Tetraploidization Increases the Contents of Functional Metabolites in Cnidium officinale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Oryzalin Treatment
2.3. Flow Cytometry
2.4. Multiplication and Acclimatization
2.5. Growth and Cytological Characterization
2.5.1. Growth Characteristics
2.5.2. Cytological Analysis
2.5.3. Pigment Quantification
2.6. Extraction and Analysis of Funtional Secondary Metabolites
2.6.1. Extraction and Determination of Phenolic Compounds by HPLC
2.6.2. Metabolite Profiling
2.7. Heat Stress Treatment
2.8. Gene Expression Analysis
2.8.1. RNA Isolation and cDNA Synthesis
2.8.2. Quantitative Real-Time PCR (qRT-PCR)
2.9. Statistical Analysis
3. Results
3.1. Chromosome Doubling
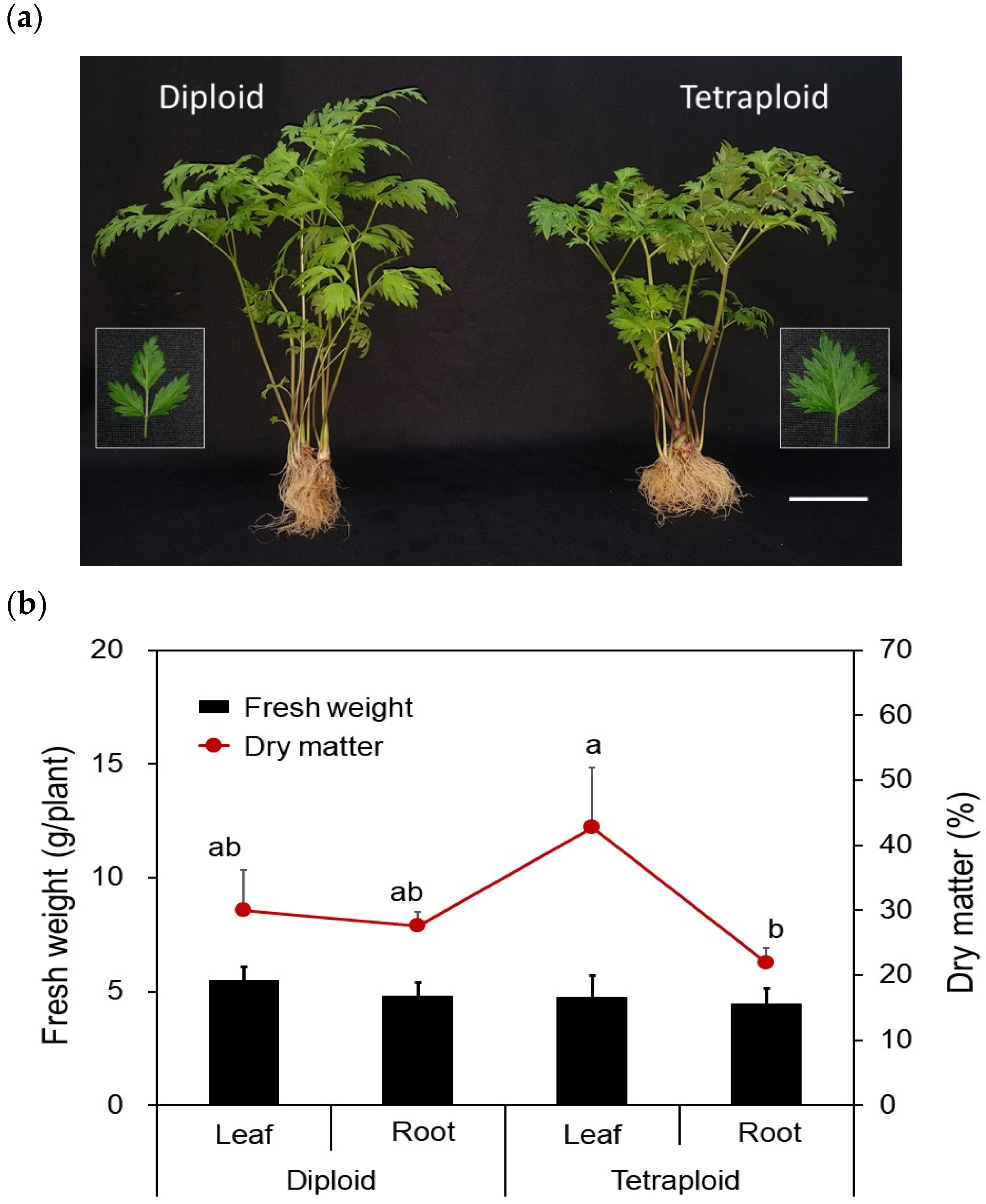
3.2. Growth and Cytological Characterization
3.3. Pigment Content Analysis
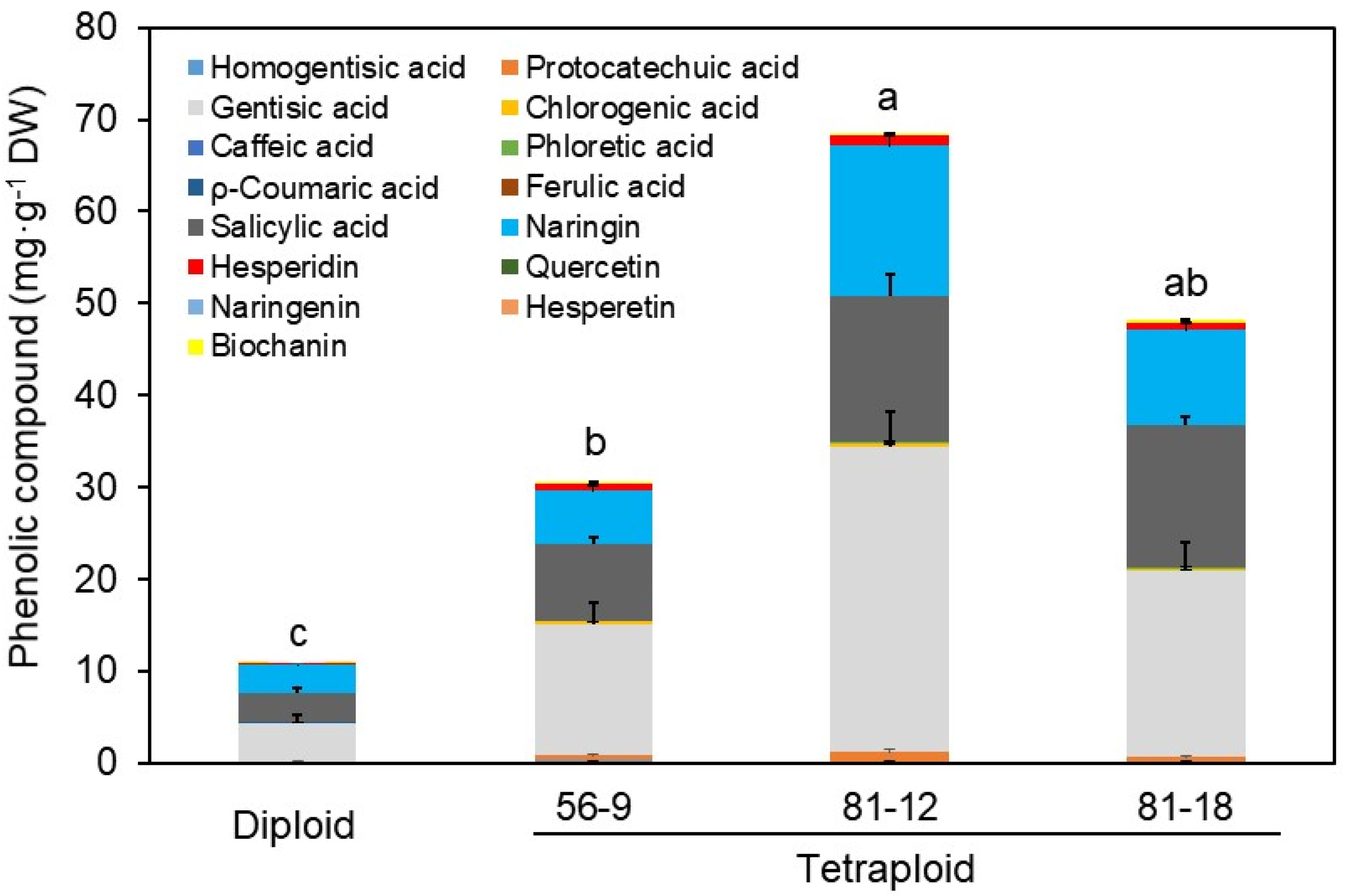
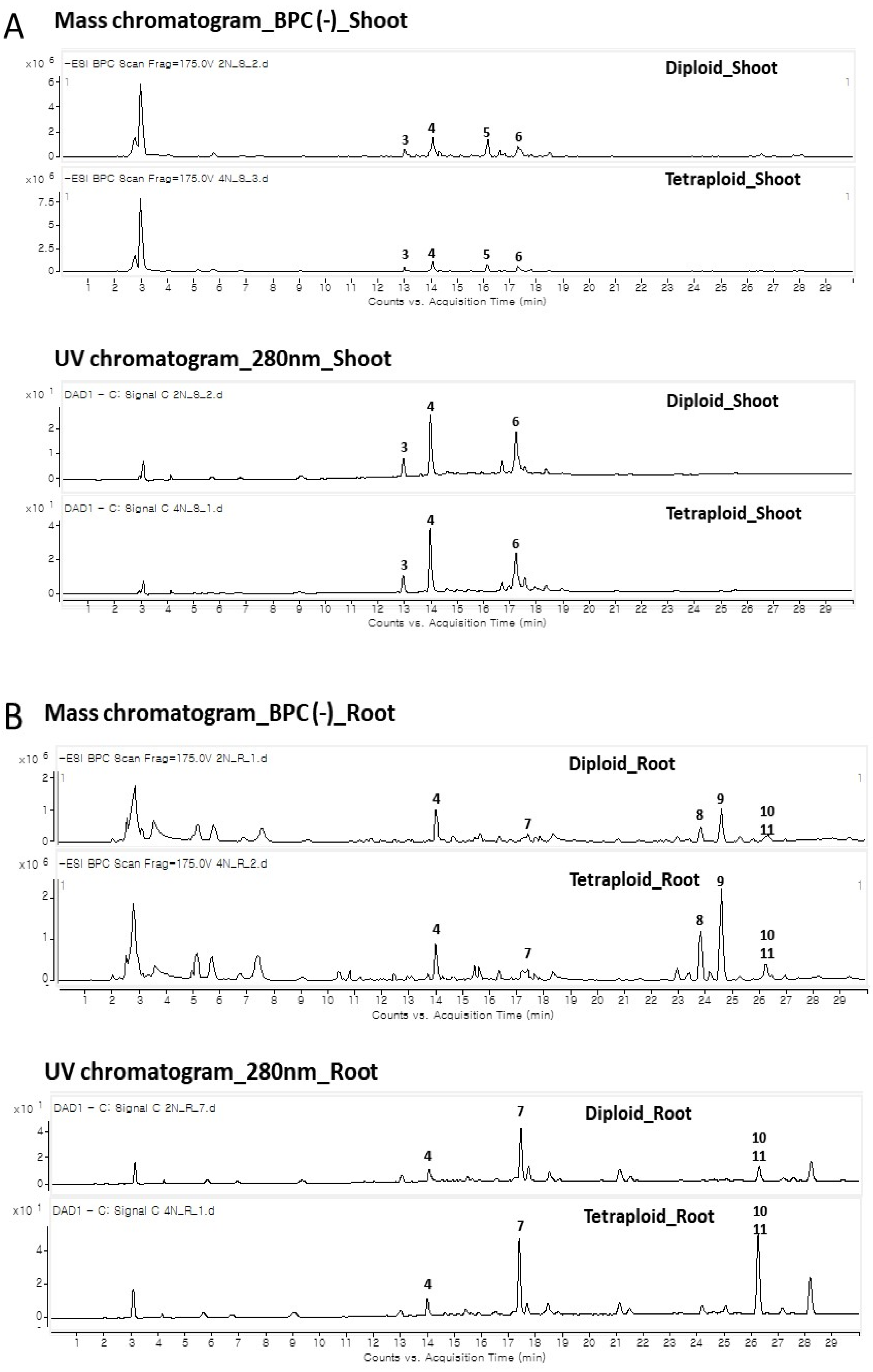
3.4. Changes in Functional Metabolites by HPLC and LC–MS
3.5. Expression Levels of Heat Stress-Responsive Genes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hop, H.T.; Arayan, L.T.; Reyes, A.W.B.; Huy, T.X.N.; Baek, E.J.; Min, W.; Lee, H.J.; Lee, C.H.; Rhee, M.H.; Kim, S. Inhibitory effect of the ethanol extract of a rice bran mixture comprising Angelica gigas, Cnidium officinale, Artemisia princeps, and Camellia sinensis on Brucella abortus uptake by professional and nonprofessional phagocytes. Microbiol. Biotechnol. 2017, 27, 1885–1891. [Google Scholar] [CrossRef] [Green Version]
- Bae, K.-E.; Choi, Y.-W.; Kim, S.-T.; Kim, Y.-K. Components of rhizome extract of Cnidium officinale Makino and their in vitro biological effects. Molecules 2011, 16, 8833–8847. [Google Scholar] [CrossRef] [Green Version]
- Adil, M.; Ren, X.; Kang, D.I.; Thi, L.T.; Jeong, B.Y. Effect of explant type and plant growth regulators on callus induction, growth and secondary metabolites production in Cnidium officinale Makino. Mol. Biol. Rep. 2018, 45, 1919–1927. [Google Scholar] [CrossRef]
- Cho, S.K.; Kwon, O.I.; Kim, C.J. Anti-inflammatory and analgesic activities of the extracts and fractinos of Cnidii Rhizoma. Korean J. Pharmacogn. 1996, 27, 282–287. [Google Scholar]
- Lee, W.S.; Shin, J.S.; Jang, D.S.; Lee, K.T. Cnidilide, an alkylphthalide isolated from the roots of Cnidium officinale, suppresses LPS-induced NO, PGE2, IL-1β, IL-6 and TNF-α production by AP-1 and NF-κB inactivation in RAW 264.7 macrophages. Int. Immunopharmacol. 2016, 40, 146–155. [Google Scholar] [CrossRef]
- Ninomiya, K.; Sakai, C.; Ninomiya, K.; Shiotani, M.; Morikawa, T. Phthalides from rhizomes of Cnidium officinale accelerate metabolism of triglyceride in hepatocytes. Planta Med. 2016, 82, S1–S381. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, J.M.; Kang, S.M.; Lee, S.M.; Seo, C.W.; Lee, I.W.; Lee, I.J. Distribution characteristics of weeds and vegetation types in Cnidium officinale field. Weed Turfgrass Sci. 2012, 4, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [Green Version]
- Driedonks, N.; Rieu, I.; Vriezen, W.H. Breeding for plant heat tolerance at vegetative and reproductive stages. Plant Reprod. 2016, 29, 67–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, A.E. Breeding for heat tolerance. Plant Breed. Rev. 1992, 10, 129–168. [Google Scholar]
- Li, W.D.; Hu, X.; Liu, J.K.; Jiang, G.M.; Li, O.; Xing, D. Chromosome doubling can increase heat tolerance in Lonicera japonica as indicated by chlorophyll fluorescence imaging. Biol. Plant 2011, 55, 279. [Google Scholar] [CrossRef]
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. Polyploidy and its key role in plant breeding. Planta 2016, 243, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.G.; Tang, T.X.; Chen, R.; Liang, C.H.; Liu, X.Y.; Wu, C.L.; Yang, Y.S.; Yang, D.P.; Wu, H. A comparative study of bioactive secondary metabolite production in diploid and tetraploid Echinacea purpurea (L.) Moench. Plant Cell Tiss. Org. 2013, 116, 323–332. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, J.; Sun, K.; Chang, D.; Bai, S.; Shen, Y.; Huang, L.; Zhang, J.; Zhang, Y.; Dong, Y. Ploidy level and DNA content of Erianthus arundinaceus as determined by flow cytometry and the association with biological characteristics. PLoS ONE 2016, 11, e0151948. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohama, N.; Sato, H.; Shinozaki, K.; Yamahuchi-Shinozaki, K. Transcriptional regulatory network of plant heat stress response. Trends Plant Sci. 2017, 22, 53–65. [Google Scholar] [CrossRef]
- Lee, G.J.; Vierling, E. A small heat shock protein cooperates with heat shock protein 70 systems to reactivate a heat-denatured protein. Plant Physiol. 2000, 122, 189–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Sci. Hortic. 2018, 133, 481–489. [Google Scholar] [CrossRef]
- Chen, G.H.; Huang, L.T.; Yap, M.N.; Lee, R.H.; Huang, Y.J.; Cheng, M.C.; Chen, S.C.G. Molecular characterization of a senescence-associated gene encoding cysteine proteinase and its gene expression during leaf senescence in sweet potato. Plant Cell Physiol. 2002, 43, 984–991. [Google Scholar] [CrossRef] [Green Version]
- Jespersen, D.; Belanger, F.C.; Huang, B. Candidate genes and molecular markers associated with heat tolerance in colonial Bentgrass. PLoS ONE 2017, 12, e0171183. [Google Scholar] [CrossRef] [Green Version]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophyll and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Gallonea, A.; Hunter, A.; Douglas, G.C. Polyploid induction in vitro using colchicine and oryzalin on Hebe ‘Oratia Beauty’: Production and characterization of the vegetative traits. Sci. Hortic. 2014, 179, 59–66. [Google Scholar] [CrossRef]
- Miguel, T.P.; Leonhardt, K.W. In Vitro polyploid induction of orchids using oryzalin. Sci. Hortic. 2011, 130, 314–319. [Google Scholar] [CrossRef]
- Sajjad, Y.; Jaskani, M.J.; Mehmood, A.; Ahmad, I.; Abbas, H. Effect of colchicine on in vitro polyploidy induction in African marigold (Tagetes erecta). Pak. J. Bot. 2013, 45, 1255–1258. [Google Scholar]
- Atichart, P. Polyploid induction by colchicine treatments and plant regeneration of Dendrobium chrysotoxum. Thai J. Agric. Sci. 2013, 46, 59–63. [Google Scholar]
- Wannakrairoj, S.; Wondyifraw, T. In vitro chromosome doubling in Korarima [Aframomum corrorima (Braun) PCM Jansen] using colchicine and oryzalin. Kassetsart J. 2013, 47, 684–694. [Google Scholar]
- Talebi, S.F.; Saharkhiz, M.J.; Kermani, M.J.; Sharafi, Y.; Raouf Fard, F. Effect of different antimitotic agents on polyploid induction of anise hyssop (Agastache foeniculum L.). Caryologia 2017, 70, 184–193. [Google Scholar] [CrossRef]
- da S. de Carvalho, M.d.J.; Gomes, V.B.; da S. Souza, A.; Aud, F.F.; Santos-Serejo, J.A.; Oliveira, E.J. Inducing autotetraploids in cassava using oryzalin and colchicine and their in vitro morphophysiological effects. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Kaensaksiri, T.; Soontornchainaksaeng, P.; Soonthornchareonnon, N.; Prathanturarug, S. In Vitro induction of polyploidy in Centella asiatica (L.) Urban. Plant Cell Tiss Org. 2011, 10, 187. [Google Scholar] [CrossRef]
- Chung, M.Y.; Kim, C.Y.; Min, J.S.; Lee, D.J.; Naing, A.H.; Chung, J.D.; Kim, C.K. In Vitro induction of tetraploids in an interspecific hybrid of Calanthe (Calanthe discolor × Calanthe sieboldii) through colchicine and oryzalin treatments. Plant Biotechnol. Rep. 2014, 8, 251–257. [Google Scholar] [CrossRef]
- Chaves, A.L.A.; Chiavegatto, R.B.; Gabilanes, M.L.; Benites, F.R.G.; Techio, V.H. Effect of polyploidy on the leaf epidermis structure of Cynodon dactylon (L.) Pers.(Poaceae). Biologia 2018, 73, 1007–1013. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant 2015, 153, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Winarto, B.; Mattjik, N.A.; Teixeira da Silva, J.A.; Purwito, A.; Marwoto, B. Ploidy screening of anthurium (Anthurium andreanum Linden ex André) regenerants derived from anther culture. Sci. Hortic. 2010, 127, 86–90. [Google Scholar] [CrossRef]
- Mansouri, H.; Bagheri, M. Induction of polyploidy and its effect on Cannabis sativa L. In Cannabis Sativa L.-Botany and Biotechnology; Chandra, S., Lata, H., ElSohly, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 365–383. [Google Scholar]
- Ciccoritti, R.; Carbone, K.; Bellato, S.; Pogna, N.; Sgrulletta, D. Content and relative composition of some phytochemicals in diploid, tetraploid and hexaploid Triticum species with potential nutraceutical properties. J. Cereal Sci. 2013, 57, 200–206. [Google Scholar] [CrossRef]
- Caruso, I.; Lepore, L.; De Tommasi, N.; Dal Piaz, F.; Frusciante, L.; Aversano, R.; Garramone, R.; Carputo, D. Secondary metabolite profile in induced tetraploids of wild Solanum commersonii Dun. Chem. Biodivers. 2011, 8, 2226–2237. [Google Scholar] [CrossRef]
- Qu, A.L.; Ding, Y.F.; Jiang, Q.; Zhu, C. Molecular mechanisms of the plant heat stress response. Biochem. Biophys. Res. Commun. 2013, 432, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef] [Green Version]




| Oryzalin | Survival Rate (%) 1 | Rate of Mixoploid Induction (%) 1 | Rate of Polyploid Induction (%) 1 | |
|---|---|---|---|---|
| Conc. (mg∙L−1) | Time (Days) | |||
| Control | 100.00 ± 0.00 a | 0.00 ± 0.00 b | 0.00 ± 0.00 c | |
| Addition in culture medium | ||||
| 1 | 1 | 100.00 ± 0.00 a | 0.00 ± 0.00 b | 0.00 ± 0.00 c |
| 2 | 86.61 ± 5.85 bc | 0.00 ± 0.00 b | 0.00 ± 0.00 c | |
| 2 | 1 | 92.86 ± 4.12 b | 3.13 ± 3.13 ab | 0.00 ± 0.00 c |
| 2 | 84.38 ± 9.38 c | 0.00 ± 0.00 b | 0.00 ± 0.00 c | |
| 4 | 1 | 89.73 ± 6.78 bc | 3.57 ± 3.57 ab | 3.13 ± 3.13 b |
| 2 | 82.59 ± 6.93 c | 9.82 ± 6.08 a | 5.00 ± 5.00 a | |
| Drop on in vitro shoot tip | 100.00 ± 0.00 a | 0.00 ± 0.00 b | 0.00 ± 0.00 c | |
| Ploidy Level | No. of Leaves per Plant 1 | No. of Shoots per Plant 1 | Leaf Area (cm2) 1 | Petiole Length (mm) 1 | Petiole Diameter (mm) 1 |
|---|---|---|---|---|---|
| Diploid | 7.57 ± 1.00 | 3.14 ± 0.86 | 38.69 ± 3.84 | 134.88 ± 4.14 a | 1.93 ± 0.03 b |
| Tetraploid | 6.50 ± 1.34 | 3.67 ± 0.49 | 37.71 ± 3.84 | 114.89 ± 2.19 b | 2.69 ± 0.08 a |
| Significance | ns | ns | ns | ** | * |
| Ploidy Level | No. of Chloroplasts per Guard Cell 1 | Chloroplast Area (μm2) 1 | Stoma Length (μm) 1 | Stoma Width (μm) 1 | Guard Cell Area (μm2) 1 |
|---|---|---|---|---|---|
| Diploids | 10.70 ± 0.04 c | 5.94 ± 0.42 ab | 26.48 ± 0.38 c | 20.13 ± 0.36 b | 184.06 ± 3.05 b |
| Mixoploids | 12.40 ± 0.04 b | 7.79 ± 0.67 a | 30.68 ± 1.56 b | 23.74 ± 0.84 a | 293.56 ± 13.75 a |
| Tetraploids | 20.80 ± 0.05 a | 4.17 ± 0.38 b | 33.60 ± 0.91 a | 25.92 ± 0.68 a | 275.42 ± 21.51 a |
| Peak No. | Expected Compounds | tR (min) | Observed m/z | Calculated m/z | Molecular Formula [M-H]− | MS/MS Fragments (m/z) | Collision Energy (eV) | UV (λmax, nm) |
|---|---|---|---|---|---|---|---|---|
| 1 | Unidentified | 10.417 | 383.1557 | 383.1559 | C15H27O11 | 101 [M-282-H]− | 20 | |
| 2 | Unidentified | 11.663 | 335.0444 | 335.0444 | C8H15O14 | 172 [M-163-H]− | 30 | 272 |
| 3 | Neochlorogenic acid(5-caffeoylquinic acid) | 12.971 | 353.0864 | 353.0864 | C16H17O9 | 191 [M-C9H6O3-H]− | 20 | 298, 324 |
| 4 | Chlorogenic acid(3-caffeoylquinic acid) | 14.033 | 353.0865 | 353.0864 | C16H17O9 | 191 [M-C9H6O3-H]− | 30 | 300, 327 |
| 5 | Quinic acid derivative | 16.163 | 417.1748 | 417.1766 | C19H29O9 a | 191 [M-180-HCOOH-H]− | 10 | |
| 6 | Dicaffeoylquinic acid | 17.288 | 515.1177 | 515.1177 | C25H23O12 | 191 [M-C9H6O3-C9H6O3-H]− | 20 | 328 |
| 7 | Unidentified | 17.471 | 193.0505 | 193.0506 | C10H10O4 | 134 [M-59-H]− | 20 | 295, 323 |
| 8 | Senkyunolide F | 23.903 | 205.0868 | 205.0870 | C12H13O3 | 161 [M-CO2-H]− | 10 | |
| 9 | Phthalide (senkyunolide B, C) | 24.652 | 203.0710 | 203.0714 | C12H11O3 | 160 [M-C3H7-H]− | 20 | |
| 10 | Unidentified | 26.340 | 223.0970 | 223.0976 | C12H15O4 | 161 [M-62-H]− | 20 | |
| 11 | Unidentified | 26.403 | 311.2228 | 311.2228 | C18H31O4 | 171 [M-140-H]− | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-E.; Han, J.-E.; Lee, H.; Kim, J.-H.; Kim, H.-H.; Lee, K.-Y.; Shin, J.-H.; Kim, H.-K.; Park, S.-Y. Tetraploidization Increases the Contents of Functional Metabolites in Cnidium officinale. Agronomy 2021, 11, 1561. https://doi.org/10.3390/agronomy11081561
Kim H-E, Han J-E, Lee H, Kim J-H, Kim H-H, Lee K-Y, Shin J-H, Kim H-K, Park S-Y. Tetraploidization Increases the Contents of Functional Metabolites in Cnidium officinale. Agronomy. 2021; 11(8):1561. https://doi.org/10.3390/agronomy11081561
Chicago/Turabian StyleKim, Hyung-Eun, Jong-Eun Han, Hyoshin Lee, Ji-Hye Kim, Hyun-Hee Kim, Ki-Yong Lee, Jae-Heyuk Shin, Hyun-Kuy Kim, and So-Young Park. 2021. "Tetraploidization Increases the Contents of Functional Metabolites in Cnidium officinale" Agronomy 11, no. 8: 1561. https://doi.org/10.3390/agronomy11081561
APA StyleKim, H.-E., Han, J.-E., Lee, H., Kim, J.-H., Kim, H.-H., Lee, K.-Y., Shin, J.-H., Kim, H.-K., & Park, S.-Y. (2021). Tetraploidization Increases the Contents of Functional Metabolites in Cnidium officinale. Agronomy, 11(8), 1561. https://doi.org/10.3390/agronomy11081561






