Management of Phosphorus in Salinity-Stressed Agriculture for Sustainable Crop Production by Salt-Tolerant Phosphate-Solubilizing Bacteria—A Review
Abstract
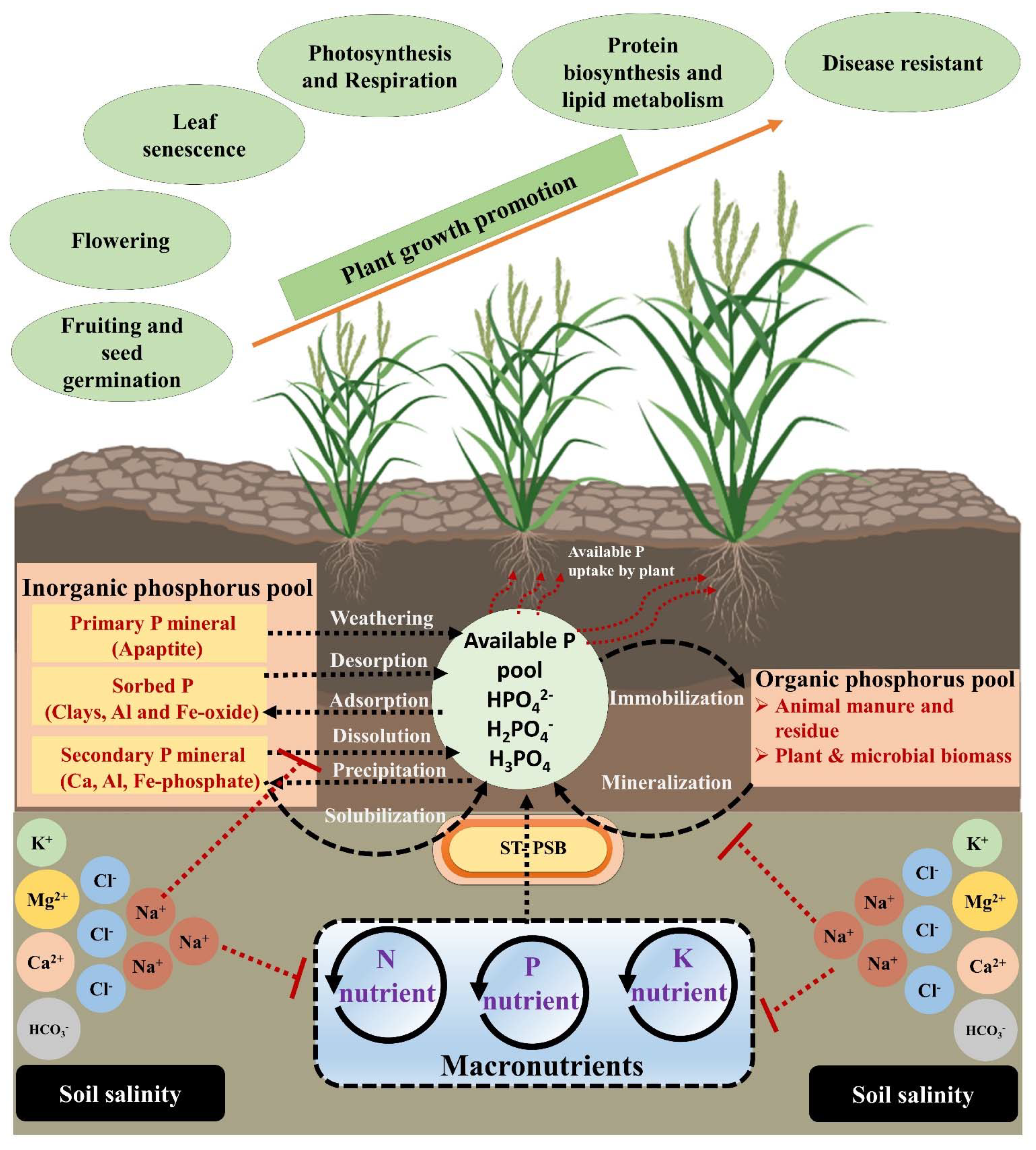
:1. Introduction
2. Soil Salinization and Its Effect on P Availability
2.1. Soil Salinization
2.2. Salinity Effect on P Availability in Soil
2.3. Effect of Salinity on P Uptake and Accumulation in the Plant
3. Plant Response to Salinity and P Deficiency
4. Diversity of Salt-Tolerant Phosphate Solubilizing Bacteria
5. Mechanism of P Mobilization by Salt-Tolerant Phosphate Solubilizing Bacteria
5.1. Mechanism of Inorganic P Solubilization
5.1.1. P-Solubilization through Acidification
5.1.2. P-Solubilization through Protonation
5.1.3. P-Solubilization through Chelation
5.1.4. Solubilization by Extracellular Polymeric Substances
5.2. Mechanism of Organic Phosphate Mineralization
5.2.1. Phosphatase Activity
5.2.2. Phytase Activity
5.2.3. Phosphonatases and C–P Lyase Activity
6. The Role of ST-PSB in Plant Growth Promotion under Salinity
6.1. Ionic Homeostasis and Nutritional Balance
6.2. Mitigation of Osmotic and Oxidative Stress
6.3. Hormonal Modulation to Salt Tolerance Plant
6.4. Reduced Plant Susceptibility to Pathogens
7. Application of ST-PSB in Sustainable Agriculture in Saline Environments
7.1. Salt Alleviator and Phosphate Biofertilizer
7.2. ST-PSB as a Biocontrol Agent
7.3. Bioremediation
8. Future Prospects
- (i)
- The comparative studies of interactions between plants and ST-PSB, which are currently lacking, would clarify about the mechanisms governing salinity stresses.
- (ii)
- Limited knowledge about the mechanisms by which ST-PSB-mediated P nutrition modifies the salt tolerance and P uptake of plants (specially P starvation gene expression), which should be further studied in the future.
- (iii)
- Since the ability of PSB to dissolve inorganic insoluble phosphate and mineralize organic phosphates decreases under salinity stress, in order to select and introduce the best ST-PSB, it is recommended that the screening of these bacteria be investigated in the presence of salinity and drought stress because in most studies these bacteria were screened under non-stress conditions.
- (iv)
- An assessment to select the best ST-PSB should be undertaken in poor soils because most these PSB are sensitive to environmental stresses. Under such conditions, PSB that can compete for limited resources should be selected.
- (v)
- Since both drought and salinity stress cause osmotic stress to plants, in order to select and introduce the best PSB, it is recommended that the efficacy of these bacteria in the presence of salinity and drought stress be investigated concurrently because most studies have investigated the effect of these bacteria on alleviating either salinity stress or drought stress in plants.
- (vi)
- Since most PSB are heterotrophic and require organic matter as a source of carbon and energy, in order to improve the efficiency of these bacteria in saline soils, it is recommended to use them along with organic matter in saline soils, which are mostly poor in organic matter.
- (vii)
- Most research has been executed on culturable PSB. Since most plant-associated microorganisms including PSB are un–culturable, more research is needed on un–culturable PSB in plant rhizosphere and endosphere, which will lead to an improved knowledge of the PSB community and the PSB-plant interactions.
- (viii)
- The expansion of basic science about the interactions between PSB and salinity-stressed plants facilitates a better understanding of decreased salinity stresses and possibly provides better forecasts about salinity-stressed plant responses. Therefore, it is anticipated that one of the most interesting research areas in the future for agricultural scientists will be to investigate the PSB mechanisms involved in decreasing salinity stress on plants.
- (ix)
- It is known that the plants inoculated with a combination of PSB and arbuscular mycorrhizal fungi (AMF) can express synergistic effects to increase salinity-stressed plant growth indices, while maintaining safe natural resources, such as P stocks. Therefore, further research to evaluate the application and the efficacy of PSB as independent inoculations or as a co–inoculation with AMF under various environmental stresses including salinity stress on various crops fertilized with different low-solubility P sources in field conditions is necessary, where the survival of PSB and AMF, as well as how the mechanisms by which they promote plant growth, are cramped by competition with the endemic microorganisms, environmental stresses, and soil conditions (phosphorus sorption capacity, soil phosphorus status, pH, etc.).
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilbert, N.; Gewin, V.; Tollefson, J.; Sachs, J.; Potrykus, I. How to feed a hungry world. Nature 2010, 466, 531–532. [Google Scholar]
- Mitter, E.K.; Tosi, M.; Obregón, D.; Dunfield, K.E.; Germida, J.J. Rethinking Crop Nutrition in Times of Modern Microbiology: Innovative Biofertilizer Technologies. Front. Sustain. Food Syst. 2021, 5, 29. [Google Scholar] [CrossRef]
- Malhotra, H.; Sharma, S.; Pandey, R. Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Singapore, 2018; pp. 171–190. [Google Scholar]
- Adhikari, A.; Khan, M.A.; Lee, K.E.; Kang, S.M.; Dhungana, S.K.; Bhusal, N.; Lee, I.J. The Halotolerant Rhizobacterium—Pseudomonas koreensis MU2 Enhances Inorganic Silicon and Phosphorus Use Efficiency and Augments Salt Stress Tolerance in Soybean (Glycine max L.). Microorganisms 2020, 8, 1256. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Qu, L.; Hong, X.; Sun, X. Isolation and characterization of a phosphate solubilizing halophilic bacterium Kushneria sp. YCWA18 from Daqiao Saltern on the coast of yellow sea of China. Evid. Based Complement. Altern. Med. 2011, 2011, 615032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talbi Zribi, O.; Abdelly, C.; Debez, A. Interactive effects of salinity and phosphorus availability on growth, water relations, nutritional status and photosynthetic activity of barley (Hordeum vulgare L.). Plant Biol. 2011, 13, 872–880. [Google Scholar] [CrossRef]
- Power, J.F.; Grunes, D.L.; Reichman, G.A.; Willis, W.O. Soil Temperature Effects on Phosphorus Availability. J. Agron. 1964, 56, 545–548. [Google Scholar] [CrossRef]
- Keith, H.; Jacobsen, K.L.; Raison, R. Effects of soil phosphorus availability, temperature and moisture on soil respiration in Eucalyptus pauciflora forest. Plant Soil 1997, 190, 127–141. [Google Scholar] [CrossRef]
- Fink, J.R.; Inda, A.V.; Tiecher, T.; Barrón, V. Iron oxides and organic matter on soil phosphorus availability. Cienc. E Agrotecnologia 2016, 40, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant. Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Tchakounté, G.V.T.; Berger, B.; Patz, S.; Becker, M.; Fankem, H.; Taffouo, V.D.; Ruppel, S. Selected Rhizosphere Bacteria Help Tomato Plants Cope with Combined Phosphorus and Salt Stresses. Microorganisms 2020, 8, 1844. [Google Scholar] [CrossRef]
- Kafi, M.; Khan, M.A. Crop and Forage Production Using Saline Waters; Daya Books; Daya Publishing House: New Delhi, India, 2008. [Google Scholar]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225246. [Google Scholar] [CrossRef]
- Hossain, M.S. Present scenario of global salt affected soils, its management and importance of salinity research. Int. Res. J. Biol. Sci 2019, 1, 1–3. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Bhowmik, P.C.; Hossain, M.; Rahman, M.M.; Prasad, M.N.V.; Ozturk, M.; Fujita, M. Potential use of halophytes to remediate saline soils. Biomed. Res. Int. 2014, 2014, 589341. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Xie, X.; Kim, H.I.; Kisugi, T.; Nomura, T.; Sekimoto, H.; Yokota, T.; Yoneyama, K. How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta 2012, 235, 1197–1207. [Google Scholar] [CrossRef] [Green Version]
- Kosová, K.; Prášil, I.T.; Vítámvás, P. Protein contribution to plant salinity response and tolerance acquisition. Int. J. Mol. Sci. 2013, 14, 6757–6789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carstensen, A.; Herdean, A.; Schmidt, S.B.; Sharma, A.; Spetea, C.; Pribil, M.; Husted, S. The impacts of phosphorus deficiency on the photosynthetic electron transport chain. Plant Physiol. 2018, 177, 271–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokrani, S.; Nabti, E.H.; Cruz, C. Current Advances in Plant Growth Promoting Bacteria Alleviating Salt Stress for Sustainable Agriculture. Appl. Sci. 2020, 10, 7025. [Google Scholar] [CrossRef]
- Besri, M. Effects of salinity on plant diseases development. In Towards the Rational Use of High Salinity Tolerant Plants; Springer: Dordrecht, The Netherlands, 1993; pp. 67–74. [Google Scholar]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Talbi Zribi, O.; Slama, I.; Trabelsi, N.; Hamdi, A.; Smaoui, A.; Abdelly, C. Combined effects of salinity and phosphorus availability on growth, gas exchange, and nutrient status of Catapodium rigidum. Arid. Land Res. Manag. 2018, 32, 277–290. [Google Scholar] [CrossRef]
- Grattan, S.R.; Grieve, C.M. Mineral element acquisition and growth response of plants grown in saline environments. Agric. Ecosyst. Environ. 1992, 38, 275–300. [Google Scholar] [CrossRef]
- Bidalia, A.; Vikram, K.; Yamal, G.; Rao, K.S. Effect of salinity on soil nutrients and plant health. In Salt Stress, Microbes, and Plant Interactions: Causes and Solution; Springer: Singapore, 2019; pp. 273–297. [Google Scholar]
- Grattan, S.R.; Grieve, C.M. Salinity–mineral nutrient relations in horticultural crops. Sci. Hortic. 1998, 78, 127–157. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, T.L. Cadmium and phosphorous fertilizers: The issues and the science. Procedia Eng. 2014, 83, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Zaidi, A.; Wani, P.A.; Oves, M. Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils. Environ. Chem. Lett. 2009, 7, 1–19. [Google Scholar] [CrossRef]
- Gyaneshwar, P.; Kumar, G.N.; Parekh, L.J.; Poole, P.S. Role of soil microorganisms in improving P nutrition of plants. Plant Soil 2002, 245, 83–93. [Google Scholar] [CrossRef]
- Walpola, B.C.; Yoon, M.H. Prospectus of phosphate solubilizing microorganisms and phosphorus availability in agricultural soils: A review. Afr. J. Microbiol. Res. 2012, 6, 6600–6605. [Google Scholar]
- Kalayu, G. Phosphate solubilizing microorganisms: Promising approach as biofertilizers. Int. J. Agron. 2019, 2019, 4917256. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Y.; Sun, J.; Shao, H. Negative interactive effects between biochar and phosphorus fertilization on phosphorus availability and plant yield in saline sodic soil. Sci. Total Environ. 2016, 568, 910–915. [Google Scholar] [CrossRef]
- Wu, L.; Wei, C.; Zhang, S.; Wang, Y.; Kuzyakov, Y.; Ding, X. MgO-modified biochar increases phosphate retention and rice yields in saline-alkaline soil. J. Clean. Prod. 2019, 235, 901–909. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, S.; Wang, J.; Ding, X. Phosphorus retention using iron (II/III) modified biochar in saline-alkaline soils: Adsorption, column and field tests. Environ. Pollut. 2020, 261, 114223. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Kheir, A.M.; Ali, M.G.; Ali, O.A.; Abdelaal, A.I.; Zhou, Z.; Wang, B.; Liu, B.; He, Z. The integrated effect of salinity, organic amendments, phosphorus fertilizers, and deficit irrigation on soil properties, phosphorus fractionation and wheat productivity. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, A.; Lal, R. The biochar dilemma. Soil Res. 2014, 52, 217–230. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, J.S.; Saxena, A.K.; Singh, D.P. Impact of PGPR inoculation on growth and antioxidant status of wheat under saline conditions. Plant Biol. 2012, 14, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.K.; Singh, D.P. Effect of salt-tolerant plant growth promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biol. 2015, 17, 288–293. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 2004, 42, 565–572. [Google Scholar] [CrossRef]
- Mishra, P.K.; Mishra, S.; Bisht, S.C.; Selvakumar, G.; Kundu, S.; Bisht, J.K.; Gupta, H.S. Isolation, molecular characterization and growth-promotion activities of a cold tolerant bacterium Pseudomonas sp. NARs9 (MTCC9002) from the Indian Himalayas. Biol. Res. 2009, 42, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Adnan, M.; Fahad, S.; Zamin, M.; Shah, S.; Mian, I.A.; Danish, S.; Zafar-ul-Hye, M.; Battaglia, M.L.; Naz, R.M.M.; Saeed, B.; et al. Coupling phosphate-solubilizing bacteria with phosphorus supplements improve maize phosphorus acquisition and growth under lime induced salinity stress. Plants 2020, 9, 900. [Google Scholar] [CrossRef]
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Allaoui, A.; Biskri, L. Plant Growth Enhancement using Rhizospheric Halotolerant Phosphate Solubilizing Bacterium Bacillus licheniformis QA1 and Enterobacter asburiae QF11 Isolated from Chenopodium quinoa Willd. Microorganisms 2020, 8, 948. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Ahmad, I.; Pichtel, J. Growth stimulation and alleviation of salinity stress to wheat by the biofilm forming Bacillus pumilus strain FAB10. Appl. Soil Ecol. 2019, 143, 45–54. [Google Scholar] [CrossRef]
- Yasmeen, T.; Ahmad, A.; Arif, M.S.; Mubin, M.; Rehman, K.; Shahzad, S.M.; Iqbal, S.; Rizwan, M.; Ali, S.; Alyemeni, M.N.; et al. Biofilm forming rhizobacteria enhance growth and salt tolerance in sunflower plants by stimulating antioxidant enzymes activity. Plant Physiol. Biochem. 2020, 156, 242–256. [Google Scholar] [CrossRef]
- Rengasamy, P. Soil salinity and sodicity. In Growing Crops with Reclaimed Wastewater; CABI Publishing: Collingwood, Australia, 2006; pp. 125–138. [Google Scholar]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; LWW: Philadelphia, PA, USA, 1954; p. 154. [Google Scholar]
- Kumar, P.; Sharma, P.K. Soil Salinity and Food Security in India. Front. Sustain. Food Syst. 2020, 4, 533781. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil salinity under climate change: Challenges for sustainable agriculture and food security. J. Environ. Manag. 2020, 280, 111736. [Google Scholar] [CrossRef]
- Abbas, R.; Rasul, S.; Aslam, K.; Baber, M.; Shahid, M.; Mubeen, F.; Naqqash, T. Halotolerant PGPR: A hope for cultivation of saline soils. J. King Saud Univ. Sci. 2019, 31, 1195–1201. [Google Scholar] [CrossRef]
- Fageria, N.K.; Gheyi, H.R.; Moreira, A. Nutrient bioavailability in salt affected soils. J. Plant Nutr. 2011, 34, 945–962. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalal, R.C. Soil organic phosphorus. Adv. Agron. 1977, 29, 83–117. [Google Scholar]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Beji, R.; Hamdi, W.; Kesraoui, A.; Seffen, M. Effects of salts on phosphorus adsorption in alkalize Tunisian soil. Euro-Mediterr. J. Environ. Integr. 2017, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Fageria, N.K.; Moreira, A. The role of mineral nutrition on root growth of crop plants. Adv. Agron. 2011, 110, 251–331. [Google Scholar]
- Nasrin, S.; Biswas, T.K.; Amin, M.S.; Khatun, M. Study of salinity effects on the inorganic phosphorus transformation in three different soil series of Ganges River Floodplain. Jahangirnagar Univ. J. Biol. Sci. 2016, 5, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Shi, Y.; Cui, X.; Yue, P.; Li, K.; Liu, X.; Tripathi, B.M.; Chu, H. Salinity is a key determinant for soil microbial communities in a desert ecosystem. MSystems 2019, 4, e00225-18. [Google Scholar] [CrossRef] [Green Version]
- Champagnol, F. Relationships between phosphate nutrition of plants and salt toxicity. Phosphorus Agric. 1979, 33, 35–43.ill. [Google Scholar]
- Sharpley, A.N.; Meisinger, J.J.; Power, J.F.; Suarez, D.L. Root extraction of nutrients associated with long-term soil management. In Limitations to Plant Root Growth; Springer: New York, NY, USA, 1992; pp. 151–217. [Google Scholar]
- Tang, H.; Niu, L.; Wei, J.; Chen, X.; Chen, Y. Phosphorus limitation improved salt tolerance in maize through tissue mass density increase, osmolytes accumulation, and Na+ uptake inhibition. Front. Plant Sci. 2019, 10, 856. [Google Scholar] [CrossRef] [Green Version]
- Phang, T.H.; Shao, G.; Liao, H.; Yan, X.; Lam, H.M. High external phosphate (Pi) increases sodium ion uptake and reduces salt tolerance of ‘Pi-tolerant’ soybean. Physiol. Plant. 2009, 135, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.P.; White, P.J. Sucrose transport in the phloem: Integrating root responses to phosphorus starvation. J Exp. Bot. 2008, 59, 93–109. [Google Scholar] [CrossRef] [PubMed]
- López-Arredondo, D.L.; Leyva-González, M.A.; González-Morales, S.I.; López-Bucio, J.; Herrera-Estrella, L. Phosphate nutrition: Improving low-phosphate tolerance in crops. Annu. Rev. Plant Biol. 2014, 65, 95–123. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Rohman, M.M.; Ahmed, I.; Molla, M.R.; Hossain, M.A.; Amiruzzaman, M. Evaluation of salt tolerant mungbean (Vigna radiata L.) Genotypes on growth through bio-molecular approaches. Bangladesh J. Agric. Res. 2019, 44, 469–492. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Yang, X.J.; Finnegan, P.M. Regulation of phosphate starvation responses in higher plants. Ann. Bot. 2010, 105, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Chen, A.; Sun, S.; Xu, G. Complex regulation of plant phosphate transporters and the gap between molecular mechanisms and practical application: What is missing? Mol. Plant 2016, 9, 396–416. [Google Scholar] [CrossRef] [Green Version]
- Aslam, M.M.; Akhtar, K.; Karanja, J.K.; Haider, F.U. Understanding the Adaptive Mechanisms of Plant in Low Phosphorous Soil. In Plant Stress Physiology; IntechOpen: London, UK, 2020. [Google Scholar]
- Qin, Y.; Druzhinina, I.S.; Pan, X.; Yuan, Z. Microbially mediated plant salt tolerance and microbiome-based solutions for saline agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Ruppel, S.; Franken, P.; Witzel, K. Properties of the halophyte microbiome and their implications for plant salt tolerance. Funct. Plant Biol. 2013, 40, 940–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etesami, H.; Beattie, G.A. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front. Microbiol. 2018, 9, 148. [Google Scholar] [CrossRef] [Green Version]
- Yan, N.; Marschner, P.; Cao, W.; Zuo, C.; Qin, W. Influence of salinity and water content on soil microorganisms. Int. Soil Water Conserv. Res. 2015, 3, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Son, H.J.; Park, G.T.; Cha, M.S.; Heo, M.S. Solubilization of insoluble inorganic phosphates by a novel salt-and pH-tolerant Pantoea agglomerans R-42 isolated from soybean rhizosphere. Bioresour. Technol. 2006, 97, 204–210. [Google Scholar] [CrossRef]
- Park, K.H.; Lee, O.M.; Jung, H.I.; Jeong, J.H.; Jeon, Y.D.; Hwang, D.Y.; Lee, C.Y.; Son, H.J. Rapid solubilization of insoluble phosphate by a novel environmental stress-tolerant Burkholderia vietnamiensis M6 isolated from ginseng rhizospheric soil. Appl. Microbiol. Biotechnol. 2010, 86, 947–955. [Google Scholar] [CrossRef]
- Srinivasan, R.; Yandigeri, M.S.; Kashyap, S.; Alagawadi, A.R. Effect of salt on survival and P-solubilization potential of phosphate solubilizing microorganisms from salt affected soils. Saudi J. Biol. Sci. 2012, 19, 427–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Wang, T.; Chi, X.; Wang, M.; Chen, N.; Chen, M.; Pan, L.; Qi, P. Isolation and characterization of halotolerant phosphate solubilizing bacteria naturally colonizing the peanut rhizosphere in salt-affected soil. Geomicrobiol. J. 2020, 37, 110–118. [Google Scholar] [CrossRef]
- Jiang, H.; Qi, P.; Wang, T.; Wang, M.; Chen, M.; Chen, N.; Pan, L.; Chi, X. Isolation and characterization of halotolerant phosphate-solubilizing microorganisms from saline soils. 3 Biotech. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.C.; Yadav, H.; Singh, S.K.; Mishra, R.R.; Sethi, B.K.; Dutta, S.K.; Thatoi, H.N. Phosphate solubilization and acid phosphatase activity of Serratia sp. isolated from mangrove soil of Mahanadi river delta, Odisha, India. J. Genet Eng. Biotechnol. 2017, 15, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.C.; Yadav, H.; Singh, S.K.; Sethi, B.K.; Mishra, R.R.; Kumari, S.; Thatoi, H. Alkaline phosphatase activity of a phosphate solubilizing Alcaligenes faecalis, isolated from Mangrove soil. Biotechnol. Res. Innov. 2017, 1, 101–111. [Google Scholar] [CrossRef]
- Noskova, Y.; Likhatskaya, G.; Terentieva, N.; Son, O.; Tekutyeva, L.; Balabanova, L. A Novel Alkaline Phosphatase/Phosphodiesterase, CamPhoD, from Marine Bacterium Cobetia amphilecti KMM 296. Mar. Drugs 2019, 17, 657. [Google Scholar] [CrossRef] [Green Version]
- Malboobi, M.A.; Owlia, P.; Behbahani, M.; Sarokhani, E.; Moradi, S.; Yakhchali, B.; Deljou, A.; Heravi, K.M. Solubilization of organic and inorganic phosphates by three highly efficient soil bacterial isolates. World J. Microbiol. Biotechnol. 2009, 25, 1471–1477. [Google Scholar] [CrossRef]
- Barua, S.; Tripathi, S.; Chakraborty, A.; Ghosh, S.; Chakrabarti, K. Characterization and crop production efficiency of diazotrophic bacterial isolates from coastal saline soils. Microbiol. Res. 2012, 167, 95–102. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, P.; Jorquera, M.A.; Sangwan, P.; Kumar, P.; Verma, A.K.; Agrawal, S. Isolation of phytase-producing bacteria from Himalayan soils and their effect on growth and phosphorus uptake of Indian mustard (Brassica juncea). World J. Microbiol. Biotechnol. 2013, 29, 1361–1369. [Google Scholar] [CrossRef]
- Kageyama, H.; Tripathi, K.; Rai, A.K.; Cha-Um, S.; Waditee-Sirisattha, R.; Takabe, T. An alkaline phosphatase/phosphodiesterase, PhoD, induced by salt stress and secreted out of the cells of Aphanothece halophytica, a halotolerant cyanobacterium. Appl. Environ. Microbiol. 2011, 77, 5178–5183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johri, J.K.; Surange, S.; Nautiyal, C.S. Occurrence of salt, pH, and temperature-tolerant, phosphate-solubilizing bacteria in alkaline soils. Curr. Microbiol. 1999, 39, 89–93. [Google Scholar] [CrossRef]
- Yue, Z.; Shen, Y.; Chen, Y.; Liang, A.; Chu, C.; Chen, C.; Sun, Z. Microbiological insights into the stress-alleviating property of an endophytic Bacillus altitudinis WR10 in wheat under low-phosphorus and high-salinity stresses. Microorganisms 2019, 7, 508. [Google Scholar] [CrossRef] [Green Version]
- Abdelmoteleb, A.; Gonzalez-Mendoza, D. Isolation and identification of phosphate solubilizing Bacillus spp. from Tamarix ramosissima rhizosphere and their effect on growth of Phaseolus vulgaris under salinity stress. Geomicrobiol. J. 2020, 37, 901–908. [Google Scholar] [CrossRef]
- Sahay, R.; Patra, D.D. Identification and performance of sodicity tolerant phosphate solubilizing bacterial isolates on Ocimum basilicum in sodic soil. Ecol. Eng. 2014, 71, 639–643. [Google Scholar] [CrossRef]
- Pradhan, A.; Pahari, A.; Mohapatra, S.; Mishra, B.B. Phosphate-Solubilizing Microorganisms in Sustainable Agriculture: Genetic Mechanism and Application. In Advances in Soil Microbiology: Recent Trends and Future Prospects; Springer: Singapore, 2017; pp. 81–97. [Google Scholar]
- Xiang, W.L.; Liang, H.Z.; Liu, S.; Luo, F.; Tang, J.; Li, M.Y.; Che, Z.M. Isolation and performance evaluation of halotolerant phosphate solubilizing bacteria from the rhizospheric soils of historic Dagong Brine Well in China. World J. Microbiol. Biotechnol. 2011, 27, 2629–2637. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Mukherjee, A.; Rastogi, R.P.; Verma, J.P. Salt-tolerant plant growth-promoting Bacillus pumilus strain JPVS11 to enhance plant growth attributes of rice and improve soil health under salinity stress. Microbiol. Res. 2021, 242, 126616. [Google Scholar] [CrossRef] [PubMed]
- Boukhris, I.; Farhat-Khemakhem, A.; Blibech, M.; Bouchaala, K.; Chouayekh, H. Characterization of an extremely salt-tolerant and thermostable phytase from Bacillus amyloliquefaciens Usint. J. Biol. Macromol. 2015, 80, 581–587. [Google Scholar] [CrossRef]
- Yadav, B.K.; Tarafdar, J.C. Efficiency of Bacillus coagulans as P biofertilizer to mobilize native soil organic and poorly soluble phosphates and increase crop yield. Arch. Agron. Soil Sci. 2012, 58, 1099–1115. [Google Scholar] [CrossRef]
- Villarreal-Chiu, J.F.; Quinn, J.P.; McGrath, J.W. The genes and enzymes of phosphonate metabolism by bacteria, and their distribution in the marine environment. Front. Microbiol. 2012, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.T.; Lee, L.Y.; Tai, C.Y.; Hung, C.H.; Chang, Y.S.; Wolfram, J.H.; Rogers, R.; Goldstein, A.H. Cloning of an Erwinia herbicola gene necessary for gluconic acid production and enhanced mineral phosphate solubilization in Escherichia coli HB101: Nucleotide sequence and probable involvement in biosynthesis of the coenzyme pyrroloquinoline quinone. J. Bacteriol. Res. 1992, 174, 5814–5819. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, A.H.; Liu, S.T. Molecular cloning and regulation of a mineral phosphate solubilizing gene from Erwinia herbicola. Nat. Biotechnol. 1987, 5, 72–74. [Google Scholar] [CrossRef]
- Deubel, A.; Gransee, A.; Merbach, W. Transformation of organic rhizodepositions by rhizosphere bacteria and its influence on the availability of tertiary calcium phosphate. J. Plant. Nutr. Soil Sci. 2000, 163, 387–392. [Google Scholar] [CrossRef]
- Goldstein, A.H. Bioprocessing of rock phosphate ore: Essential technical considerations for the development of a successful commercial technology. In Proceedings of the 4th International Fertilizer Association Technical Conference; IFA: Paris, French, 2000. [Google Scholar]
- Zhao, K.; Penttinen, P.; Zhang, X.; Ao, X.; Liu, M.; Yu, X.; Chen, Q. Maize rhizosphere in Sichuan, China, hosts plant growth promoting Burkholderia cepacia with phosphate solubilizing and antifungal abilities. Microbiol. Res. 2014, 169, 76–82. [Google Scholar] [CrossRef]
- Illmer, P.; Schinner, F. Solubilization of inorganic calcium phosphates—Solubilization mechanisms. Soil Biol. Biochem. 1995, 27, 257–263. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Fu, L.; Gao, Y.; Zhao, H.; Zhou, W. Aerobic-heterotrophic nitrogen removal through nitrate reduction and ammonium assimilation by marine bacterium Vibrio sp. Y1-5. Bioresour. Technol. 2017, 230, 103–111. [Google Scholar] [CrossRef] [PubMed]
- McNaught, A.D. Compendium of Chemical Terminology; Blackwell Science: Oxford, UK, 1997. [Google Scholar]
- Prabhu, N.; Borkar, S.; Garg, S. Phosphate solubilization by microorganisms: Overview, mechanisms, applications and advances. In Advances in Biological Science Research; Academic Press: Cambridge, MA, USA, 2019; pp. 161–176. [Google Scholar]
- Park, K.H.; Lee, C.Y.; Son, H.J. Mechanism of insoluble phosphate solubilization by Pseudomonas fluorescens RAF15 isolated from ginseng rhizosphere and its plant growth-promoting activities. Lett. Appl. Microbiol. 2009, 49, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.W.; Leong, J.; Teintze, M.; Schroth, M.N. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar] [CrossRef]
- Schiessl, K.T.; Janssen, E.M.L.; Kraemer, S.M.; McNeill, K.; Ackermann, M. Magnitude and mechanism of siderophore-mediated competition at low iron solubility in the Pseudomonas aeruginosa pyochelin system. Front. Microbiol. 2017, 8, 1964. [Google Scholar] [CrossRef] [Green Version]
- Andrews, S.C.; Robinson, A.K.; Rodríguez-Quiñones, F. Bacterial iron homeostasis. FEMS Microbiol. Rev. 2003, 27, 215–237. [Google Scholar] [CrossRef]
- Abbas, G.; Saqib, M.; Akhtar, J. Interactive effects of salinity and iron deficiency on different rice genotypes. J. Plant Nutr. Soil Sci. 2015, 178, 306–311. [Google Scholar] [CrossRef]
- Moscovici, M. Present and future medical applications of microbial exopolysaccharides. Front. Microbiol. 2015, 6, 1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, O.Y.; Raaijmakers, J.M.; Kuramae, E.E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Diwan, B. Bacterial exopolysaccharide mediated heavy metal removal: A review on biosynthesis, mechanism and remediation strategies. Biotechnol. Rep. 2017, 13, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Shultana, R.; Kee Zuan, A.T.; Yusop, M.R.; Saud, H.M. Characterization of salt-tolerant plant growth-promoting rhizobacteria and the effect on growth and yield of saline-affected rice. PLoS ONE 2020, 15, e0238537. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.K.; Singh, J.S.; Singh, D.P. Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition. Pedosphere 2011, 21, 214–222. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, W.; Ge, Y. Exopolysaccharide: A novel important factor in the microbial dissolution of tricalcium phosphate. World J. Microbiol. Biotechnol. 2008, 24, 1059–1065. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Aparna, K.; Dotaniya, C.K.; Singh, M.; Regar, K.L. Role of soil enzymes in sustainable crop production. In Enzymes in Food Biotechnology; Academic Press: Cambridge, MA, USA, 2019; pp. 569–589. [Google Scholar]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef] [Green Version]
- Coleman, J.E. Structure and mechanism of alkaline phosphatase. Annu. Rev. Biophys. Biomol. Struct. 1992, 21, 441–483. [Google Scholar] [CrossRef]
- Millán, J.L. Alkaline phosphatases. Purinergic Signal. 2006, 2, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Kier, L.D.; Weppelman, R.M.; Ames, B.N. Regulation of nonspecific acid phosphatase in Salmonella: PhoN and phoP genes. J. Bacteriol. Res. 1979, 138, 155–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaller, M.C.; Berlutti, F.; Schippa, S.; Iori, P.; Passariello, C.; Rossolini, G.M. Heterogeneous patterns of acid phosphatases containing low-molecular-mass polypeptides in members of the family Enterobacteriaceae. Int. J. Syst. Evol. Microbiol. 1995, 45, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Neal, A.L.; Blackwell, M.; Akkari, E.; Guyomar, C.; Clark, I.; Hirsch, P.R. Phylogenetic distribution, biogeography and the effects of land management upon bacterial non-specific Acid phosphatase Gene diversity and abundance. Plant Soil 2018, 427, 175–189. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, H.; Fraga, R.; Gonzalez, T.; Bashan, Y. Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant Soil 2006, 287, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Rosales, E.; Pazos, M.; Sanromán, M.Á. Solid-state fermentation for food applications. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 319–355. [Google Scholar]
- Mullaney, E.J.; Daly, C.B.; Ullah, A.H. Advances in phytase research. Adv. Appl. Microbiol. 2000, 47, 157–199. [Google Scholar]
- Richardson, A.E.; Hadobas, P.A.; Hayes, J.E. Extracellular secretion of Aspergillus phytase from Arabidopsis roots enables plants to obtain phosphorus from phytate. Plant J. 2001, 25, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.E.; Hadobas, P.A.; Simpson, R.J. Phytate as a source of phosphorus for the growth of transgenic Trifolium subterraneum. In Plant Nutrition; Springer: Dordrecht, The Netherlands, 2001; pp. 560–561. [Google Scholar]
- Seweryn, P.; Van, L.B.; Kjeldgaard, M.; Russo, C.J.; Passmore, L.A.; Hove-Jensen, B.; Jochimsen, B.; Brodersen, D.E. Structural insights into the bacterial carbon–phosphorus lyase machinery. Nature 2015, 525, 68–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilangumaran, G.; Smith, D.L. Plant growth promoting rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Salinity, Growth and Phytohormones; Springer: Berlin, Germany, 2002. [Google Scholar]
- Niu, X.; Bressan, R.A.; Hasegawa, P.M.; Pardo, J.M. Ion homeostasis in NaCl stress environments. Plant Physiol. 1995, 109, 735–742. [Google Scholar] [CrossRef] [Green Version]
- Etesami, H.; Glick, B.R. Halotolerant plant growth–promoting bacteria: Prospects for alleviating salinity stress in plants. Environ. Exp. Bot. 2020, 178, 104124. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Nawaz, S. Mitigation of salinity-induced negative impact on the growth and yield of wheat by plant growth-promoting rhizobacteria in naturally saline conditions. Ann. Microbiol. 2013, 63, 225–232. [Google Scholar] [CrossRef]
- Rojas-Tapias, D.; Moreno-Galván, A.; Pardo-Díaz, S.; Obando, M.; Rivera, D.; Bonilla, R. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Appl. Soil Ecol. 2012, 61, 264–272. [Google Scholar] [CrossRef]
- Etesami, H. Plant–microbe interactions in plants and stress tolerance. In Plant Life under Changing Environment; Academic Press: Cambridge, MA, USA, 2020; pp. 355–396. [Google Scholar]
- Zhang, H.; Kim, M.S.; Sun, Y.; Dowd, S.E.; Shi, H.Z.; Pare, P.W. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter Hkt. Mol. Plant-Microbe Interact. 2008, 21, 737–744. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.; Mitra, A.; Roy, M. Halomonas rhizobacteria of Avicennia marina of Indian Sundarbans promote rice growth under saline and heavy metal stresses through exopolysaccharide production. Front. Microbiol. 2019, 10, 1207. [Google Scholar] [CrossRef] [Green Version]
- Gupta, G.; Parihar, S.S.; Ahirwar, N.K.; Snehi, S.K.; Singh, V. Plant growth promoting rhizobacteria (PGPR): Current and future prospects for development of sustainable agriculture. J. Microb. Biochem. Technol. 2015, 7, 96–102. [Google Scholar]
- Safdarian, M.; Askari, H.; Nematzadeh, G.; Adriano, S.O.F.O. Halophile plant growth-promoting rhizobacteria induce salt tolerance traits in wheat seedlings (Triticum aestivum L.). Pedosphere 2020, 30, 684–693. [Google Scholar] [CrossRef]
- Sarkar, A.; Ghosh, P.K.; Pramanik, K.; Mitra, S.; Soren, T.; Pandey, S.; Mondal, M.H.; Maiti, T.K. A halotolerant Enterobacter sp. displaying ACC deaminase activity promotes rice seedling growth under salt stress. Res. Microbiol. 2018, 169, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kulkarni, J.; Jha, B. Halotolerant rhizobacteria promote growth and enhance salinity tolerance in peanut. Front. Microbiol. 2016, 7, 1600. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, U.; Chakraborty, B.N.; Chakraborty, A.P.; Dey, P.L. Water stress amelioration and plant growth promotion in wheat plants by osmotic stress tolerant bacteria. World J. Microbiol. Biotechnol. 2013, 29, 789–803. [Google Scholar] [CrossRef]
- Pandey, S.; Gupta, S. Diversity analysis of ACC deaminase producing bacteria associated with rhizosphere of coconut tree (Cocos nucifera L.) grown in Lakshadweep islands of India and their ability to promote plant growth under saline conditions. J. Biotechnol. 2020, 324, 183–197. [Google Scholar] [CrossRef]
- Fatima, T.; Mishra, I.; Verma, R.; Arora, N.K. Mechanisms of halotolerant plant growth promoting Alcaligenes sp. involved in salt tolerance and enhancement of the growth of rice under salinity stress. 3 Biotech. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sukweenadhi, J.; Balusamy, S.R.; Kim, Y.J.; Lee, C.H.; Kim, Y.J.; Koh, S.C.; Yang, D.C. A growth promoting bacteria, Paenibacillus yonginensis DCY84T enhanced salt stress tolerance by activating defense-related systems in Panax ginseng. Front. Plant Sci. 2018, 9, 813. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Geng, X.; Xie, R.; Fu, L.; Jiang, J.; Gao, L.; Sun, J. The endophytic bacteria isolated from elephant grass (Pennisetum purpureum Schumach) promote plant growth and enhance salt tolerance of Hybrid Pennisetum. Biotechnol. Biofuels 2016, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Fan, P.; Chen, D.; He, Y.; Zhou, Q.; Tian, Y.; Gao, L. Alleviating salt stress in tomato seedlings using Arthrobacter and Bacillus megaterium isolated from the rhizosphere of wild plants grown on saline–alkaline lands. Int. J. Phytoremediation 2016, 18, 1113–1121. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.M.; Kim, K.M.; Lee, I.J. Rhizobacteria AK1 remediates the toxic effects of salinity stress via regulation of endogenous phytohormones and gene expression in soybean. Biochem. J. 2019, 476, 2393–2409. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Akram, M.S.; Khan, M.A.; Zubair, M.; Shah, S.M.; Ismail, M.; Shabir, G.; Basheer, S.; Aslam, K.; Tariq, M. A phytobeneficial strain Planomicrobium sp. MSSA-10 triggered oxidative stress responsive mechanisms and regulated the growth of pea plants under induced saline environment. J. Appl. Microbiol. 2018, 124, 1566–1579. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Bano, A. Isolation of PGPRs from rhizospheric soil of halophytes and its impact on maize (Zea mays L.) under induced soil salinity. Can. J. Microbiol. 2015, 11, 1–7. [Google Scholar]
- Habib, S.H.; Kausar, H.; Saud, H.M. Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. Biomed Res. Int. 2016, 6284547. [Google Scholar] [CrossRef] [Green Version]
- Tewari, S.; Arora, N.K. Fluorescent Pseudomonas sp. PF17 as an efficient plant growth regulator and biocontrol agent for sunflower crop under saline conditions. Symbiosis 2016, 68, 99–108. [Google Scholar] [CrossRef]
- Ghorai, S.; Pal, K.K.; Dey, R. Alleviation of salinity stress in groundnut by application of PGPR. Int. J. Res. Eng. Technol. 2015, 2, 742–750. [Google Scholar]
- Qin, S.; Zhang, Y.J.; Yuan, B.; Xu, P.Y.; Xing, K.; Wang, J.; Jiang, J.H. Isolation of ACC deaminase-producing habitat-adapted symbiotic bacteria associated with halophyte Limonium sinense (Girard) Kuntze and evaluating their plant growth-promoting activity under salt stress. Plant Soil 2014, 374, 753–766. [Google Scholar] [CrossRef]
- He, A.L.; Niu, S.Q.; Zhao, Q.; Li, Y.S.; Gou, J.Y.; Gao, H.J.; Suo, S.Z.; Zhang, J.L. Induced salt tolerance of perennial ryegrass by a novel bacterium strain from the rhizosphere of a desert shrub Haloxylon ammodendron. Int. J. Mol. Sci. 2018, 19, 469. [Google Scholar] [CrossRef] [Green Version]
- Saxena, S.C.; Kaur, H.; Verma, P.; Petla, B.P.; Andugula, V.R.; Majee, M. Osmoprotectants: Potential for crop improvement under adverse conditions. In Plant Acclimation to Environmental Stress; Springer: New York, NY, USA, 2013; pp. 197–232. [Google Scholar]
- Riyazuddin, R.; Verma, R.; Singh, K.; Nisha, N.; Keisham, M.; Bhati, K.K.; Kim, S.T.; Gupta, R. Ethylene: A master regulator of salinity stress tolerance in plants. Biomolecules 2020, 10, 959. [Google Scholar] [CrossRef]
- Tao, J.J.; Chen, H.W.; Ma, B.; Zhang, W.K.; Chen, S.Y.; Zhang, J.S. The role of ethylene in plants under salinity stress. Front. Plant Sci. 2015, 6, 1059. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.W.; Drew, M.C. Ethylene and plant responses to stress. Physiol. Plant. 1997, 100, 620–630. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 963401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadeem, S.M.; Ahmad, M.; Naveed, M.; Imran, M.; Zahir, Z.A.; Crowley, D.E. Relationship between in vitro characterization and comparative efficacy of plant growth-promoting rhizobacteria for improving cucumber salt tolerance. Arch. Microbiol. 2016, 198, 379–387. [Google Scholar] [CrossRef]
- Mapelli, F.; Marasco, R.; Rolli, E.; Barbato, M.; Cherif, H.; Guesmi, A.; Ouzari, I.; Daffonchio, D.; Borin, S. Potential for plant growth promotion of rhizobacteria associated with Salicornia growing in Tunisian hypersaline soils. Biomed Res. Int. 2013, 2013, 248078. [Google Scholar]
- Patel, D.; Jha, C.K.; Tank, N.; Saraf, M. Growth enhancement of chickpea in saline soils using plant growth-promoting rhizobacteria. J. Plant Growth Reg. 2012, 31, 53–62. [Google Scholar] [CrossRef]
- Desale, P.; Patel, B.; Singh, S.; Malhotra, A.; Nawani, N. Plant growth promoting properties of Halobacillus sp. and Halomonas sp. in presence of salinity and heavy metals. J. Basic Microbiol. 2014, 54, 781–791. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.A.; Benková, E. Cytokinin cross-talking during biotic and abiotic stress responses. Front. Plant Sci. 2013, 4, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arkhipova, T.N.; Prinsen, E.; Veselov, S.U.; Martinenko, E.V.; Melentiev, A.I.; Kudoyarova, G.R. Cytokinin producing bacteria enhance plant growth in drying soil. Plant Soil 2007, 292, 305–315. [Google Scholar] [CrossRef]
- Liu, F.; Xing, S.; Ma, H.; Du, Z.; Ma, B. Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl. Microbiol. Biotechnol. 2013, 97, 9155–9164. [Google Scholar] [CrossRef]
- Wang, G.L.; Que, F.; Xu, Z.S.; Wang, F.; Xiong, A.S. Exogenous gibberellin altered morphology, anatomic and transcriptional regulatory networks of hormones in carrot root and shoot. BMC Plant Biol. 2015, 15, 290. [Google Scholar] [CrossRef] [Green Version]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd-Allah, E.F.; Hashem, A. Phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef]
- Ryu, H.; Cho, Y.G. Plant hormones in salt stress tolerance. J. Plant Biol. 2015, 58, 147–155. [Google Scholar] [CrossRef]
- Golan, Y.; Shirron, N.; Avni, A.; Shmoish, M.; Gepstein, S. Cytokinins induce transcriptional reprograming and improve Arabidopsis plant performance under drought and salt stress conditions. Front. Environ. Sci. 2016, 4, 63. [Google Scholar] [CrossRef] [Green Version]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Jha, P.N. A halotolerant bacterium Bacillus licheniformis HSW-16 augments induced systemic tolerance to salt stress in wheat plant (Triticum aestivum). Front. Plant Sci. 2016, 7, 1890. [Google Scholar] [CrossRef]
- Nandi, M.; Selin, C.; Brawerman, G.; Fernando, W.D.; de Kievit, T. Hydrogen cyanide, which contributes to Pseudomonas chlororaphis strain PA23 biocontrol, is upregulated in the presence of glycine. Biol. Control 2017, 108, 47–54. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Prakash, A.; Johri, B.N. Induced systemic resistance (ISR) in plants: Mechanism of action. Indian J. Microbiol. 2007, 47, 289–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Loon, L.C.; Bakker, P.A.H.M.; Pieterse, C.M.J. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998, 36, 453–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halfeld-Vieira, B.D.A.; Vieira Júnior, J.R.; Romeiro, R.D.S.; Silva, H.S.A.; Baracat-Pereira, M.C. Induction of systemic resistance in tomato by the autochthonous phylloplane resident Bacillus cereus. Pesqui. Agropecuária Bras. 2006, 41, 1247–1252. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.S.; Siddiqui, Z.A. Role of plant growth promoting rhizobacteria in biocontrol of plant diseases and sustainable agriculture. In Plant Growth and Health Promoting Bacteria; Springer: Berlin/Heidelberg, Germany, 2010; pp. 157–195. [Google Scholar]
- Dineshkumar, R.; Kumaravel, R.; Gopalsamy, J.; Sikder, M.N.A.; Sampathkumar, P. Microalgae as bio-fertilizers for rice growth and seed yield productivity. Waste Biomass Valorization 2018, 9, 793–800. [Google Scholar] [CrossRef]
- Chaudhary, T.; Dixit, M.; Gera, R.; Shukla, A.K.; Prakash, A.; Gupta, G.; Shukla, P. Techniques for improving formulations of bioinoculants. 3 Biotech. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mącik, M.; Gryta, A.; Frąc, M. Biofertilizers in agriculture: An overview on concepts, strategies and effects on soil microorganisms. Adv. Agron. 2020, 162, 31–87. [Google Scholar]
- Phua, C.K.H.; Khairuddin, A.R. Multifunctional Liquid Bio Fertilizer as an Innovative Agronomic Input for Modern Agriculture; Office of Scientific and Technical Information, U.S. Department of Energy: Washington, DC, USA, 2010. [Google Scholar]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Pandey, A.; Trivedi, P.; Kumar, B.; Palni, L.M.S. Characterization of a phosphate solubilizing and antagonistic strain of Pseudomonas putida (B0) isolated from a sub-alpine location in the Indian Central Himalaya. Curr. Microbiol. 2006, 53, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Dastager, S.G.; Deepa, C.K.; Puneet, S.C.; Nautiyal, C.S.; Pandey, A. Isolation and characterization of plant growth-promoting strain Pantoea NII-From Western Ghat Forest soil, India. Lett. Appl. Microbiol. 2009, 49, 20–25. [Google Scholar] [CrossRef]
- Goswami, D.; Dhandhukia, P.; Patel, P.; Thakker, J.N. Screening of PGPR from saline desert of Kutch: Growth promotion in Arachis hypogea by Bacillus licheniformis A2. Microbiol. Res. 2014, 169, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Paul, S.C.; Parveen, S.; Alam, S.; Rahman, N.; Jannat, B.; Hoque, S.; Rahman, M.T.; Karim, M.M. Isolation and identification of salt-tolerant plant-growth-promoting rhizobacteria and their application for rice cultivation under salt stress. Can. J. Microbiol. 2020, 66, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Chen, Y.; Chen, C.; Ma, K.; Tian, E.; Wang, Y.; Liu, H.; Sun, Z. Endophytic Bacillus altitudinis WR10 alleviates Cu toxicity in wheat by augmenting reactive oxygen species scavenging and phenylpropanoid biosynthesis. J. Hazard. Mater. 2021, 405, 124272. [Google Scholar] [CrossRef]
- Kadmiri, I.M.; Chaouqui, L.; Azaroual, S.E.; Sijilmassi, B.; Yaakoubi, K.; Wahby, I. Phosphate-solubilizing and auxin-producing rhizobacteria promote plant growth under saline conditions. Arab. J. Sci. Eng. 2018, 43, 3403–3415. [Google Scholar] [CrossRef]
- Joe, M.M.; Devaraj, S.; Benson, A.; Sa, T. Isolation of phosphate solubilizing endophytic bacteria from Phyllanthus amarus Schum & Thonn: Evaluation of plant growth promotion and antioxidant activity under salt stress. J. Appl. Res. Med. Aroma 2016, 3, 71–77. [Google Scholar]
- Naz, I.; Bano, A. Biochemical, molecular characterization and growth promoting effects of phosphate solubilizing Pseudomonas sp. isolated from weeds grown in salt range of Pakistan. Plant Soil 2010, 334, 199–207. [Google Scholar] [CrossRef]
- Verma, M.; Singh, A.; Dwivedi, D.H.; Arora, N.K. Zinc and phosphate solubilizing Rhizobium radiobacter (LB2) for enhancing quality and yield of loose-leaf lettuce in saline soil. J. Environ. Sustain. 2020, 3, 209–218. [Google Scholar] [CrossRef]
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Benjelloun, S.; Allaoui, A.; Biskri, L. Rhizospheric Phosphate Solubilizing Bacillus atrophaeus GQJK17 S8 Increases Quinoa Seedling, Withstands Heavy Metals, and Mitigates Salt Stress. Sustainability 2021, 13, 3307. [Google Scholar] [CrossRef]
- Jin, H.; Mitchum, M.; Panstruga, R.; Stone, J. Focus Issue Editorial: Biotic Stress. Plant Physiol. 2019, 179, 1193–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chojak-Koźniewska, J.; Kuźniak, E.; Zimny, J. The effects of combined abiotic and pathogen stress in plants: Insights from salinity and Pseudomonas syringae pv lachrymans interaction in cucumber. Front. Plant Sci. 2018, 9, 1691. [Google Scholar] [CrossRef] [PubMed]
- Ahemad, M. Phosphate-solubilizing bacteria-assisted phytoremediation of metalliferous soils: A review. 3 Biotech. 2015, 5, 111–121. [Google Scholar] [CrossRef] [Green Version]


| Salt Tolerant PSB | Source of Phosphate | Mode of Action for P Solubilization and Mineralization | Reference |
|---|---|---|---|
| Pantoea agglomerans R-42 | Ca3(PO4)2, CaHPO4, Hydroxyapatite, AlPO4, FePO4 | pH reduced; OC ND | [76] |
| Burkholderia vietnamiensis M6 | pH reduced; gluconic and 2-ketogluconic acid | [77] | |
| Aerococcus sp. strain PSBCRG1-1, Pseudomonas aeruginosa strain PSBI3-1 | Ca3(PO4)2 | ND | [78] |
| Pseudomonas koreensis MU2 | malic acid, citric acid, acetic acid, and tartaric acid | [4] | |
| Ensifer sesbaniae, Gordonia terrae, Bacillus Sp., Acinetobacter Sp. | Ca3(PO4)2, AlPO4, FePO4, and lecithin | pH reduced | [79] |
| Bacillus sp., Pseudomonas sp., Streptomyces sp., Arthrobacter sp., Providencia rettgeri sp. and Acinetobacter sp. | Ca3(PO4)2, AlPO4, and FePO4, | pH reduced; gluconic acid, formic acid, malic acid, lactic acid, succinic acid, citric acid and propionic acid | [80] |
| Serratia sp. | Ca3(PO4)2, p-nitrophenyl phosphate | malic acid, lactic acid and acetic acid; acid phosphatase | [81] |
| Alcaligenes faecalis | pH reduced, oxalic acid, citric acid, malic acid, succinic acid and acetic acid; alkaline phosphatase | [82] | |
| Kushneria sp. YCWA18 | Ca3(PO4)2 and Lecithin | pH reduced; OC ND | [5] |
| Bacillus megaterium | Ca3(PO4)2 and Lecithin | [93] | |
| Cobetia amphilecti | p-nitrophenyl phosphate (pNPP) and guanosine 5-triphosphate (GTP) | alkaline phosphatase/phosphodiesterase activity | [83] |
| Pantoea agglomerans strain P5, Microbacterium laevaniformans strain P7 and Pseudomonas putida strain P13 | Ca3(PO4)2; 5-bromo-4-choloro-3-indolyl phosphate (BCIP) | pH reduced; phosphatase | [84] |
| Agrobacterium sp. & Bacillus sp. | Ca3(PO4)2, AlPO4; beta-Glycerophosphate | pH reduced; acid phosphatase | [85] |
| Bacillus pumilus strain JPVS11 | ND | alkaline phosphatase and acid phosphatase | [94] |
| Bacillus amyloliquefaciens US573 | Phytic acid | phytase | [95] |
| Acromobacter sp. PB-01, Tetrathiobacter sp. PB-03 and Bacillus sp. PB-13 | Ca3(PO4)2; Na-phytate | pH reduced; phytase | [86] |
| Bacillus coagulans | K2HPO4, CaHPO4, Ca3(PO4)2; glycerophosphate and phytate | alkaline phosphatase and acid phosphatase; phytase | [96] |
| Bacillus altitudinis WR10 | Ca-phytate | [89] | |
| Aphanothece halophytica | ND | alkaline phosphatase | [87] |
| Marine bacterial community | C-P lyase | [97] |
| Salt Tolerant PSB | PGP Characters | Mode of Action for Alleviation of Salt Stress | Inoculant Plant | Reference |
|---|---|---|---|---|
| Bacillus pumilus FAB10 | EPS, IAA, NH3, siderophore, PS, and HCN | Biofilm development; enhancement of CAT, SOD, GR and decrease MDA; maintain Na+/K+ concentration & increase root colonization | Wheat (Triticum aestivum) | [44] |
| Halomonas sp. Exo1 | EPS, IAA, NH3, N2 fixation, siderophore, PS, and HCN | Sequestration of Na+ by EPS; increasing P and N uptake | Rice (Oryza sativa) varietyJarava | [139] |
| Bacillus sp., Burkholderia sp., Enterobacter sp., Microbacterium sp., and Paenibacillus sp. | EPS, IAA, and PS | Na+ binds to EPS-decrease Na+ uptake; aggregate RAS; increase proline | Wheat (Triticum aestivum) cultivar K7903 | [116] |
| Enterobacter asburiae | IAA, siderophore, ACC deaminase, and PS | reducing Na+ content and ethylene production; up-regulating the expression of HKT1, H+PPase, NHX7, CAT, and APX | Wheat (Triticum aestivum L. cv. drya) | [141] |
| Enterobacter sp. P23 | EPS, IAA, NH3, siderophore, PS, ACC deaminase, SA, and HCN | Reduce ethylene stress; increase protein and soluble sugar content | Rice seedling (Oryza sativa) | [142] |
| Bacillus subtilis SU47 and Arthrobacter sp. SU18 | EPS, IAA, PS and GA | Increase total soluble sugar, proline, P and K+ uptake; reduce the Na+ uptake and enhance CAT, GR & APX activity | Wheat (Triticum aestivum) cultivar Raj-3077 | [38] |
| Bacillus licheniformis and Pseudomonas plecoglossicid | IAA, PS, and ACC deaminase. | Enhance the CAT, GPX and SOD activity; increase accumulation of phenolic, proline and ascorbic acid molecules, protein, P and K in grains and decrease the MDA accumulation | Sunflower (Helianthus annuus cv. Hysun-33) | [45] |
| Klebsiella Sp., Pseudomonas Sp., Agrobacterium Sp., and Ochrobactrum Sp. | IAA, N2 fixation, PS ACC deaminase and acetylene reduction. | Reduce ethylene stress; lower shoot and root Na+/K+ ratio; increased shoot and root Ca2+ accumulation; enhance the expression of antioxidant genes APX, CAT, and SOD and maintain of ROS level | Pea nut (Arachis hypogaea) | [143] |
| Ochrobactrum pseudogregnonense and Bacillus safensis | IAA, siderophore, PS, and ACC deaminase | Enhancement SOD, POD, APX, CAT and GR; increase accumulation of carotenoids and ascorbate; increase proline and P uptake | Six varieties of wheat (Triticum aestivum) | [144] |
| Pseudomonas putida and Bacillus paramycoides | IAA, NH3, siderophore, PS, and ACC deaminase | Reduce stress ethylene & MDA content; increase water content. | French bean (Phaseolus vulgaris) | [145] |
| Alcaligenes sp. AF7 | EPS, IAA, siderophore, PS, Zn solubilization, and GA | EPS trapping Na+ from soil; increase water holding capacity | Rice plant (Oryza sativa) | [146] |
| Paenibacillus yonginensis DCY84T | IAA, siderophore, PS, and anti-bacterial activity | Increase sugars, proline, polyamine accumulation; enhance SOD, GPX, APX, and CAT activity; maintain moister content; decrease MDA, H2O2 level, and ABA accumulation | Panax ginseng | [147] |
| Sphingomonas sp., Pantoea sp, Bacillus sp., and Enterobacter sp. | PS, N2 fixation, IAA, NH3, siderophore, ACC deaminase | Colonization inside the root and mitigate salt stress | Hybrid Pennisetum (Pennisetum americanum × P. purpureum Schumach) | [148] |
| Arthrobacter sp. and Bacillus megaterium | IAA and PS | β-propeller phytase- BPP, choline oxidase- codA(a), β-amylase-amyG, γ-glutamyl kinase (γ-GK), and γ-glutamyl-phosphate reductase (γ-GPR)-proBA and HCN synthase-hcnBC | Tomato (Lycopersicon esculentum Mill.) | [149] |
| Arthrobacter woluwensis AK1 | IAA, siderophores, PS, and GA | Decrease the Na+ and increase the Ca2+ concentration; increase GSH and SOD activity, decrease ABA; upregulate GmLAX, GmAKT2, GmST1, and GmSALT3; downregulate GmNHX1 and GmCLC1 | Soybean (Glycine max) | [150] |
| Bacillus licheniformis and Enterobacter asburiae | IAA, NH3, siderophore, PS, and HCN | Enhance P and K+ uptake and decreased plant Na+ uptake; biofilm development | Quinoa plant (Chenopodium quinoa) | [43] |
| Pseudomonas lurida | IAA, siderophore, PS, and HCN | Enhance uptake N, P and K | wheat (Triticum aestivum cv. VL 829) | [41] |
| Planomicrobium sp. MSSA-10 | IAA, PS and ACC deaminase | Decrease level of MDA & H2O2; up-regulation CAT, POD, proline, phenolic activities; improve N, P, and K uptake | Pea plants (Pisum sativum L) | [151] |
| Arthrobacter pascens and Bacillus sp. | Siderophore and PS | Enhance the accumulation proline, sugar and amino acid; increase SOD, POD, CAT and APX activity | Maize, Zea mays variety (Rakaposhi) | [152] |
| Enterobacter sp. and Bacillus megaterium | IAA, N2 fixation, PS, and ACC deaminase | Reduce ethylene stress and increase APX, CAT, and SOD activity | Okra (Abelmoschus esculentus L) | [153] |
| Pseudomonas sp. PF17 | IAA, siderophore, pyocyanin, PS, HCN and chitinase, β-1, 3 glucanase | Enhance the RAS/RT ratio; biocontrol potentiality | Sunflower crop (Helianthus annuus) | [154] |
| Pseudomonas aeruginosa AMAAS57 and Pseudomonas aeruginosa BM6. | IAA, NH3, PS, and HCN | Increase carbohydrate level, phenol, free amino acid; decrease electrolytic leakage; and antifungal activity | Ground nut (Arachis hypogaea) | [155] |
| Azotobacter chroococcum | IAA, N2 fixation and PS | Increase K+/Na+ ratio and content of polyphenol | Corn (Zea mays) | [136] |
| Bacillus sp., Pseudomonas sp., Klebsiell sp., Serratia sp., Arthrobacter sp., Streptomyces sp., Isoptericola sp., and Microbacterium sp. | IAA, N2 fixation, PS, and ACC deaminase | maintain the ethylene stress level; increase the accumulation of flavonoid production. | Limonium sinense (Girard) Kuntze | [156] |
| Pseudomonas sp. M30–35 | IAA, PS, N2 fixation | Increase CAT activity in leaf; decrease MDA content and REC; enhance accumulation soluble sugar and proline; and increase K+/Na+ concentration | Ryegrass (Lolium perenne) | [157] |
| Application | Name of the ST-PSB | Response | Effective Crop | Reference |
|---|---|---|---|---|
| Biocontrol Agent | Pseudomonas sp. PF17 | Antifungal- Macrophomina phaseolina | Sunflower (Helianthus annuus) | [154] |
| Pseudomonas putida | Antifungal- Alternaria alternata and Fusarium oxysporum | Maize (Zea mays var. QPM-1) | [186] | |
| Pantoea sp. NII-186 | Antifungal-Penicillium chrysogenum, Aspergillus niger, Geotrichum candidum | Not detected | [187] | |
| Bacillus licheniformis HSW-164 | Antibacterial-Enterobacter sp., Erwinia carotovora, Klebsiella pneumoniae, and Escherichia coli; Antifungal- Aspergillus flavus, Fusarium graminearum, Fusarium oxysporum, and Penicillium citrinum; | Wheat (Triticum aestivum L., variety C-309) | [175] | |
| Pseudomonas aeruginosa AMAAS57 and Pseudomonas aeruginosa BM6. | Antifungal-Aspergillus niger, Aspergillus flavus, and Sclerotium rolfsii | Ground nut (Arachis hypogaea) | [155] | |
| Bacillus licheniformis A2 | Antifungal- Fusariun oxysporum | [188] | ||
| Bioremediation | Halomonas sp. Exo1 | Biosorption of arsenic through EPS | Rice (Oryza sativa) variety Jarava | [139] |
| Halomonas sp. MAN5, Halobacillus sp. ADN1, and Halobacillus sp. MAN6 | Assist phytoremediation (Sesuvium portulacastrum) against cadmium, nickel, mercury, and silver | Sesuvium portulacastrum | [166] | |
| Bacillus aryabhattai, Achromobacter denitrificans and Ochrobactrum intermedium | Resistant to Ni and Cu | Rice (Oryza sativa L.) | [189] | |
| Bacillus altitudinis WR10 | Alleviated Cu toxicity | Wheat (Triticum aestivum L.) | [190] | |
| Salt alleviator and Phosphate biofertilizer | Achromobacter piechaudii | Increase fresh and dry weights of plant seedlings grown in the presence of up to 172 mM NaCl salt. | Tomato (Solanum lycopersicum L) | [40] |
| Arthrobacter sp. and Bacillus sp. | Increased plant growth in P-deficiency and salt-affected (12 ds m−1) soils by 47–115%. | Tomato (Solanum lycopersicum L., cultivar Harzfeuer F1) | [12] | |
| Pseudomonas fluorescens Ms-01 Azosprillum brasilense DSM1690 | Plant height, shoot and root weight are significantly increased by the inoculation of individual and co inoculation under salinity 7.49 ± 1.2 ds m−1 | Wheat (T. aestivum L. var. Amal) | [191] | |
| Acinetobacter sp. and Bacillus sp. | Both the endophytes alone or in combination promoted a greater vigor index, germination (%), plant biomass, P content compared to un- inoculated control under 160 mM NaCl. | Phyllanthus amarus | [192] | |
| Bacillus altitudinis WR10 | The inoculated plant Significantly improved the seed germination rate, root dry weight, and enhance P availability from organic under salinity stress (200/400 mM NaCl) and phosphorus stress. | Wheat (Triticum aestivum L.) | [89] | |
| Bacillus licheniformis and Enterobacter asburiae | Enhanced plant development/growth under 400 mM NaCl | Quinoa plant (Chenopodium quinoa) | [43] | |
| Bacillus megaterium AL-18 and Bacillus cereus AL-19 | Single and co inoculation of the strains in plant were significantly increased root length, plant height, root and shoot dry weight, phosphate content in plants and photosynthetic pigments under salt stress conditions | Common bean (Phaseolus vulgaris cv. Karnac) | [90] | |
| Pseudomonas mendocina Khsr2, Pseudomonas stutzeri Khsr3 and Pseudomonas putida Khsr4 | Root/shoot length and Root/shoot dry weight of the plant increase under normal and NaCl (20 ds m−1) stress. | Maize (Zea mays L.) | [193] | |
| Rhizobium radiobacter LB2 | Enhance all parameter of plant growth with improved nutritional content in comparison to control under saline condition (EC~4.8 ds/m.) | Lettuce (Lactuca sativa) | [194] | |
| Pseudomonas dimnuta, Xanthomanas sp. and Exiguobacterium sp. | Increased the plant growth with respect to number of branches, height, dry matter accumulation and P content of plants under sodic condition | Ocimum basilicum | [91] | |
| Bacillus atrophaeus S8 and Enterobacter sp. QE3 | Inoculation increase germination rate and seedling growth under salinity. | Quinoa (Chenopodium quinoa wild.) | [195] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations All authors have read and agreed to the published version of the manuscript. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dey, G.; Banerjee, P.; Sharma, R.K.; Maity, J.P.; Etesami, H.; Shaw, A.K.; Huang, Y.-H.; Huang, H.-B.; Chen, C.-Y. Management of Phosphorus in Salinity-Stressed Agriculture for Sustainable Crop Production by Salt-Tolerant Phosphate-Solubilizing Bacteria—A Review. Agronomy 2021, 11, 1552. https://doi.org/10.3390/agronomy11081552
Dey G, Banerjee P, Sharma RK, Maity JP, Etesami H, Shaw AK, Huang Y-H, Huang H-B, Chen C-Y. Management of Phosphorus in Salinity-Stressed Agriculture for Sustainable Crop Production by Salt-Tolerant Phosphate-Solubilizing Bacteria—A Review. Agronomy. 2021; 11(8):1552. https://doi.org/10.3390/agronomy11081552
Chicago/Turabian StyleDey, Gobinda, Pritam Banerjee, Raju Kumar Sharma, Jyoti Prakash Maity, Hassan Etesami, Arun Kumar Shaw, Yi-Hsun Huang, Hsien-Bin Huang, and Chien-Yen Chen. 2021. "Management of Phosphorus in Salinity-Stressed Agriculture for Sustainable Crop Production by Salt-Tolerant Phosphate-Solubilizing Bacteria—A Review" Agronomy 11, no. 8: 1552. https://doi.org/10.3390/agronomy11081552
APA StyleDey, G., Banerjee, P., Sharma, R. K., Maity, J. P., Etesami, H., Shaw, A. K., Huang, Y.-H., Huang, H.-B., & Chen, C.-Y. (2021). Management of Phosphorus in Salinity-Stressed Agriculture for Sustainable Crop Production by Salt-Tolerant Phosphate-Solubilizing Bacteria—A Review. Agronomy, 11(8), 1552. https://doi.org/10.3390/agronomy11081552







