Morphological Characterization of Southern Jalisco, Mexico, Pomegranate Genotypes Using AFLP Markers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. DNA Isolation
2.3. AFLP Analysis
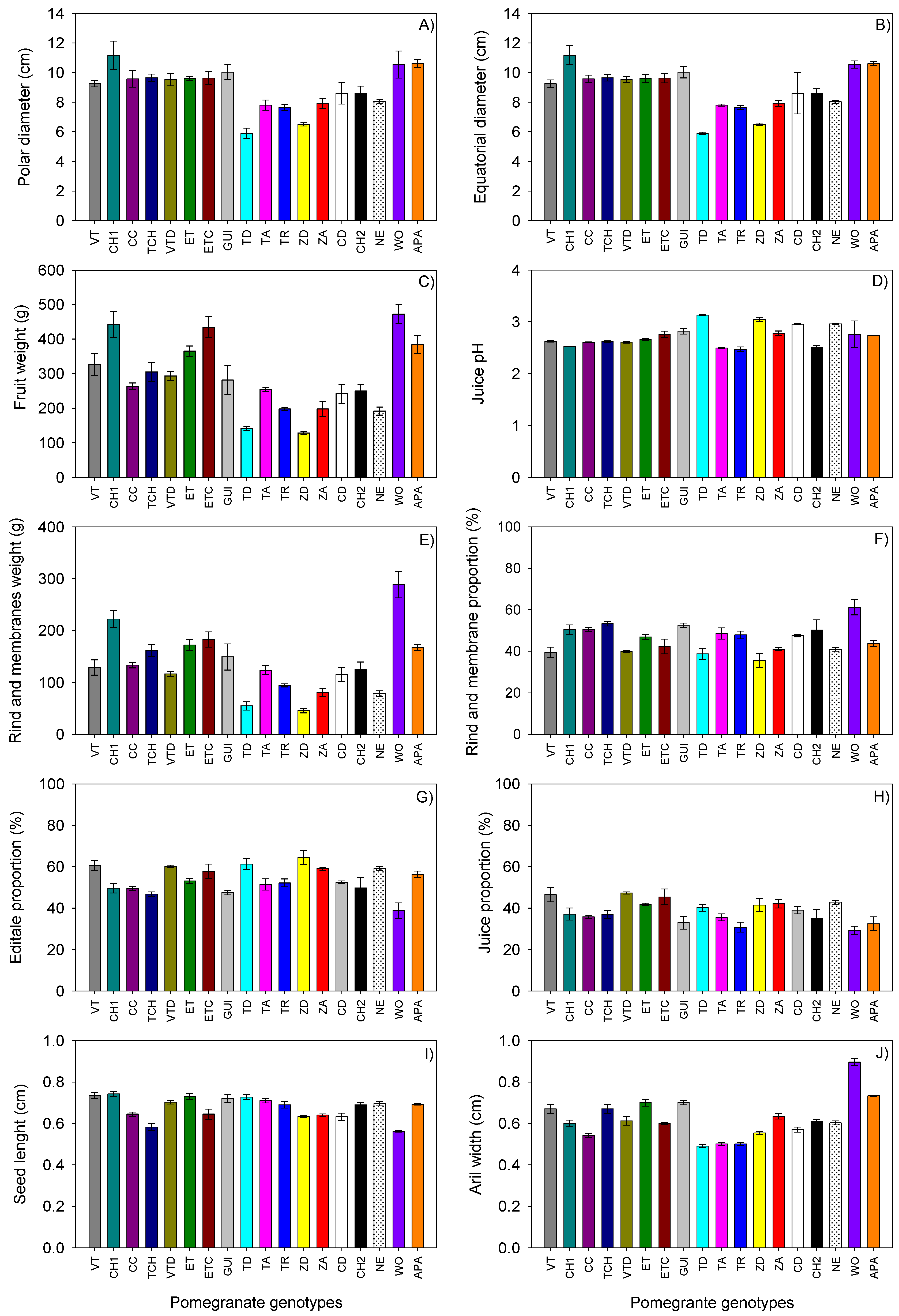
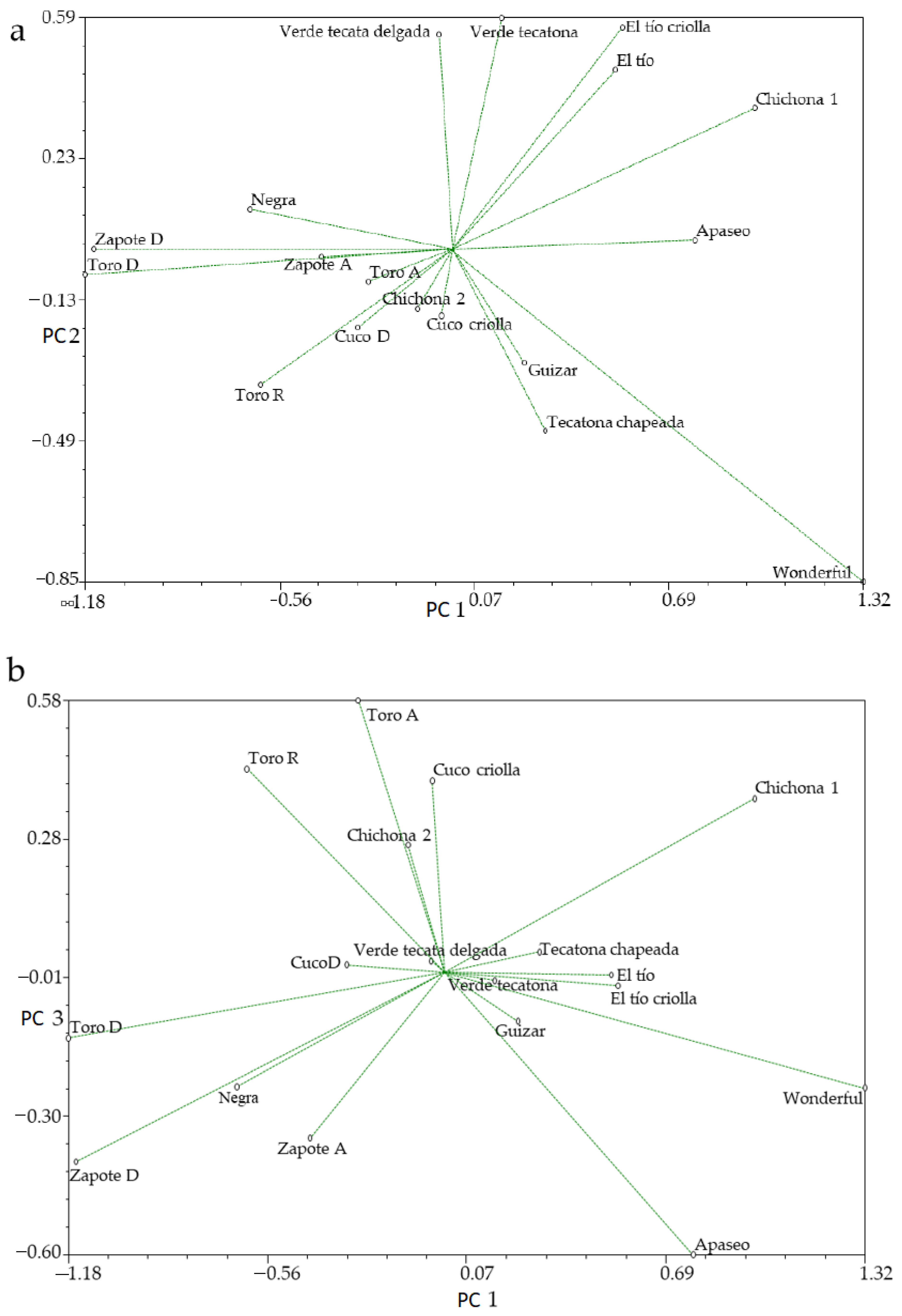
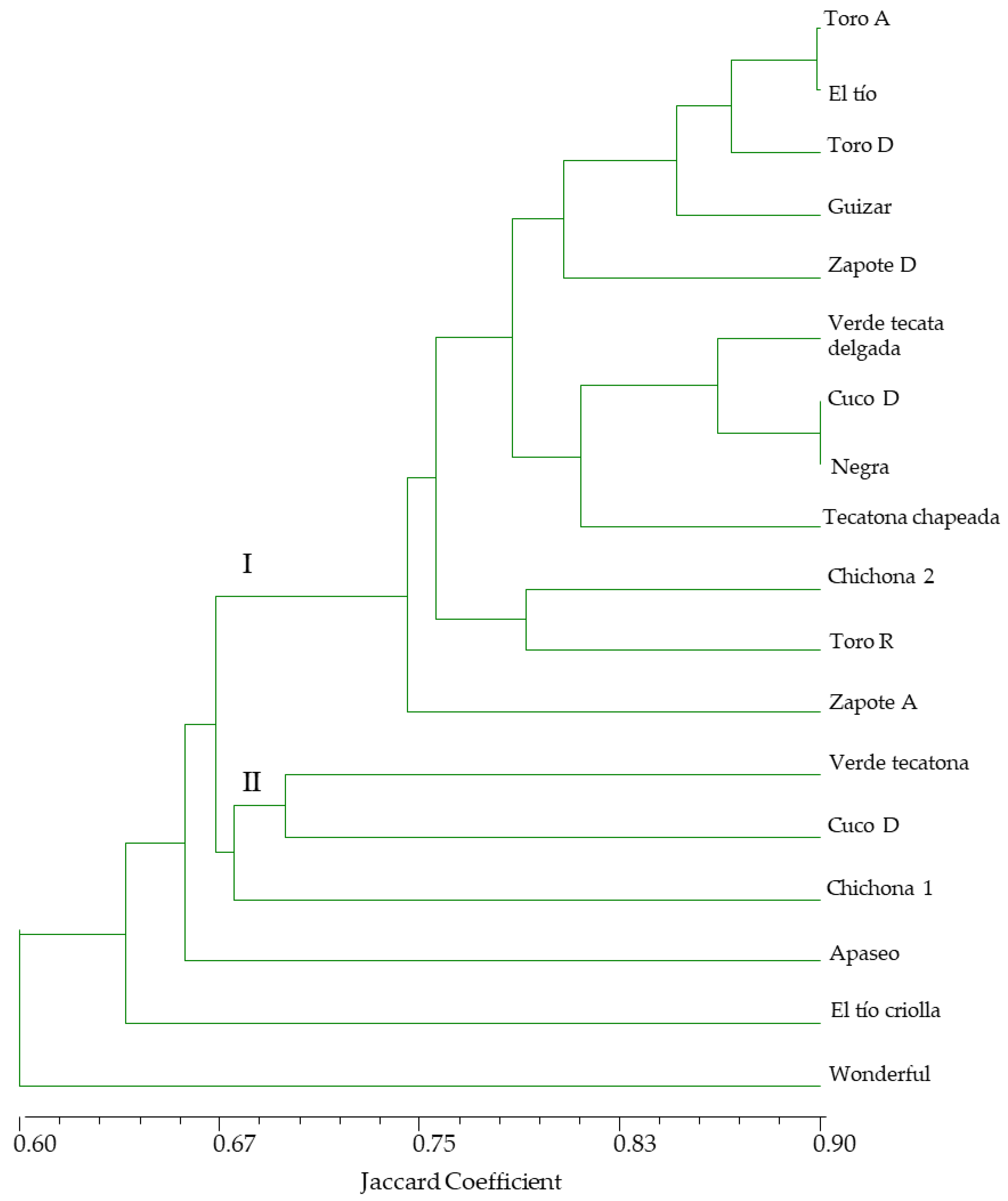
3. Results and Discussion
3.1. Principal Components Analysis
3.1.1. Morphological Analysis
3.1.2. AFLP Analysis
Level of Polymorphism
3.2. Correlation between Morphology and AFLP
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tavallali, H.; Bahmanzadegan, A.; Tavallali, V.; Rowshan, V. Phytochemical Investigation of Punica granatum Pomace: As Source of Bioactive and Medicinal Natural Products. Adv. Med. Plant Res. 2020, 8, 1–13. [Google Scholar]
- Melgarejo, M.P. El Granado, su Problemática Producción, Economía, Industrialización, Alimentación, Salud y Usos. In El Granado. I Jornadas Nacionales Sobre el Granado: Producción, Economía, Industrialización, Alimentación y Salud; Melgarejo, M.P., Hernández, G.F., Legua, M.P., Eds.; Universidad Miguel Hernández de Elche: Orihuela, Mexico, 2010; pp. 7–26. [Google Scholar]
- Rios-Corripio, G.; Guerrero-Beltrán, J.Á. Antioxidant and Physicochemical Characteristics of Unfermented and Fermented Pomegranate (Punica granatum L.) Beverages. J. Food Sci. Technol. 2019, 56, 132–139. [Google Scholar] [CrossRef]
- Jaisinghani, R.N.; Makhwana, S.; Kanojia, A. Study on Antibacterial and Flavonoid Content of Ethanolic Extract of Punica Granatum (Pomegranate) Peel. Microbiol. Res. 2018, 9, 6–9. [Google Scholar] [CrossRef] [Green Version]
- Soloklui, A.A.G.; Gharaghani, A.; Oraguzie, N.; Ramezanian, A. Shelf Life and Biochemical Changes of Ready-To-Eat Arils among Nineteen Iranian Pomegranate Cultivars (Punica granatum L.) during Storage. J. Food Sci. Technol. 2019, 56, 1416–1426. [Google Scholar] [CrossRef]
- Martini, N. Pomegranate. J. Prim. Health Care 2020, 12, 293–294. [Google Scholar] [CrossRef]
- Mahajan, D.; Bhat, Z.F.; Kumar, S. Pomegranate (Punica granatum) Rind Extract as a Novel Preservative in Cheese. Food Biosci. 2015, 12, 47–53. [Google Scholar] [CrossRef]
- Todaro, A.; Cavallaro, R.; Malfa, S.; Continella, A.; Gentile, A.; Fischer, U.A.; Carle, R.; Spagna, G. Anthocyanin Profile and Antioxidant Activity of Freshly Squeezed Pomegranate (Punica granatum L.) Juices of Sicilian and Spanish Provenances. Ital. J. Food Sci. 2016, 28, 464–479. [Google Scholar] [CrossRef]
- Khadivi, A.; Ayenehkar, D.; Kazemi, M.; Khaleghi, A. Phenotypic and Pomological Characterization of a Pomegranate (Punica granatum L.) Germplasm Collection and Identification of the Promising Selections. Sci. Hort. 2018, 238, 234–245. [Google Scholar] [CrossRef]
- Lucci, P.; Pacetti, D.; Loizzo, M.R.; Frega, N.G. Punica granatum cv. Dente di Cavallo Seed Ethanolic Extract: Antioxidant and Antiproliferative Activities. Food Chem. 2015, 167, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.N.; Bandawane, D.D.; and Mhetre, N.K. Pomegranate (Punica granatum Linn.) Leaves Attenuate Disturbed Glucosehomeostasis and Hyperglycemia Mediated Hyperlipidemia and Oxidativestress in Streptozotocin Induced Diabetic Rats. Eur. J. Integr. Med. 2014, 6, 307–332. [Google Scholar] [CrossRef]
- Zarfeshany, A.; Asgary, S.; and Javanmard, S. Potent Health Effects of Pomegranate. Adv. Biomed. Res. 2014, 3, 100. [Google Scholar] [CrossRef] [PubMed]
- Moga, M.A.; Dimienescu, O.G.; Bălan, A.; Dima, L.; Toma, S.I.; Bîgiu, N.F.; Blidaru, A. Pharmacological and Therapeutic Properties of Punica granatum Phytochemicals: Possible Roles in Breast Cancer. Molecules 2021, 26, 1054. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz-Mármol, F.; Nuncio-Jauregui, N.; García-Sanchez, F.; Martínez-Nicolás, J.J.; Hernandez, F. Characterization of Twenty Pomegranate (Punica granatum L) Cultivars Grown in Spain: Aptitudes for Fresh Consumption and Processing. Sci. Hort. 2017, 219, 152–160. [Google Scholar] [CrossRef]
- Puneeth, H.R.; Chandra, S.S.P. A Review on Potential Therapeutic Properties of Pomegranate (Punica granatum L.). Plant Sci. Today 2020, 7, 9–16. [Google Scholar] [CrossRef]
- SIAP (Servicio de Información Agroalimentaria y Pesquera). Servicio de Información Estadística Agroalimentaria y Pesquera. 2015. Available online: http://www.siap.gob.mx/cierre-de-la-produccion-agricola-por-cultivo/ (accessed on 24 April 2016).
- Patil, P.G.; Singh, N.V.; Parashuram, S.; Bohra, A.; Sowjanya, R.; Gaikwad, N.; Mundewadikar, D.M.; Sangnure, V.R.; Jamma, S.M.; Injal, A.S.; et al. Genome-Wide Characterization and Development of Simple Sequence Repeat Markers for Genetic Studies in Pomegranate (Punica granatum L.). Trees 2020, 34, 987–998. [Google Scholar] [CrossRef]
- IPGRI. Regional Report CWANA 1999–2000; International Plant Genetic Resources Institute: Rome, Italy, 2001; pp. 20–28. [Google Scholar]
- Martinez-Nicolas, J.J.; Melgarejo, P.; Legua, P.; García-Sanchez, F.; Hernández, F. Genetic Diversity of Pomegranate Germplasm Collection from Spain Determined by Fruit, Seed, Leaf and Flower Characteristics. PeerJ 2016, 4, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Sarkhosh, A.; Zamani, Z.; Fatahi, R.; Ranjbar, H. Evaluation of Genetic Diversity among Iranian Soft-Seed Pomegranate Accessions by Fruit and RAPD Markers. Sci. Hort. 2009, 121, 313–319. [Google Scholar] [CrossRef]
- Tapia-Campos, E.; Castañeda-Saucedo, M.C.; Ramírez-Anaya, J.P.; Alarcón-Domínguez, K.; Valdés-Miramontes, E.H.; Nuñez-Maciel, O. Physical-Chemical Characterization of Fourteen Pomegranate Genotypes of Southern Jalisco, Mexico. Sci. Hort. 2016, 199, 163–169. [Google Scholar] [CrossRef]
- Mansour, E.; Ben, K.A.; Triki, T.; Abid, M.; Bachar, K.; Ferchichi, A. Evaluation of Genetic Diversity among South Tunisian Pomegranate (Punica granatum L.) Accessions Using Fruit Traits and RAPD Markers. J. Agric. Sci. Technol. 2015, 17, 109–119. [Google Scholar]
- Shinde, A.S.; Mahajan, S.R.; Kakde, S.H. RAPD Based Molecular Diversity Analysis of Different Varieties of Pomegranate (Punica granatum L.). Int. J. Agric. Sci. 2015, 11, 141–145. [Google Scholar] [CrossRef]
- Narzary, D.; Mahar, K.S.; Rana, T.S.; Rana De, S.A. Analysis of Genetic Diversity among Wild Pomegranates in Western Himalayas, Using PCR Methods. Sci. Hort. 2009, 121, 237–242. [Google Scholar] [CrossRef]
- Sinjare, D.Y.K.; Jubrael, J.M. AFLP Markers for Genetic Diversity Evaluation of Pomegranate (Punica granatum L.) in Duhok Province, Kurdistan Region–Iraq. Bull. UASVM. Hortic. 2020, 77, 77–83. [Google Scholar] [CrossRef]
- Jbir, R.; Hasnaoui, N.; Mars, M.; Marrakchi, M.; Trifi, M. Characterization of Tunsian Pomegranate (Punica granatum L.) Cultivars Using Amplified Fragment Length Polymorphism Analysis. Sci. Hort. 2008, 115, 231–237. [Google Scholar] [CrossRef]
- Yuan, Z.; Yin, Y.; Qu, J.; Shu, L.; Li, Y. Population Genetic Diversity in Chinese Pomegranate (Punica granatum L) Cultivars Revealed by Flourescent-AFLP Markers. J. Genet. Genom. 2007, 34, 1061–1071. [Google Scholar] [CrossRef]
- Melgarejo, P.; Martínez, J.J.; Hernández, F.; Martínez, R.; Legua, P.; Oncina, R.; Martínez-Murcia, A. Cultivar Identification Using 18S-28S rDNA Intergenic Spacer-RFLP in Pomegranate (Punica granatum L.). Sci. Hort. 2009, 120, 500–503. [Google Scholar] [CrossRef]
- Hasnaoui, N.; Buonamici, A.; Sebastiani, F.; Mars, M.; Zhang, D.; Vendramin, G.G. Molecular Genetic Diversity of Punica granatum L. (Pomegranate) as Revealed by Microsatellite DNA Markers (SSR). Gene 2012, 493, 105–112. [Google Scholar] [CrossRef]
- Kadam, M.B.; Patil, R.S.; Supe, V.S. Morphological and Molecular Characterization of Pomegramate (Punica granatum L.) Cultivars (Maharashtra). J. Pharmacogn. Phytochem. 2018, 7, 1247–1254. [Google Scholar]
- Beghè, D.; Fabbri, A.; Petruccelli, R.; Marieschi, M.; Torelli, A.; Ganino, T. Morphological and Molecular Characterization of Ancient Pomegranate (Punica granatum L.) Accessions in Northern Italy. Adv. Hortic. Sci. 2019, 33, 581–592. [Google Scholar] [CrossRef]
- Zamani, Z.; Zarei, A.; Fatahi, R. Characterization of Progenies Derived from Pollination of Pomegranate cv. Malase-ToursheSaveh Using Fruit and RAPD Molecular Markers. Sci. Hort. 2010, 124, 67–73. [Google Scholar] [CrossRef]
- SAS Institute. SAS Versión 9, 13 Para Windows; SAS Institute: Cary, NC, USA, 2007. [Google Scholar]
- Rohlf, F.J. NTSYS-pc: Numerical Taxonomy and Multivariate Analysis System; Version 2.1; Exceter Sofware: New York, NY, USA, 2000. [Google Scholar]
- Saghai-Maroof, M.A.; Soliman, K.; Jorgensen, R.A.; Allard, R.W. Ribosomal DNA Spacer-Length Polymorphisms in Barley: Medelian Inheritance, Chromosomal Location, and Population Dynamics. Proc. Natl. Acad. Sci. USA 1984, 81, 8014–8018. [Google Scholar] [CrossRef] [Green Version]
- Vos, P.; Hogers, R.; Bleeker, M.; Reijans, M.; Van De Lee, T.; Hornes, M.; Frijters, A.; Pot, J.; Peleman, J.; Kuiper, M. AFLP: A New Technique for DNA Fingerprinting. Nucleic Acids Res. 1995, 23, 4407–4414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassam, B.J.; Caetano-Anolles, G. Silver Staining of DNA in Polyacrylamide Gels. Appl. Biochem. Biotech. 1993, 42, 181–188. [Google Scholar] [CrossRef]
- Caliskan, O.; and Bayazit, S. Morpho-pomological and Chemical Diversity of Pomegranate Accessions Grown in Eastern Mediterranean Region of Turkey. J. Agric. Sci. Technol. 2013, 15, 1449–1460. [Google Scholar]
- Martínez, J.J.; Hernández, F.; Abdelmajid, H.; Legua, P.; Martínez, R.; Amine, A.R.; Melgarejo, P. Physico-Chemical Characterization of Six Pomegranate Cultivars from Morocco: Processing and Fresh Market Aptitudes. Sci. Hort. 2012, 140, 100–106. [Google Scholar] [CrossRef]
- Hernández, F.; Legua, L.; Martínez, R.; Melgarejo, P.; Martínez, J.J. Fruit Quality Characterization of Seven Pomegranate Accessions (Punica granatum L.) Grown in Southeast of Spain. Sci. Hort. 2014, 175, 174–180. [Google Scholar] [CrossRef]
- Al-Said, F.A.; Opara, L.U.; Al-Yahyai, R.A. Physico-Chemical and Textural Quality Attributes of Pomegranate Cultivars (Punica granatum L.) Grown in the Sultanate of Oman. J. Food Eng. 2009, 90, 129–134. [Google Scholar] [CrossRef]
- Martínez, J.J.; Melgarejo, P.; Hernández, F.; Salazar, D.M.; Martínez, R. Seed Characterization of Five New Pomegranate (Punica granatum L.) Varieties. Sci. Hort. 2006, 110, 241–246. [Google Scholar] [CrossRef]
- Orak, H.H.; Yagar, H.; Isbilir, S.S. Comparison of Antioxidant Activities of Juice, Peel, and Seed of Pomegranate (Punica granatum L.) and Inter-Relationships with Total Phenolic, Tannin, Anthocyanin, and Flavonoid Contents. Food Sci. Biotechnol. 2012, 21, 373–387. [Google Scholar] [CrossRef]

| Genotypes | Taste | Geographical Coordinates | Altitude |
|---|---|---|---|
| Zapote D | sweet | 20°07′56″ N 103°32′04″ W | 1354 |
| Zapote A | sour | 20°07′56″ N 103°32′04″ W | 1354 |
| Verde tecatona | sour | 19°34′17″ N 103°30′36″ W | 1781 |
| Cuco D | sweet | 19°34′17″ N 103°30′36″ W | 1781 |
| Chichona 1 | sour | 19°34′17″ N 103°30′36″ W | 1781 |
| Cuco criolla | sour | 19°34′17″ N 103°30′36″ W | 1781 |
| Tecatona chapeada | sour | 19°34′17″ N 103°30′36″ W | 1781 |
| Verde tecata delgada | sour | 19°34′17″ N 103°30′36″ W | 1781 |
| Chichona 2 | sour | 19°41′20″ N 103°30′26″ W | 1563 |
| El tío | sweet-sour | 19°43′08″ N 103°28′11″ W | 1511 |
| El tío criolla | sour | 19°43′08″ N 103°28′11″ W | 1511 |
| Guizar | sweet-sour | 19°43′08″ N 103°28′11″ W | 1511 |
| Negra | sour | 19°37′21″ N 103°24′42″ W | 1298 |
| Toro D | sweet | 19°41′51″ N 103°29′33″ W | 1527 |
| Toro A | sour | 19°41′51″ N 103°29′33″ W | 1527 |
| Toro R | sweet-sour | 19°41′51″ N 103°29′33″ W | 1527 |
| Wonderful | sweet-sour | 19°41′20″ N 103°30′26″ W | 1563 |
| Apaseo | sweet | 19°41′20″ N 103°30′26″ W | 1563 |
| Characters | Mean | Std Dev | Min | Max | PC1 | PC2 | PC3 |
|---|---|---|---|---|---|---|---|
| Polar diameter (PD, cm) | 8.92 | 1.42 | 5.9 | 11.18 | 0.9402 | 0.0767 | 0.0368 |
| Equatorial diameter (DE, cm) | 8.14 | 1.25 | 6.13 | 10.48 | 0.9112 | 0.194 | 0.1072 |
| Fruit weight (FW, g), | 287.33 | 101.28 | 128.4 | 472.4 | 0.9705 | 0.1411 | 0.0209 |
| Rind thickness (RT, mm) | 0.4154 | 0.0917 | 0.278 | 0.625 | 0.7675 | −0.0832 | 0.067 |
| Number of seeds per fruit (SN) | 579.06 | 124.51 | 397 | 811.5 | 0.6579 | 0.3968 | 0.3295 |
| Rind and membrane weight (RMW, g) | 135.43 | 59.35 | 45.53 | 288.52 | 0.9595 | −0.1621 | 0.081 |
| Aril weight (ArW, g) | 151.90 | 49.36 | 82.88 | 252.13 | 0.8377 | 0.4844 | −0.0545 |
| Juice per fruit (JU, mL) | 110.72 | 40.58 | 53.21 | 197.93 | 0.8068 | 0.5159 | 0.0144 |
| Seed weight (fibrous part, SeW, mg) | 41.066 | 13.32 | 29.2 | 84 | 0.647 | 0.1866 | −0.2075 |
| Juice pH | 2.73 | 0.1948 | 2.47 | 3.13 | −0.4512 | −0.0756 | −0.6798 |
| Total soluble solids (TSS, Brix) | 14.95 | 1.2634 | 12.7 | 17 | 0.3317 | −0.0751 | −0.0032 |
| Edible proportion (EP, %) | 53.88 | 6.47 | 38.77 | 64.48 | −0.6268 | 0.6592 | −0.3612 |
| Juice proportion (JP, %) | 38.49 | 5.35 | 29.32 | 47.29 | −0.2798 | 0.8077 | −0.1791 |
| Seed proportion (PRS, %) | 15.34 | 4.092 | 9.75 | 23.01 | −0.6313 | −0.0395 | −0.3014 |
| Rind and membrane proportion (PRRM, %) | 46.12 | 6.4741 | 35.52 | 61.23 | 0.6268 | −0.6592 | 0.3612 |
| Weight of one seed (WOS, g) | 0.0721 | 0.0771 | 0.0534 | 0.1157 | 0.5502 | −0.4118 | −0.5064 |
| Aril width (AW, cm) | 0.6215 | 0.0990 | 0.49 | 0.8961 | 0.7541 | −0.234 | −0.4917 |
| Aril length (AL, cm) | 0.9123 | 0.0785 | 0.8 | 1.096 | 0.5837 | 0.2795 | −0.3502 |
| Thickness | 0.5200 | 0.1090 | 0.3 | 0.7 | 0.672 | 0.1753 | −0.4335 |
| Seed width (fibrous part, SW, g) | 0.3150 | 0.0435 | 0.245 | 0.415 | 0.3483 | −0.4136 | −0.4296 |
| Seed length (SL, cm) | 0.6764 | 0.0526 | 0.5616 | 0.7425 | −0.1406 | 0.6102 | 0.2444 |
| Eigenvalue | 9.750 | 3.200 | 2.078 | ||||
| Proportion | 46.432 | 15.2419 | 9.8962 | ||||
| Cumulative | 46.432 | 61.6739 | 71.5702 |
| Primer Combination | Total Fragments | Polymorphic Fragments | Polymorphism % |
|---|---|---|---|
| Mse + CTA/Eco +AGG | 48 | 32 | 66 |
| Mse + CAT/Eco + ACC | 44 | 22 | 50 |
| Mse + CAG/Eco + ACC | 27 | 17 | 63 |
| Mse + CAG/Eco + ACT | 67 | 50 | 74 |
| Mse + CAT/Eco + AGG | 92 | 78 | 84 |
| Mse + CTA/Eco + ACC | 37 | 30 | 81 |
| Total | 315 | 229 | 72.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tapia-Campos, E.; del Pilar Ramírez-Anaya, J.; Cavazos-Garduño, A.; Serrano-Niño, J.C.; Fragoso-Jiménez, J.C.; Castañeda-Saucedo, M.C. Morphological Characterization of Southern Jalisco, Mexico, Pomegranate Genotypes Using AFLP Markers. Agronomy 2021, 11, 1449. https://doi.org/10.3390/agronomy11081449
Tapia-Campos E, del Pilar Ramírez-Anaya J, Cavazos-Garduño A, Serrano-Niño JC, Fragoso-Jiménez JC, Castañeda-Saucedo MC. Morphological Characterization of Southern Jalisco, Mexico, Pomegranate Genotypes Using AFLP Markers. Agronomy. 2021; 11(8):1449. https://doi.org/10.3390/agronomy11081449
Chicago/Turabian StyleTapia-Campos, Ernesto, Jessica del Pilar Ramírez-Anaya, Adriana Cavazos-Garduño, Julio C. Serrano-Niño, Javier Cuauhtémoc Fragoso-Jiménez, and Ma. Claudia Castañeda-Saucedo. 2021. "Morphological Characterization of Southern Jalisco, Mexico, Pomegranate Genotypes Using AFLP Markers" Agronomy 11, no. 8: 1449. https://doi.org/10.3390/agronomy11081449
APA StyleTapia-Campos, E., del Pilar Ramírez-Anaya, J., Cavazos-Garduño, A., Serrano-Niño, J. C., Fragoso-Jiménez, J. C., & Castañeda-Saucedo, M. C. (2021). Morphological Characterization of Southern Jalisco, Mexico, Pomegranate Genotypes Using AFLP Markers. Agronomy, 11(8), 1449. https://doi.org/10.3390/agronomy11081449







