Water Table Fluctuation and Methane Emission in Pineapples (Ananas comosus (L.) Merr.) Cultivated on a Tropical Peatland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description for Soil Methane Emission from Field Cultivated with Pineapples
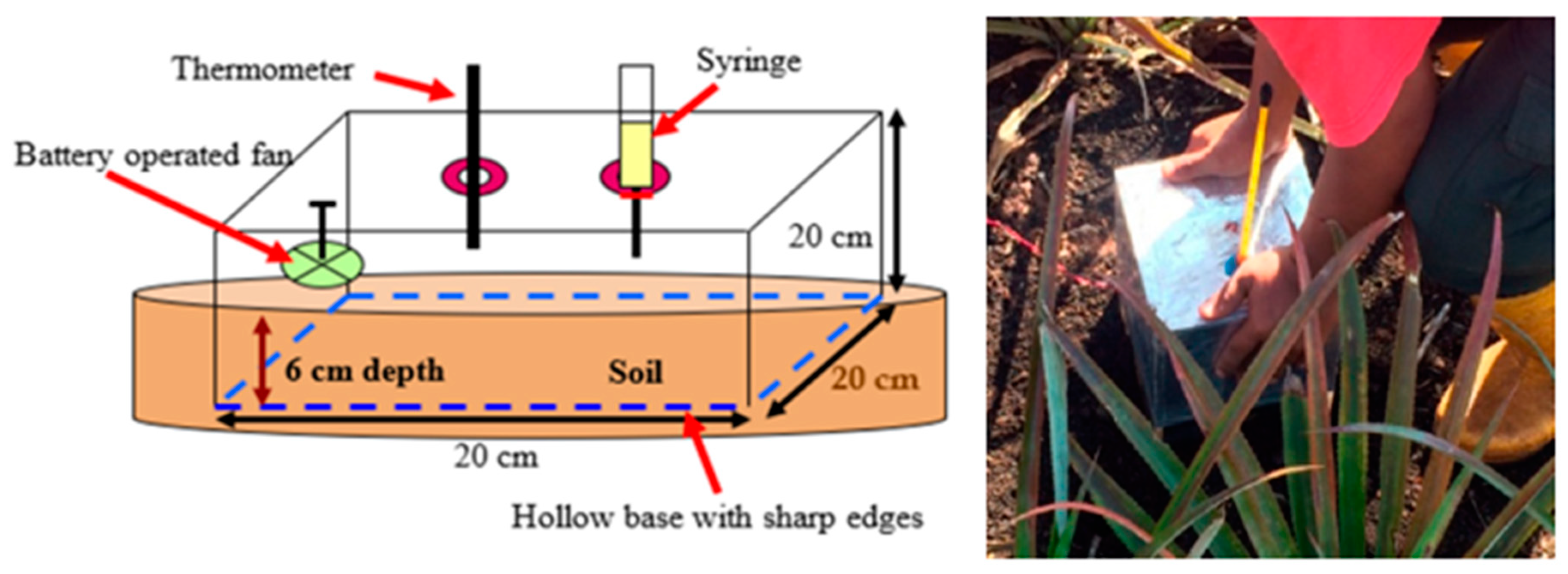
2.2. Establishment of Lysimeters for Methane Estimation under Simulated Water Table Fluctuation
2.3. Estimation of Soil Methane Emission during Water Table Fluctuation
2.4. Statistical Analysis
3. Results
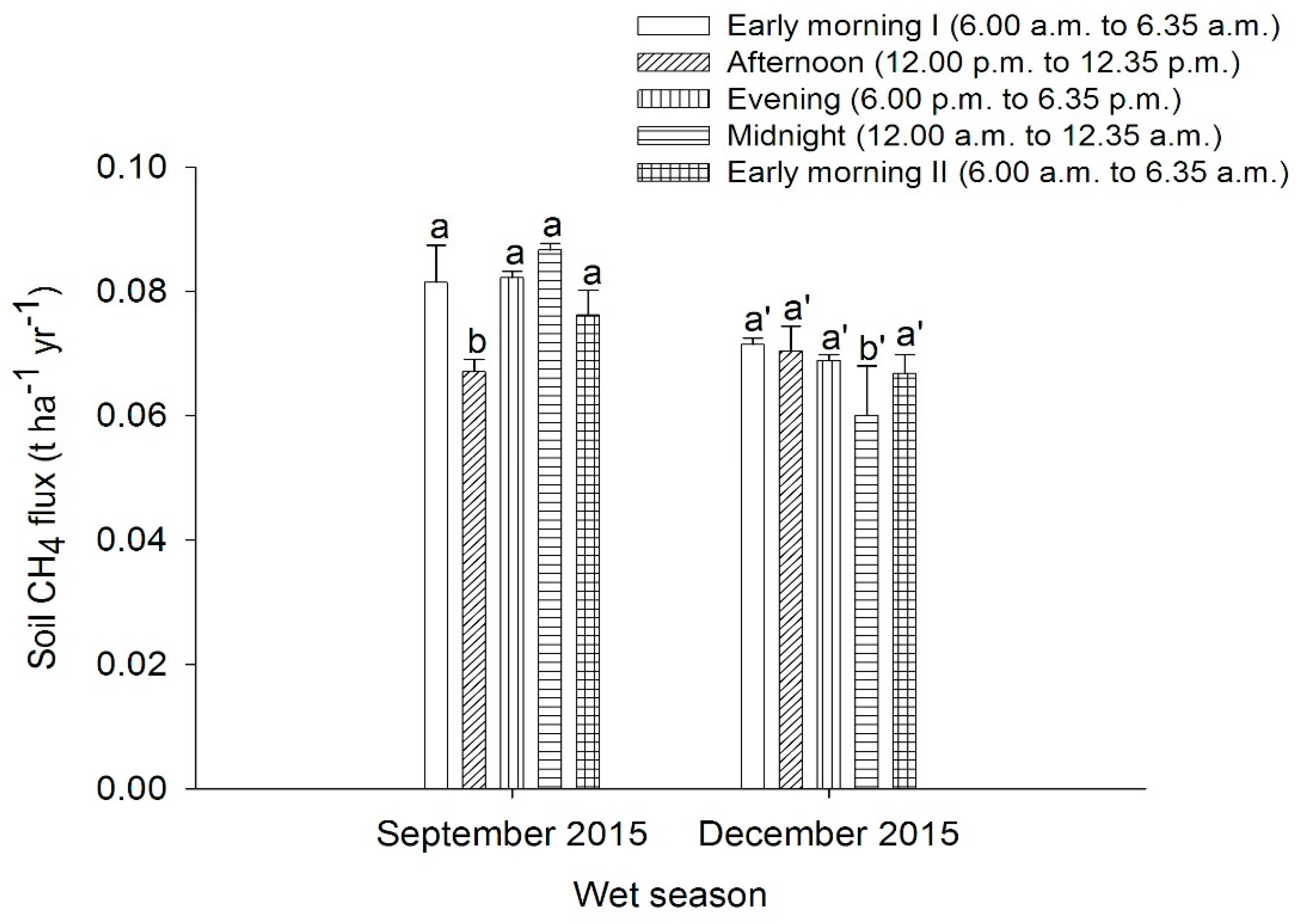
3.1. Soil Methane Emissions from Peat Soils Grown with Pineapple under Open Field Cultivation System in the Dry and Wet Seasons
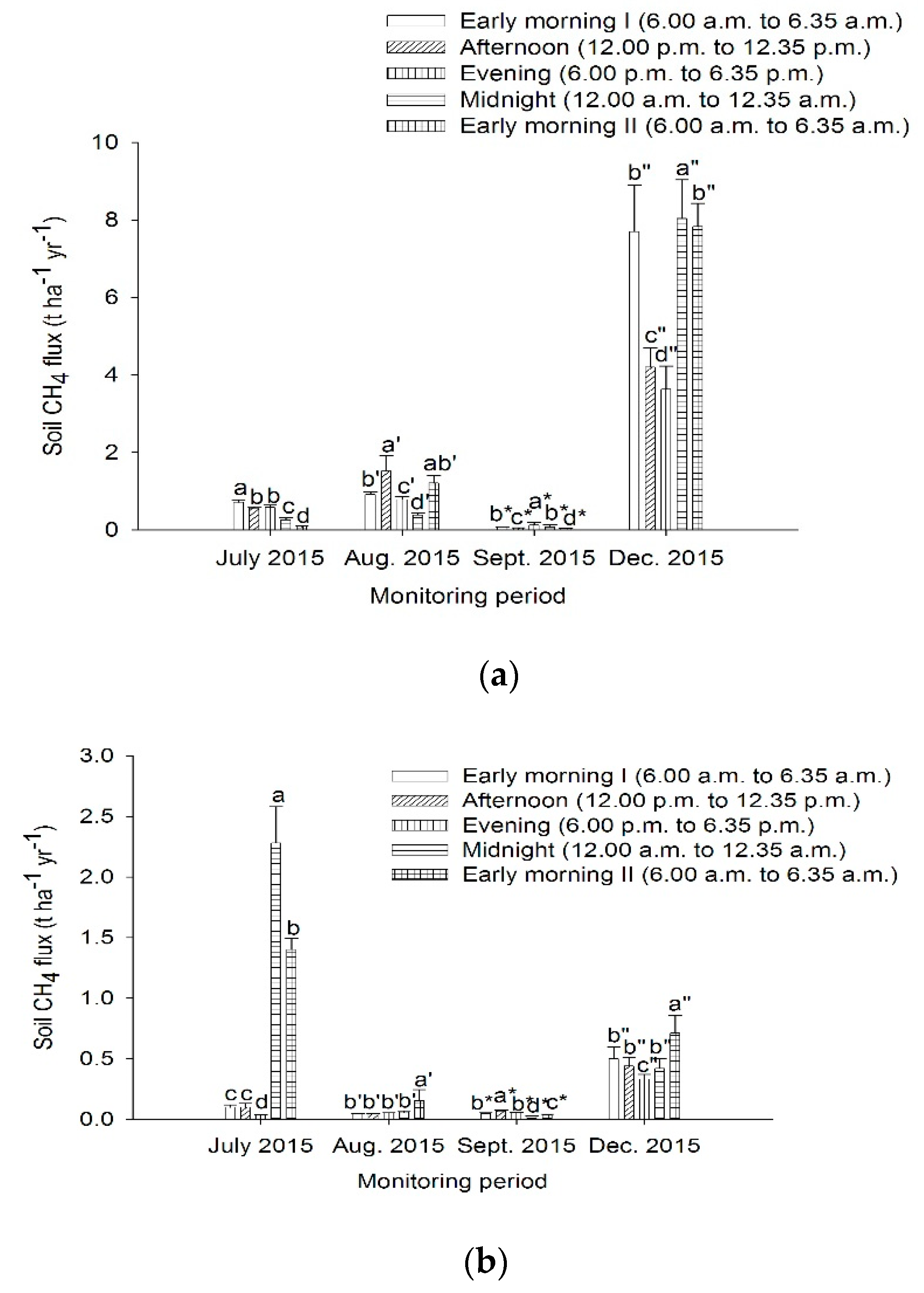
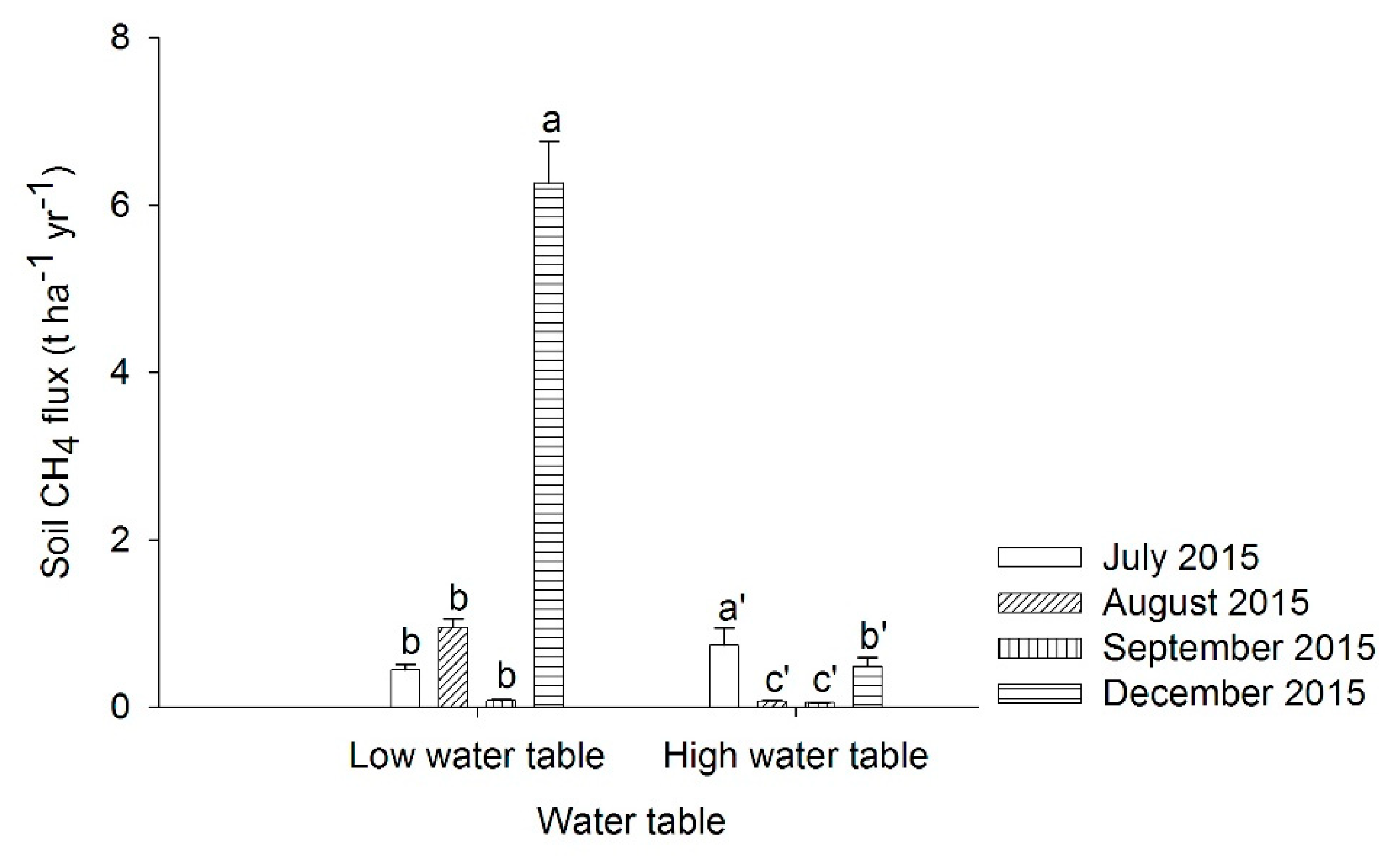
3.2. Soil Methane Emissions from Peat Soils Cultivated with Pineapples in Lysimeters under Simulated Water Table Fluctuation in the Dry and Wet Seasons
4. Discussion
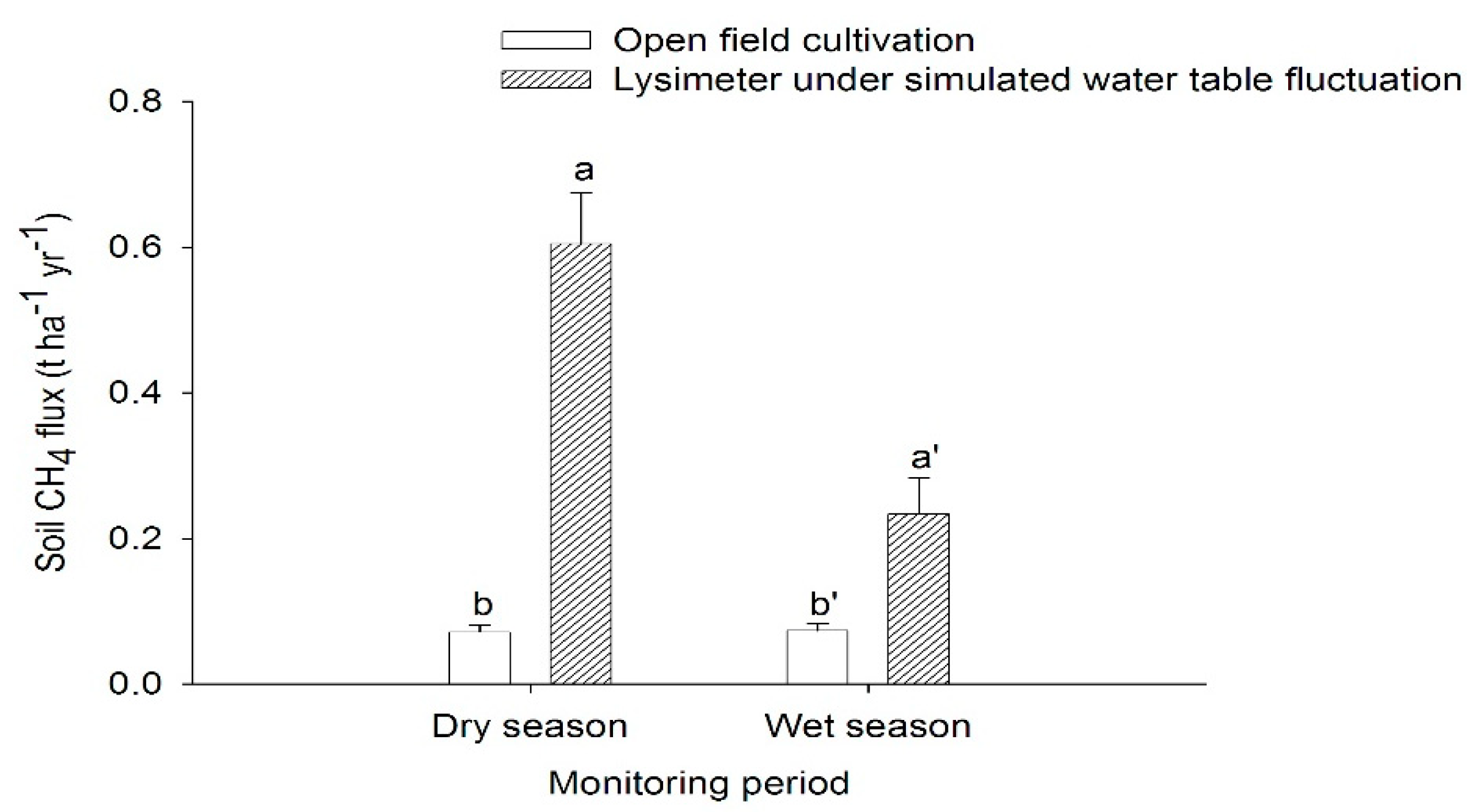
4.1. Soil Methane Emissions from Peat Soils Grown with Pineapple under Open Field Cultivation System in the Dry and Wet Seasons
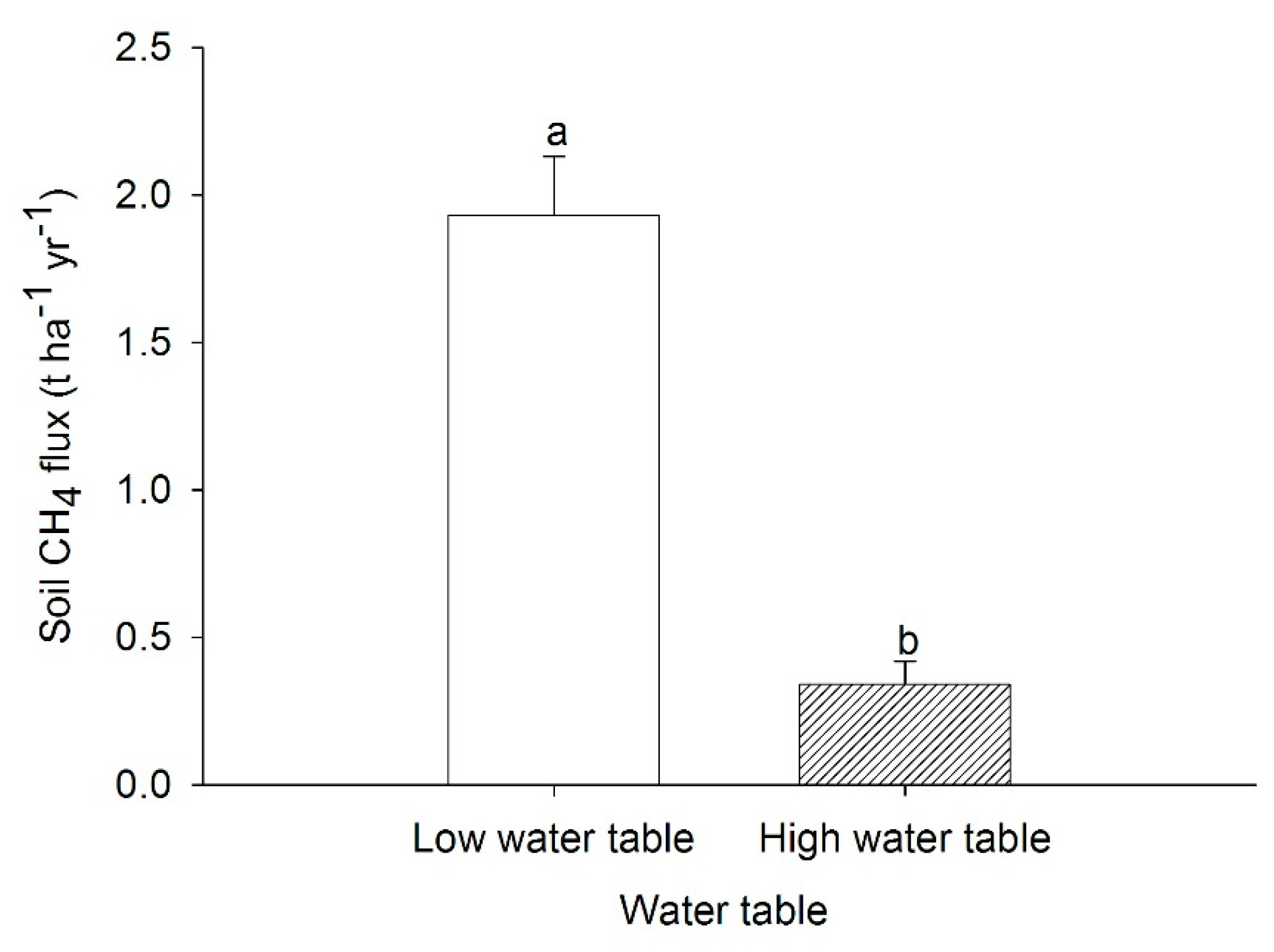
4.2. Soil Methane Emissions from Peat Soils Cultivated with Pineapples in Lysimeters under Simulated Water Table Fluctuation in the Dry and Wet Seasons
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joosten, H.; Sirin, A.; Couwenberg, J.; Laine, J.; Smith, P. The role of peatlands in climate regulation. In Peatland Restoration and Ecosystem Services: Science, Policy and Practice; Cambridge University Press: Cambridge, UK, 2016; pp. 63–76. [Google Scholar]
- Chen, X.; Tung, K.K. Varying planetary heat sink led to global-warming slowdown and acceleration. Science 2014, 6199, 897–903. [Google Scholar] [CrossRef]
- Snyder, C.S.; Tom, B.; Jensen, T.L.; Paul, E.F. Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agric. Ecosys. Environ. 2009, 133, 247–266. [Google Scholar] [CrossRef]
- Nisbet, E.; Manning, M.R.; Dlugockenky, E.J.; Fisher, R.E.; Lowry, D.; Michel, S.E. Very strong atmospheric methane growth in the 4 years 2014–2017: Implications for the Paris Agreement. Glob. Biogeochem. Cycles 2019, 33, 318–342. [Google Scholar] [CrossRef]
- Dlugokencky, E.J.; Lang, P.M.; Crotwell, A.M.; Mund, J.W.; Crotwell, M.J.; Thoning, K.W. Atmospheric Methane Dry Air Mole Fractions from the NOAA ESRL Carbon Cycle Cooperative Global Air Sampling Network, 1983–2016. In Division N-EGM, Editor. Version: 2017-07-28. 2017. Available online: Ftp://aftp.cmdl.noaa.gov/data/trace_gases/ch4/flask/surface/ (accessed on 29 May 2021).
- Ismail, A.B.; Zulkefli, M.; Salma, I.; Jamaludin, J.; Mohamad, H.M.J. Selection of land clearing technique and crop type as preliminary steps in restoring carbon reserve in tropical peatland under agriculture. In Proceedings of the 12th International Peat Congress, Tullamore, Ireland, 8–13 June 2008. Int Peat Society. [Google Scholar]
- Kechavarzi, C.; Dawson, Q.; Bartlett, M.; Leeds-Harrison, B.P. The role of soil moisture, temperature, and nutrient amendment on CO2 efflux from agricultural peat soil microcosms. Geoderma 2010, 154, 203–210. [Google Scholar] [CrossRef]
- Mäkiranta, P.; Minkkinen, K.; Hytönen, J.; Laine, J. Factors causing temporal and spatial variation in heterotrophic and rhizospheric components of soil respiration in afforested organic soil croplands in Finland. Soil Biol. Biochem. 2008, 40, 1592–1600. [Google Scholar] [CrossRef]
- Fenner, N.; Ostle, J.N.; McNamara, N.; Sparks, T.; Harmens, H.; Reynolds, B.; Freeman, C. Elevated CO2 effects on peatland plant community carbon dynamics and DOC production. Ecosystems 2007, 10, 635–647. [Google Scholar] [CrossRef]
- Collins, W.J.; Fry, M.M.; Yu, H.; Fuglestvedt, J.S.; Shindell, D.T.; West, J.J. Global and regional temperature-change potentials for near-term climate forcers. Atmos. Chem. Phys. 2013, 13, 2471–2485. [Google Scholar] [CrossRef] [Green Version]
- Saunois, M.; Jackson, R.B.; Bousquet, P.; Poulter, B.; Canadell, J.G. The growing role of methane in anthropogenic climate change. Environ. Res. Lett. 2017, 11, 120207. [Google Scholar] [CrossRef] [Green Version]
- Leifeld, J. Prologue paper: Soil carbon losses from land-use change and the global agricultural greenhouse gas budget. Sci. Total Environ. 2013, 465, 3–6. [Google Scholar] [CrossRef]
- Berglund, Ö.; Berglund, K. Influence of water table level and soil properties on emissions of greenhouse gases from cultivated peat soil. Soil Biol. Biochem. 2011, 43, 923–931. [Google Scholar] [CrossRef] [Green Version]
- Van Huissteden, J.; Petrescu, A.M.R.; Hendriks, D.M.D.; Rebel, K.T. Modelling the effect of water-table management on CO2 and CH4 fluxes from peat soils. Netherland J. Geosci. 2006, 85, 3–18. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; von Lampe, M.; van Tongeren, F. Climate change and trade in agriculture. Food Policy 2010, 36, S9–S13. [Google Scholar] [CrossRef]
- Vaghefi, N.; Mad Nasir, S.; Alias, R.; Khalid, A.R. Impact of climate change on food security in Malaysia: Economic and policy adjustments for rice industry. J. Integr. Environ. Sci. 2016, 13, 19–35. [Google Scholar] [CrossRef]
- Melling, L.; Hatano, R.; Goh, K.J. Methane fluxes from three ecosystems in tropical peatland of Sarawak, Malaysia. Soil Biol. Biochem. 2005, 37, 1445–1453. [Google Scholar] [CrossRef]
- Couwenberg, J.; Dommain, R.; Joosten, H. Greenhouse gas fluxes from tropical peatlands in south-east Asia. Glob. Chang. Biol. 2010, 16, 1715–1732. [Google Scholar] [CrossRef]
- Ahmed, O.H.; Liza Nuriati, L.K.C. Greenhouse Gas Emission and Carbon Leaching in Pineapple Cultivation on Tropical Peat Soil; Universiti Putra Malaysia Press: Serdang, Malaysia, 2019; pp. 1–145. [Google Scholar]
- Furukawa, Y.; Inubushi, K.; Ali, M.; Itang, A.M.; Tsuruta, H. Effect of changing groundwater levels caused by land-use changes on greenhouse gas fluxes from tropical peat lands. Nutr. Cycl. Agroecosyst. 2005, 71, 81–91. [Google Scholar] [CrossRef]
- Deshmukh, C.; Serça, D.; Delon, C.; Tardif, R.; Demarty, M.; Jarnot, C.; Guérin, F. Physical controls on CH4 emissions from a newly flooded subtropical freshwater hydroelectric reservoir: Nam Theun 2. Biogeosciences 2014, 11, 4251–4269. [Google Scholar] [CrossRef] [Green Version]
- Burrows, H.E.; Bubier, L.J.; Mosedale, A.; Cobb, W.G.; Crill, M.P. Net ecosystem exchange of carbon dioxide in a temperate poor fen: A comparison of automated and manual chamber techniques. Biogeochemistry 2005, 76, 21–45. [Google Scholar] [CrossRef]
- Abdul, H.; Kazuyuki, I.; Yuichiro, F.; Erry, P.; Muhammad, R.; Haruo, T. Greenhouse gas emissions from tropical peatlands of Kalimantan, Indonesia. Nutr. Cyc. Agroecosyst. 2005, 71, 73–80. [Google Scholar]
- Zulkefli, M.; Nuriati, L.L.; Ismail, A.B. Soil CO2 flux from tropical peatland under different land clearing techniques. J. Trop. Agric. Food Sci. 2010, 38, 131–137. [Google Scholar]
- Raziah, M.L.; Alam, A.R. Status and impact of pineapple technology on mineral soil. Econ. Tech. Manag. Rev. 2010, 5, 11–19. [Google Scholar]
- Barba, J.; Bradford, M.A.; Brewer, P.E.; Bruhn, D.; Covey, K.; Haren, J.; Vargas, R. Methane emissions from tree stems: A new frontier in the global carbon cycle. New Phytol. 2018, 222, 18–28. [Google Scholar] [CrossRef]
- Sjögersten, S.; Aplin, P.; Gauci, V.; Peacock, M.; Siegenthaler, A.; Turner, B.L. Temperature response of ex-situ greenhouse gas emissions from tropical peatlands: Interactions between forest type and peat moisture conditions. Geoderma 2018, 324, 47–55. [Google Scholar] [CrossRef]
- Hu, Y.G.; Feng, Y.L.; Zhang, Z.S.; Huang, L.; Zhang, P.; Xu, B.X. Greenhouse gases fluxes of biological soil crusts and soil ecosystem in the artificial sand-fixing vegetation region in Shapotou area. Chin. J. Appl. Ecol. 2014, 25, 61–68. [Google Scholar]
- Ehrenfeld, J.G. Encyclopedia of Biodiversity, 2nd ed.; Levin, S., Ed.; Academic Press: Cambridge, MA, USA; Princeton University: Princeton, NJ, USA, 2013; pp. 109–128. [Google Scholar]
- Inubushi, K.; Otake, S.; Furukawa, Y.; Shibasaki, N.; Ali, M.; Itang, A.M.; Tsuruta, H. Factors influencing methane emission from peat soil: Comparison of tropical and temperate wetlands. Nutr. Cycl. Agroecosyst. 2005, 71, 93–99. [Google Scholar] [CrossRef]
- Kazemian, S.; Huat, B.B.K.; Prasad, A.; Barghchi, M. State of an art review of peat: General perspective. Int. J. Phys. Sci. 2011, 6, 1974–1981. [Google Scholar]
- International Atomic Energy Agency (IAEA). Manual on measurement of methane and nitrous oxide emissions from agriculture. In Sampling Techniques and Sample Handling; IAEA-TECDOC-674; IAEA: Vienna, Austria, 1992; pp. 45–67. [Google Scholar]
- Rachwal, M.; Fernando, G.; Zanatta, J.A.; Dieckow, J.; Denega, G.L.; Curcio, G.R.; Bayer, C. Methane fluxes from waterlogged and drained Histosols of highland areas. Rev. Bras. Cienc. Solo 2014, 38, 486–494. [Google Scholar] [CrossRef] [Green Version]
- Le Mer, J.; Roger, P. Production, oxidation, emission, and consumption of methane by soils: A review. Eur. J. Soil Biol. 2001, 37, 25–50. [Google Scholar] [CrossRef]
- Farmer, J.; Matthews, R.; Smith, J.U.; Smith, P.; Singh, B.K. Assessing existing peatland models for their applicability for modelling greenhouse gas emissions from tropical peat soils. Curr. Opin. Environ. Sustain. 2011, 3, 339–349. [Google Scholar] [CrossRef]
- Pangala, S.R.; Enrich-Prast, A.; Basso, L.S.; Peixoto, R.B.; Bastviken, D.; Hornibrook, E.R.C.; Gauci, V. Large emissions from floodplain trees close the Amazon methane budget. Nature 2017, 552, 230–234. [Google Scholar] [CrossRef]
- Jauhiainen, J.; Silvennoinen, H. Diffusion GHG fluxes at tropical peatland drainage canal water surfaces. Suo 2012, 63, 93–105. [Google Scholar]
- Luta, W.; Ahmed, O.H.; Heng, R.K.J.; Choo, L.N.K. Water table fluctuation and carbon dioxide emission from a tropical peat soil cultivated with pineapples (Ananas comosus (L.) Merr.). Int. J. Biosci. 2017, 10, 172–178. [Google Scholar]
- Kløve, B.; Sveistrup, T.E.; Hauge, A. Leaching of nutrients and emission of greenhouse gases from peatland cultivation at Bodin, Northern Norway. Geoderma 2010, 154, 219–232. [Google Scholar] [CrossRef]
- Kløve, B.; Berglund, K.; Berglund, Ö.; Weldon, S.; Maljanen, M. Future options for cultivated Nordic peat soils: Can land management and rewetting control greenhouse gas emissions? Environ. Sci. Pol. 2017, 69, 85–93. [Google Scholar] [CrossRef]
- Wang, B.; Hou, L.; Liu, W.; Wang, Z. Non-microbial methane emissions from soils. Atmos. Environ. 2013, 80, 290–298. [Google Scholar] [CrossRef]
- Allen, S.J.; McKay, G.; Porter, J.F. Adsorption isotherm models for basic dye adsorption by peat in single binary component systems. J. Colloid Interf. Sci. 2004, 280, 322–333. [Google Scholar] [CrossRef]
- Udin, A.B.M.H.; Sujari, A.M.A.; Nawi, M.A.M. Effectiveness of peat coagulant for the removal of textile dyes from aqueous solution and textile wastewater. Malay J. Chem. 2003, 5, 034–043. [Google Scholar]
- Zulfikar, M.A.; Novita, E.; Hertadi, R.; Djajanti, S.D. Removal of humic acid from peat water using untreated powdered eggshell as a low cost adsorbent. Int. J. Environ. Sci. Technol. 2013, 10, 1357–1366. [Google Scholar] [CrossRef] [Green Version]
- Jassal, R.S.; Black, T.A.; Roy, R.; Ethier, G. Effect of nitrogen fertilization on soil CH4 and N2O fluxes and soil and bole respiration. Geoderma 2011, 162, 182–186. [Google Scholar] [CrossRef]





| Variable | Soil Temperature (°C) | |||
|---|---|---|---|---|
| Dry Season Wet Season | ||||
| July 2015 | August 2015 | September 2015 | December 2015 | |
| Early morning I (6:00 a.m. to 6:35 a.m.) | 30.7 a | 28.3 a | 29.0 a | 31.0 a |
| Afternoon (12:00 p.m. to 12:35 p.m.) | 25.3 a | 27.0 a | 25.7 a | 25.0 a |
| Evening (6:00 p.m. to 6:35 p.m.) | 28.3 a | 29.3 a | 29.3 a | 29.3 a |
| Midnight (12:00 a.m. to 12:35 a.m.) | 25.3 a | 28.3 a | 28.3 a | 27.0 a |
| Early morning II (6:00 a.m. to 6:35 a.m.) | 26.7 a | 28.5 a | 28.5 a | 26.7 a |
| Soil CH4 emission | r = −0.1191 p = 0.6725 | r = 0.2209 p = 0.4286 | r = −0.1386 p = 0.6224 | r = −0.0529 p = 0.8513 |
| Variable | Soil Temperature (°C) | |||||||
|---|---|---|---|---|---|---|---|---|
| Dry Season | Wet Season | |||||||
| July 2015 | August 2015 | September 2015 | December 2015 | |||||
| Low Water Table | High Water Table | Low Water Table | High Water Table | Low Water Table | High Water Table | Low Water Table | High Water Table | |
| Early morning I (6:00 a.m. to 6:35 a.m.) | 32.7 a | 32.3 a,b | 27.5 a,b | 28.3 a | 28.3 a | 27.7 a | 29.2 a | 30.3 a |
| Afternoon (12:00 p.m. to 12:35 p.m.) | 24.3 a | 25.0 a | 27.2 b | 29.0 a | 29.0 a | 28.7 a | 27.8 a | 28.0 a |
| Evening (6:00 p.m. to 6:35 p.m.) | 32.7 a | 36.0 a | 29.3 a,b | 29.3 a | 28.3 a | 30.0 a | 26.3 a | 30.7 a |
| Midnight (12:00 a.m. to 12:35 a.m.) | 28.0 a | 24.7 b | 28.3 a,b | 28.2 a | 29.3 a | 28.7 a | 29.7 a | 28.7 a |
| Early morning II (6:00 a.m. to 6:35 a.m.) | 32.0 a | 28.3 a,b | 29.7 a | 27.7 a | 28.2 a | 28.0 a | 27.0 a | 30.0 a |
| Soil CH4 emission | r = 0.2904 p = 0.2938 | r = −0.0609 p = 0.8292 | r = 0.4051 p = 0.1342 | r = −0.0299 p = 0.9156 | r = −0.0183 p = 0.9484 | r = −0.0014 p = 0.9959 | r = −0.1583 p = 0.5731 | r = −0.4843 p = 0.0674 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luta, W.; Ahmed, O.H.; Omar, L.; Heng, R.K.J.; Choo, L.N.L.K.; Jalloh, M.B.; Musah, A.A.; Abdu, A. Water Table Fluctuation and Methane Emission in Pineapples (Ananas comosus (L.) Merr.) Cultivated on a Tropical Peatland. Agronomy 2021, 11, 1448. https://doi.org/10.3390/agronomy11081448
Luta W, Ahmed OH, Omar L, Heng RKJ, Choo LNLK, Jalloh MB, Musah AA, Abdu A. Water Table Fluctuation and Methane Emission in Pineapples (Ananas comosus (L.) Merr.) Cultivated on a Tropical Peatland. Agronomy. 2021; 11(8):1448. https://doi.org/10.3390/agronomy11081448
Chicago/Turabian StyleLuta, Wendy, Osumanu Haruna Ahmed, Latifah Omar, Roland Kueh Jui Heng, Liza Nuriati Lim Kim Choo, Mohamadu Boyie Jalloh, Adiza Alhassan Musah, and Arifin Abdu. 2021. "Water Table Fluctuation and Methane Emission in Pineapples (Ananas comosus (L.) Merr.) Cultivated on a Tropical Peatland" Agronomy 11, no. 8: 1448. https://doi.org/10.3390/agronomy11081448
APA StyleLuta, W., Ahmed, O. H., Omar, L., Heng, R. K. J., Choo, L. N. L. K., Jalloh, M. B., Musah, A. A., & Abdu, A. (2021). Water Table Fluctuation and Methane Emission in Pineapples (Ananas comosus (L.) Merr.) Cultivated on a Tropical Peatland. Agronomy, 11(8), 1448. https://doi.org/10.3390/agronomy11081448








