Diversity of Leaf Stomatal Traits among Coffea canephora Pierre ex A. Froehner Genotypes" Agronomy 11, no. 6: 1126. https://doi.org/10.3390/agronomy11061126
APA StyleDubberstein, D., Oliveira, M. G., Aoyama, E. M., Guilhen, J. H., Ferreira, A., Marques, I., Ramalho, J. C., & Partelli, F. L. (2021). Diversity of Leaf Stomatal Traits among Coffea canephora Pierre ex A. Froehner Genotypes. Agronomy, 11(6), 1126. https://doi.org/10.3390/agronomy11061126









