Factors Determining the Variability of Performance of Bio-Control Agents against Root-Knot Nematodes in Vegetable Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Plants and Nematode Inoculation
- Seedlings (1.0–2.5 g);
- Young plants (3.5–5 g);
- Adult plants (8.5–10 g).
2.2. Treatments of Plants by BCAs
- Tomato: 1, 0.04, 0.18 g/plant for Myco, Tellus and Tusal, respectively;
- Egg plant: 1, 0.6, 0.4 g/plant;
- Pepper: 1, 0.04, 0.08 g/plant.
2.3. Measurements of Plant Growth and Nematode Infection Factors
2.4. Experimental Design and Statistical Analysis
3. Results
3.1. Effects of Myco on Plant Fitness and Nematode Infection in Tomato Plants at Different Growth Stages
3.2. Effects of Myco on Fitness and Nematode Infection on Young Plants of Different Vegetable Species
3.3. Effects of BCF-Based Formulates on Plant Fitness and Nematode Infection
3.4. Effects on Plant Fitness and Nematode Infection in Plants Grown from Seeds Germinated in BCA-Enriched Soils
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mejias, J.; Truong, N.M.; Abad, P.; Favery, B.; Quentin, M. Plant proteins and processes targeted by parasitic nematode effectors. Front. Plant Sci. 2019, 10, 970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gheysen, G.; Mitchum, M.G. How nematodes manipulate plant development pathways for infection. Curr. Opin. Plant Biol. 2011, 14, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Molinari, S. Natural genetic and induced plant resistance, as a control strategy to plant-parasitic nematodes alternative to pesticides. Plant Cell Rep. 2011, 30, 311–323. [Google Scholar] [CrossRef]
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, Mycorrhizal and Endophytic Fungi. Front. Microbiol. 2020, 11, 992. [Google Scholar] [CrossRef]
- Molinari, S.; Leonetti, P. Bio-control agents activate plant immune response and prime susceptible tomato against root-knot nematodes. PLoS ONE 2019, 14, e0213230. [Google Scholar] [CrossRef] [PubMed]
- Pocurull, M.; Fullana, A.M.; Ferro, M.; Valero, P.; Escudero, N.; Saus, E.; Gabaldón, T.; Sorribas, F.J. Commercial formulates of Trichoderma induce systemic plant resistance to Meloidogyne incognita in tomato and the effect is additive to that of the Mi-1.2 resistance gene. Front. Microbiol. 2020, 10, 3042. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, P.; Zonno, M.C.; Molinari, S.; Altomare, C. Induction of SA-signaling pathway and ethylene biosynthesis in Trichoderma harzianum-treated tomato plants after infection of the root-knot nematode Meloidogyne incognita. Plant Cell. Rep. 2017, 36, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Vos, C.; Schouteden, N.; van Tuinenb, D.; Chatagnier, O.; Elsenc, A.; De Waele, D.; Panisa, B.; Gianinazzi-Pearson, V. Mycorrhiza-induced resistance against the root–knot nematode Meloidogyne incognita involves priming of defense gene responses in tomato. Soil Biol. Biochem. 2013, 60, 45–54. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, H.; Di, Z.; Zhu, X.; Wang, Y.; Duan, Y.; Xuan, Y.; Chen, L. Isolation and identification of bacteria from rhizosphere soil and their effect on plant growth promotion and root-knot nematode disease. Biol. Control 2018, 119, 12–19. [Google Scholar] [CrossRef]
- Igiehon, N.O.; Babalola, O.O. Biofertilizers and sustainable agriculture: Exploring arbuscular mycorrhizal fungi. Appl. Microbiol. Biotechnol. 2017, 101, 4871–4881. [Google Scholar] [CrossRef] [PubMed]
- Alori, E.T.; Dare, M.O.; Babalola, O.O. Microbial inoculants for soil quality and plant health. In Sustainable Agriculture Review; Lichtfouse, E., Ed.; Springer: Cham, Switzerland, 2017; Volume 22, pp. 281–307. [Google Scholar]
- Schouteden, N.; De Waele, D.; Panis, B.; Vos, C.M. Arbuscular mycorrhizal fungi for the biocontrol of plant-parasitic nematodes: A review of the mechanisms involved. Front. Microbiol. 2015, 6, 1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molinari, S.; Lamberti, F.; Crozzoli, R.; Sharma, S.B.; Sanchez Portales, L. Isozyme patterns of exotic Meloidogyne spp. populations. Nematol. Medit. 2005, 33, 61–65. [Google Scholar]
- Walters, D.R.; Walsh, D.; Newton, A.C.; Lyon, G.D. Induced resistance for plant disease control: Maximizing the efficacy of resistance elicitors. Phytopathology 2005, 95, 1368–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molinari, S. Bioassays on Plant-nematode interactions. In Plant Bioassays; Narwal, S.S., Sampietro, D.A., Catalàn, C.A.N., Vattuone, M.A., Politycka, B., Eds.; Enfield Science Publisher: Enfield, NH, USA, 2009; pp. 293–326. [Google Scholar]
- Veresoglou, S.D.; Rillig, M.C. Suppression of fungal and nematode plant pathogens through arbuscular mycorrhizal fungi. Biol. Lett. 2012, 8, 214–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djian-Caporalino, C.; Molinari, S.; Palloix, A.; Ciancio, A.; Fazari, A.; Marteu, N.; Ris, N.; Castagnone-Sereno, P. The reproductive potential of the root-knot nematode Meloidogyne incognita is affected by selection for virulence against major resistance genes from tomato and pepper. Eur. J. Plant Pathol. 2011, 131, 431–440. [Google Scholar] [CrossRef]
- Pozo, M.J.; Azcón-Aguilar, C. Unraveling mycorrhiza-induced resistance. Curr. Opin. Plant Biol. 2007, 10, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Molinari, S.; Fanelli, E.; Leonetti, P. Expression of tomato salicylic acid (SA)-responsive pathogenesis-related genes in Mi-1-mediated and SA-induced resistance to root-knot nematodes. Mol. Plant Pathol. 2014, 15, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Salvioli, A.; Bonfante, P. Systems biology and “omics” tools: A cooperation for next-generation mycorrhizal studies. Plant Sci. 2013, 203–204, 107–114. [Google Scholar] [CrossRef] [PubMed]
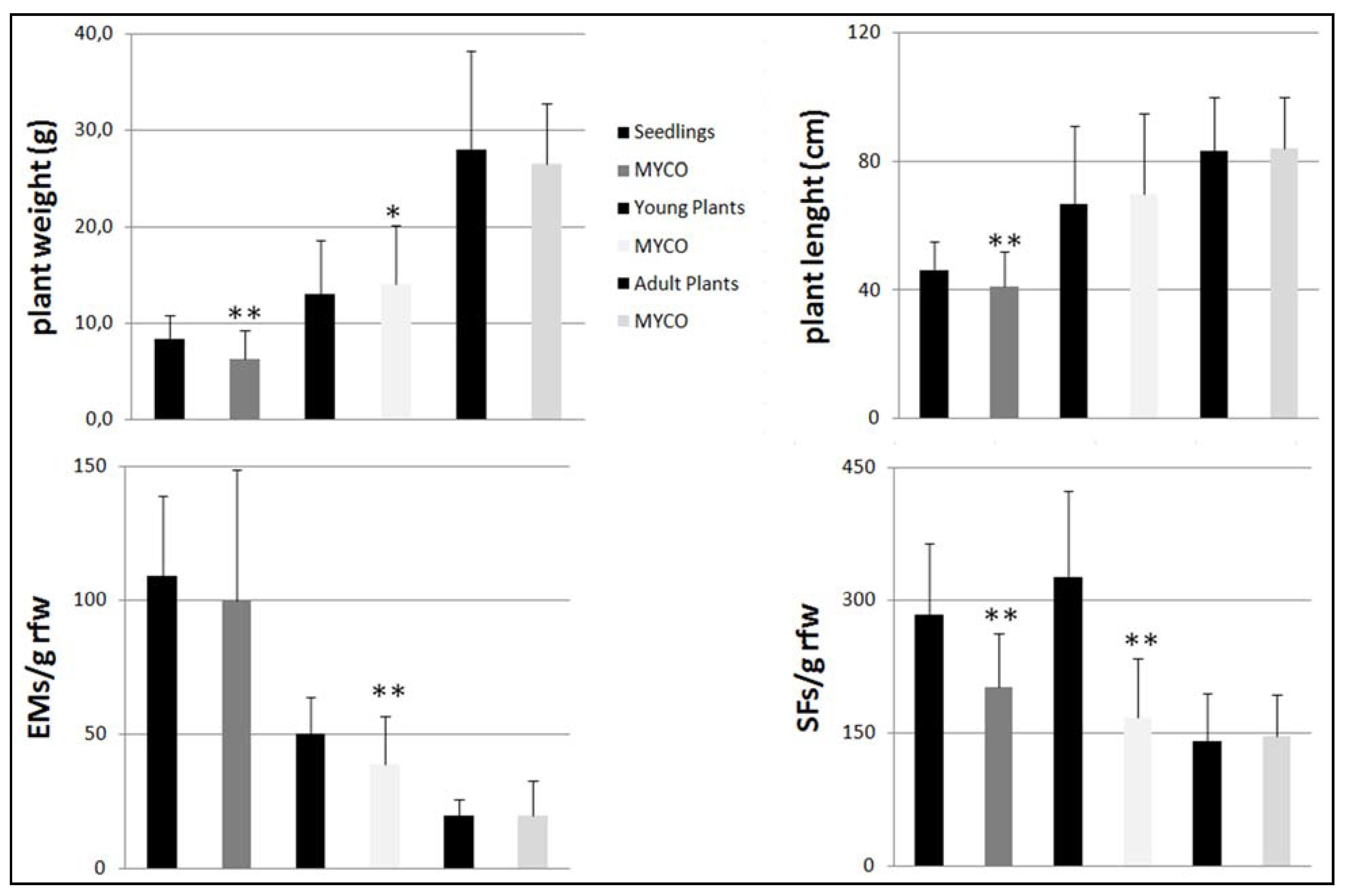
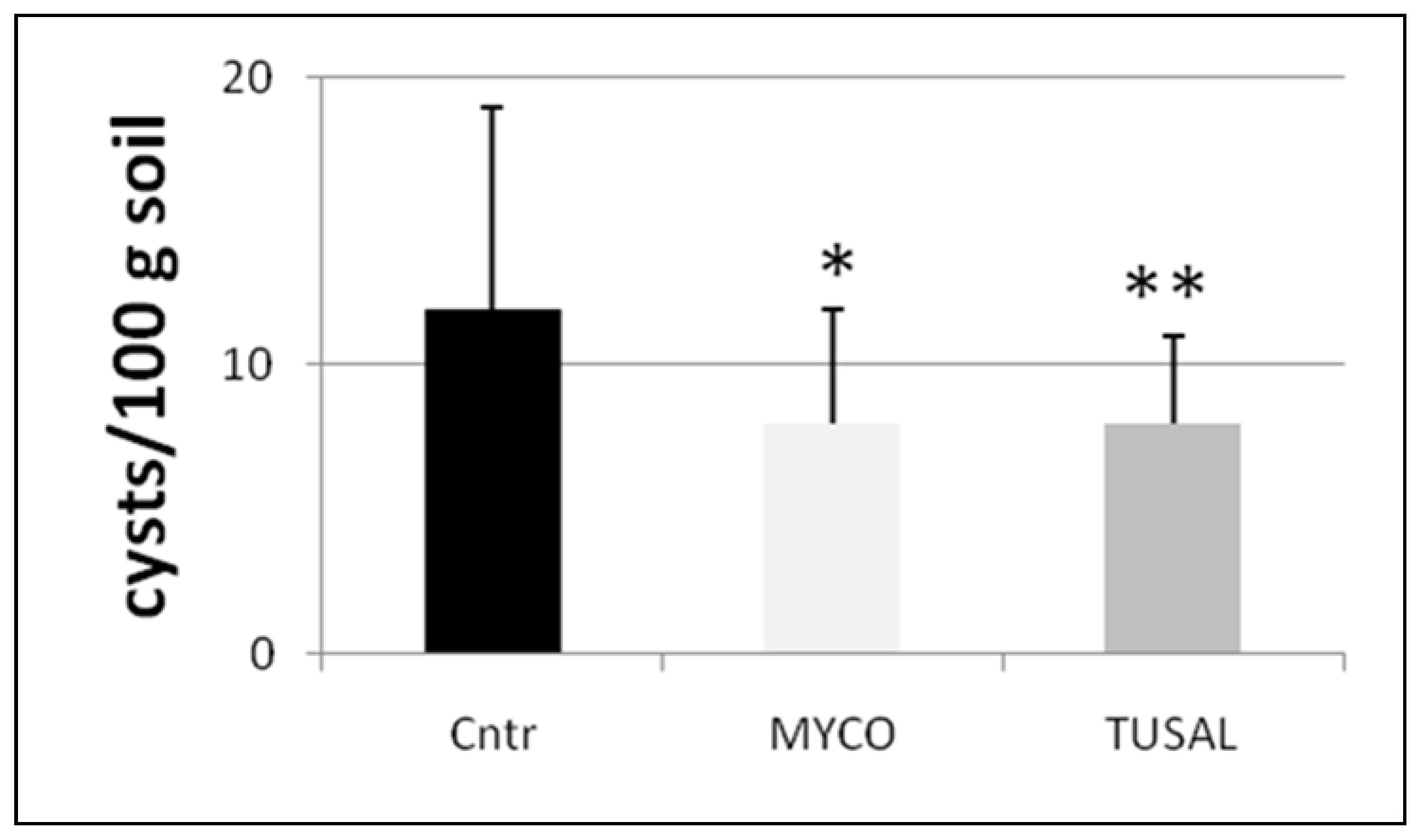
| PW | PL | EMs/g rfw | SFs/g rfw | FF | RP | |
|---|---|---|---|---|---|---|
| Untreated sus. tomato | 13.0 ± 5.7 | 67 ± 24 | 50 ± 14 | 326 ± 98 | 422 ± 91 | 128 ± 65 |
| Myco-treated | 14.1 ± 6.1 * (+8) | 70 ± 25 | 39 ± 18 ** (−22) | 167 ± 67 ** (−49) | 489 ± 130 | 82 ± 33 ** (−36) |
| Untreated res. tomato | 14.2 ± 4.6 | 78 ± 26 | 42 ± 28 | 166 ± 79 | 386 ± 96 | 116 ± 56 |
| Myco-treated | 16.3 ± 4.8 ** (+14) | 80 ± 18 | 32 ± 23 * (−23) | 116 ± 57 * (−30) | 399 ± 105 | 72 ± 36 ** (−38) |
| Untreated eggplant | 10.5 ± 4.1 | 42 ± 6 | 61 ± 26 | 263 ± 154 | 330 ± 159 | 243 ± 115 |
| Myco-treated | 10.7 ± 4.7 | 44 ± 8 | 46 ± 20 ** (−25) | 207 ± 135 ** (−21) | 327 ± 169 | 168 ± 106 ** (−31) |
| Untreated pepper | 10.3 ± 5.4 | 48 ± 9 | 48 ± 23 | 244 ± 113 | 264 ± 104 | 182 ± 102 |
| Myco-treated | 10.6 ± 5.0 | 48 ± 7 | 31 ± 26 ** (−35) | 161 ± 115 ** (−34) | 443 ± 186 * (+68) | 143 ± 95 * (−21) |
| BCA-Plant Interaction | PW | PL | EMs/g rfw | SFs/g rfw | Repr./Devel. |
|---|---|---|---|---|---|
| Untreated sus. tomato | 8.1 ± 2.6 | 53 ± 8 | 37 ± 14 | 94 ± 49 | 0.3 ± 0.1 |
| Tellus-treated (0.04 g/plant) | 8.1 ± 2.6 | 50 ± 9 * (−6) | 25 ± 15 * (−34) | 39 ± 13 ** (−59) | 0.5 ± 0.3 * (+51) |
| Untreated eggplant | 8.8 ± 2.5 | 44 ± 9 | 38 ± 20 | 111 ± 50 | 0.3 ± 0.1 |
| Tellus-treated (0.6 g/plant) | 10.5 ± 3.7 ** (+20) | 49 ± 11 ** (+12) | 32 ± 22 * (−16) | 87 ± 47 ** (−21) | 0.3 ± 0.1 |
| Untreated pepper | 8.9 ± 2.8 | 43 ± 5 | 32 ± 12 | 112 ± 41 | 0.4 ± 0.1 |
| Tellus-treated(0.04 g/plant) | 7.5 ± 2.6 ** (−15) | 41 ± 6 | 12 ± 10 ** (−63) | 35 ± 31 ** (−69) | 0.5 ± 0.3 * (+23) |
| Untreated sus. tomato | 9.9 ± 3.6 | 48 ± 11 | 34 ± 19 | 108 ± 55 | 0.35 ± 0.2 |
| Tusal-treated (0.18 g/plant) | 11.1 ± 3.7 * (+12) | 50 ± 11 * (+6) | 24 ± 18 ** (−31) | 48 ± 30 ** (−56) | 0.55 ± 0.4 * (+56) |
| Untreated eggplant | 7.2 ± 1.5 | 44 ± 8 | 54 ± 29 | 99 ± 43 | 0.7 ± 0.4 |
| Tusal-treated (0.4 g/plant) | 8.0 ± 2.8 * (+12) | 47 ± 11 * (+7) | 36 ± 17 ** (−34) | 74 ± 37 * (−25) | 0.6 ± 0.3 |
| Untreated pepper | 8.1 ± 2.4 | 44 ± 5 | 37 ± 25 | 103 ± 48 | 0.4 ± 0.2 |
| Tusal-treated (0.08 g/plant) | 8.4 ± 3.1 | 44 ± 5 | 24 ± 18 * (−35) | 44 ± 31 ** (−57) | 0.6 ± 0.2 * (+50) |
| Untreated | Myco-Treated | Tellus-Treated | Tusal-Treated | |
|---|---|---|---|---|
| Tomato | ||||
| PW | 11.1 ± 5.3 | 9.9 ± 4.0 ** (−11) | 9.5 ± 4.3 ** (−14) | 10.6 ± 6.5 |
| PL | 48 ± 9 | 48 ± 8 | 45 ± 7 ** (−7) | 45 ± 10 ** (−7) |
| EMs/g rfw | 39 ± 25 | 25 ± 15 ** (−36) | 23 ± 15 ** (−41) | 23 ± 16 ** (−41) |
| SFs/g rfw | 239 ± 112 | 158 ± 52 ** (−34) | 110 ± 70 ** (-54) | 188 ± 49 ** (−22) |
| FF | 232 ± 136 | 212 ± 111 | 234 ± 152 | 230 ± 114 |
| RP | 87 ± 35 | 51 ± 20 ** (−42) | 43 ± 25 ** (−50) | 53 ± 33 * (−40) |
| Eggplant | ||||
| PW | 7.4 ± 3.0 | 6.0 ± 1.5** (−20) | 8.0 ± 3.2 | 7.8 ± 2.1 |
| PL | 40 ± 7 | 39 ± 5 | 42 ± 8 ** (+7) | 43 ± 7 ** (+8) |
| EMs/g rfw | 47 ± 26 | 34 ± 23 * (−26) | 23 ± 14 ** (−51) | 36 ± 25 ** (−23) |
| SFs/g rfw | 168 ± 45 | 106 ± 31 ** (−37) | 122 ± 36 ** (−27) | 102 ± 25 ** (−39) |
| FF | 248 ± 70 | 268 ± 90 | 301 ± 105 * (+21) | 243 ± 56 |
| RP | 139 ± 50 | 75 ± 33 ** (−46) | 73 ± 38 ** (−48) | 119 ± 26 * (−15) |
| Pepper | ||||
| PW | 9.4 ± 2.2 | 8.2 ± 0.9* (−13) | 7.7 ± 1.1 * (−18) | 8.5 ± 1.0 |
| PL | 38 ± 4 | 36 ± 3 | 35 ± 3 | 31 ± 3 ** (−19) |
| EMs/g rfw | 52 ± 8 | 57 ± 16 | 21 ± 14 ** (−61) | 41 ± 14 * (−21) |
| SFs/g rfw | 238 ± 72 | 139 ± 74 ** (−42) | 72 ± 24 ** (−70) | 77 ± 26 ** (−68) |
| FF | 201 ± 58 | 208 ± 56 | 453 ± 107 ** (+126) | 257 ± 110 * |
| RP | 87 ± 38 | 94 ± 32 | 75 ± 26 | 69 ± 24 * (−21) |
| Untreated | Myco-Treated | Tellus-Treated | Tusal-Treated | |
|---|---|---|---|---|
| PW | 11.2 ± 3.0 | 12.4 ± 3.2 | 10.8 ± 2.6 | 13.9 ± 3.7 ** (+24) |
| PL | 45 ± 7 | 47 ± 7 | 46 ± 10 | 49 ± 8 ** (+9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molinari, S. Factors Determining the Variability of Performance of Bio-Control Agents against Root-Knot Nematodes in Vegetable Plants. Agronomy 2021, 11, 1602. https://doi.org/10.3390/agronomy11081602
Molinari S. Factors Determining the Variability of Performance of Bio-Control Agents against Root-Knot Nematodes in Vegetable Plants. Agronomy. 2021; 11(8):1602. https://doi.org/10.3390/agronomy11081602
Chicago/Turabian StyleMolinari, Sergio. 2021. "Factors Determining the Variability of Performance of Bio-Control Agents against Root-Knot Nematodes in Vegetable Plants" Agronomy 11, no. 8: 1602. https://doi.org/10.3390/agronomy11081602
APA StyleMolinari, S. (2021). Factors Determining the Variability of Performance of Bio-Control Agents against Root-Knot Nematodes in Vegetable Plants. Agronomy, 11(8), 1602. https://doi.org/10.3390/agronomy11081602






