Fruit Set in Avocado: Pollen Limitation, Pollen Load Size, and Selective Fruit Abortion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pollen Load at the Male and Female Stages during the Avocado Flowering Season
2.2. Embryo and Endosperm Development in Flowers Pollinated at the Female and Male Stages
2.3. Effect of Pollen Load on Fruit Set
2.4. Fate of the Fruits Developed after Self- and Cross-Pollination
2.5. Fruit Set and Pollination Date
2.6. Characteristics of Fruits Derived from Self- and Cross-Fertilization
3. Results
3.1. Pollen Deposition at the Male and Female Stages during the Flowering Season
3.2. Embryo and Endosperm Development in Flowers Pollinated at the Female and Male Stages
3.3. Effect of Pollen Load Size on Fruit Set
3.4. Fate of Fruits Resulting from Selfing vs. Crossing
3.5. Fruit Set According to the Date of Anthesis
3.6. Effect of the Genotype of the Male Parent on Fruit Size
4. Discussion
4.1. The Effect of Pollen Load on Fruit Set
4.2. Pollination during the Dichogamous Cycle of the Avocado Flowers
4.3. Pollination during the Flowering Season
4.4. Embryo Development in Flowers Pollinated during the Male Phase
4.5. Fruits Resulting from Self- and Cross-Fertilization
4.6. Pollen Parent Effect on Fruit and Seed Characteristics
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galindo-Tovar, M.E.; Ogata-Aguilar, N.A.; Arzate-Fernandez, M. Some aspects of avocado (Persea americana Mill.) diversity and domestication in Mesoamerica. Genet. Resour. Crop. Evol. 2008, 55, 441–450. [Google Scholar] [CrossRef]
- Chen, H.; Morrell, P.L.; Ashworth, V.E.T.M.; De La Cruz, M.; Clegg, V. Tracing the geographic origins of major avocado cultivars. J. Hered. 2009, 100, 56–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litz, R.E.; Pliego-Alfaro, F.; Palomo-Ríos, E.; Mercado, J.A.; Pliego, C.; Barceló-Muñoz, A.; López-Gómez, R.; Hormaza, J.I. Persea americana, Avocado. In Biotechnology of Fruit and Nut Crops; Litz, R.E., Pliego-Alfaro, F., Hormaza, J.I., Eds.; CABI: Wallingford, UK, 2020; pp. 258–281. [Google Scholar]
- Cameron, S.H.; Mueller, R.T.; Wallace, A. Nutrient composition and seasonal losses of avocado trees. Calif. Avocado Soc. Yearb. 1952, 37, 201–209. [Google Scholar]
- Sedgley, M. Anatomical investigation of abscissed avocado flowers and fruitlets. Ann. Bot. 1980, 46, 771–777. [Google Scholar] [CrossRef]
- Lahav, E.; Zamet, D.N. Flower, fruitlets and fruit drop in avocado trees. Rev. Chapingo Ser. Hortic. 1999, 5, 95–100. [Google Scholar]
- Garner, L.C.; Ashworth, V.E.T.M.; Clegg, M.T.; Lovatt, C.J. The impact of outcrossing on yields of ‘Hass’ avocado. J. Am. Soc. Hortic. Sci. 2008, 133, 631–722. [Google Scholar] [CrossRef]
- Garner, L.C.; Lovatt, C.J. Physiological factors affecting flower and fruit abscission of ‘Hass’ avocado. Sci. Hortic. 2016, 199, 32–40. [Google Scholar] [CrossRef]
- Boldingh, H.I.; Alcaraz, M.L.; Thorp, T.G.; Minchin, P.E.H.; Gould, N.; Hormaza, J.I. Carbohydrate and boron content of styles of ‘Hass’ avocado (Persea americana Mill.) flowers at anthesis can affect final fruit set. Sci. Hortic. 2016, 198, 125–131. [Google Scholar] [CrossRef]
- Alcaraz, M.L.; Hormaza, J.I. Influence of physical distance between cultivars on yield, outcrossing rate and selective fruit drop in avocado (Persea americana Mill., Lauraceae). Ann. Appl. Biol. 2011, 158, 354–361. [Google Scholar] [CrossRef]
- Alcaraz, M.L.; Hormaza, J.I. Optimization of controlled pollinations in avocado (Persea americana, Lauraceae). Sci. Hortic. 2014, 180, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Alcaraz, M.L.; Rodrigo, J.; Hormaza, J.I. Pistil starch reserves at anthesis correlate with final flower fate in avocado (Persea americana Mill.). PLoS ONE 2013, 8, e78467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezuidenhout, N.M.; Du Toit, E.S.; Robbertse, P.J. Finding the best polliniser for ‘Hass’ avocado and the effect of honeybees as pollinators. S. Afr. Avocado Grow. Assoc. Yearb. 2016, 39, 70–75. [Google Scholar]
- Ashman, T.L.; Knight, T.M.; Steets, J.A.; Amarasekare, P.; Burd, M.; Campbell, D.R.; Dudash, M.R. Pollen limitation of plant reproduction: Ecological and evolutionary causes and consequences. Ecology 2004, 85, 2408–2421. [Google Scholar] [CrossRef] [Green Version]
- Brewbaker, J.L.; Majumder, S.K. Cultural studies of the pollen population effect and the self-incompatibility inhibition. Am. J. Bot. 1961, 48, 457–464. [Google Scholar] [CrossRef]
- Lee, C.L. Pollen germination, pollen tube growth and fertilization behaviour of Prunus domestica. II. Pollen tube growth in the style. Gartenbauwissenschaf 1980, 45, 241–248. [Google Scholar]
- Hormaza, J.I.; Herrero, M. Dynamics of pollen tube growth under different competition regimes. Sex. Plant Reprod. 1996, 9, 153–160. [Google Scholar] [CrossRef]
- Pasonen, H.L.; Käpylä, M. Pollen–pollen interactions in Betula pendula in vitro. New Phytol. 1998, 138, 481–487. [Google Scholar] [CrossRef]
- Chen, Y.F.; Matsubayashi, Y.; Sakagami, Y. Peptide growth factor phytosulfokine-a contributes to the pollen population effect. Planta 2000, 211, 752–755. [Google Scholar] [CrossRef]
- Stephenson, A.G.; Travers, S.E.; Mena-Ali, J.I.; Winsor, J.A. Pollen performance before and during the autotrophic-heterotrophic transition of pollen tube growth. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 1009–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Losada, J.M.; Herrero, M. Glycoprotein composition along the pistil of Malus x domestica and the modulation of pollen tube growth. BMC Plant Biol. 2014, 14, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hornby, C.A.; Charles, W.B. Pollen germination as affected by variety and number of pollen grains. Tomato Grow. Coop. 1996, 16, 11–12. [Google Scholar]
- Jennings, D.L.; Topham, P.B. Some consequences of raspberry pollen dilution for its germination and fruit development. New Phytol. 1971, 70, 371–380. [Google Scholar] [CrossRef]
- Levin, D.A. Gametophytic competition in Phlox. In Gamete Competition in Plants and Animals; Mulcahy, D.L., Ed.; North Holland: Amsterdam, The Netherlands, 1975; pp. 207–218. [Google Scholar]
- Cruzan, M. Pollen tube distributions in Nicotiana glauca: Evidence for density dependent growth. Am. J. Bot. 1986, 73, 902–907. [Google Scholar] [CrossRef]
- Winsor, J.A.; Davis, L.E.; Stephenson, A.G. The relationship between pollen load and fruit maturation and the effect of pollen load on offspring vigor in Cucurbita pepo. Am. Nat. 1987, 129, 643–656. [Google Scholar] [CrossRef]
- Taylor, L.P.; Hepler, P.K. Pollen germination and tube growth. Ann. Rev. Plant Physiol. 1997, 48, 461–491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tateishi, N.; Tanabe, K. Pollen density on the stigma affects endogenous gibberellin metabolism, seed and fruit set, and fruit quality in Pyrus pyrifolia. J. Exp. Bot. 2010, 61, 4291–4302. [Google Scholar] [CrossRef] [Green Version]
- Ganeshaiah, K.N.; Shaanker, R.U. Regulation of seed number and female incitation of mate competition by a pH-dependant proteinaceous inhibitor of pollen grain germination in Leucaena leucocephala. Oecologia 1988, 75, 110–113. [Google Scholar] [CrossRef]
- Shoval, S. Pollination Rate and Pollen tube Growth of Avocado in Relation to Yield. Master’s Thesis, The Hebrew University of Jerusalen, Rehovot, Israel, 1987. [Google Scholar]
- Davenport, T.L. Pollen deposition on avocado stigmas in Southern Florida. HortScience 1989, 24, 844–845. [Google Scholar]
- Ish-am, G. Interrelationship between Avocado Flowering and Honey Bees and Its Implication on the Avocado Fruitfulness in Israel. Ph.D. Thesis, Tel-Aviv University, Tel-Aviv, Israel, 1994. [Google Scholar]
- Katz, E. Avocado Productivity: Pollination, Pollenizers, Fruit Set and Abscission. Master’s Thesis, The Hebrew University of Jerusalem, Faculty of Agriculture, Rehovot, Israel, 1995. [Google Scholar]
- Mulcahy, D.L.; Mulcahy, G.B. The effects of pollen competition. Am. Sci. 1987, 75, 44–50. [Google Scholar]
- Barnes, D.K.; Cleveland, R.W. Pollen tube growth of diploid alfalfa in vitro. Crop. Sci. 1963, 3, 291–295. [Google Scholar] [CrossRef]
- Pfahler, P.L. Fertilization ability of maize pollen grains. II. Pollen genotype, female sporophyte and pollen storage interactions. Genetics 1967, 57, 513–521. [Google Scholar] [CrossRef]
- Marshall, D.L.; Ellstrand, N.C. Sexual selection in Raphanus sativus: Experimental data on the nonrandom fertilization, maternal choice, and consequences of multiple paternity. Am. Nat. 1986, 127, 446–461. [Google Scholar] [CrossRef]
- Krauss, S.L. The realized effect of postpollination sexual selection in a natural plant population. Proc. Biol. Sci. 2000, 267, 1925–1929. [Google Scholar] [CrossRef] [Green Version]
- Marshall, D.L.; Shaner, M.G.M.; Oliveras, J.P. Effects of pollen load size on seed paternity in wild radish: The roles of pollen competition and mate choice. Evolution 2007, 61, 1925–1937. [Google Scholar] [CrossRef]
- Shaner, M.G.; Marshall, D.L. How robust is nonrandom mating in wild radish: Do small pollen loads coupled with more competing pollen donors lead to random mating? Am. J. Bot. 2007, 94, 266–273. [Google Scholar] [CrossRef]
- Jones, A.G.; Ratterman, N.L. Mate choice and sexual selection: What have we learned since Darwin? Proc. Natl. Acad. Sci. USA 2009, 106, 10001–10008. [Google Scholar] [CrossRef] [Green Version]
- Lankinen, Å.; Madjidian, J.A. Enhancing pollen competition by delaying stigma receptivity: Pollen deposition schedules affect siring ability, paternal diversity, and seed production in Collinsia heterophylla (Plantaginaceae). Am. J. Bot. 2011, 98, 1191–1200. [Google Scholar] [CrossRef]
- Moore, J.C.; Pannell, J.R. Sexual selection in plants. Curr. Biol. 2011, 21, R176–R182. [Google Scholar] [CrossRef] [Green Version]
- Lankinen, Å.; Green, K.K. Using theories of sexual selection and sexual conflict to improve our understanding of plant ecology and evolution. AoB Plants 2015, 7, plv008. [Google Scholar] [CrossRef] [Green Version]
- Lankinen, Å.; Lindström, S.A.M.; D’Hertefeldt, T. Variable pollen viability and effects of pollen load size on components of seed set in cultivars and feral populations of oilseed rape. PLoS ONE 2018, 13, e0204407. [Google Scholar] [CrossRef]
- Delph, L.F. Pollen competition is the mechanism underlying a variety of evolutionary phenomena in dioecious plants. New Phytol. 2019, 224, 1075–1079. [Google Scholar] [CrossRef]
- Mazer, S.J.; Hove, A.A.; Miller, B.S.; Barbet-Massin, M. The joint evolution of mating system and pollen performance: Predictions regarding male gametophytic evolution in selfers vs outcrossers. Perspect. Plant Ecol. 2010, 12, 31–41. [Google Scholar] [CrossRef]
- Nasrallah, J.B. Recognition and rejection of self in plant reproduction. Science 2002, 296, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Barrett, S.C.H. Mating strategies in flowering plants: The outcrossing-selfing paradigm and beyond. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 991–1004. [Google Scholar] [CrossRef]
- Johansen, D.A. Plant Microtechnique; McGraw-Hill: New York, NY, USA, 1940. [Google Scholar]
- Sabatini, D.D.; Bensch, K.; Barrnett, R.J. Cytochemistry and electron microscopy. Preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J. Cell Biol. 1963, 17, 19–58. [Google Scholar] [CrossRef]
- Feder, N.; O’Brien, T.P. Plant microtechnique. Some principles and new methods. Am. J. Bot. 1968, 55, 123–142. [Google Scholar] [CrossRef]
- Hormaza, J.I. Molecular characterization and similarity relationships among apricot (Prunus armeniaca L.) genotypes using simple sequence repeats. Theor. Appl. Genet. 2002, 104, 321–328. [Google Scholar] [CrossRef]
- Sharon, D.; Cregan, P.B.; Mhameed, S.; Kuharska, M.; Hillel, I.; Lahav, E.; Lavi, U. An integrated genetic linkage map of avocado. Theor. Appl. Genet. 1997, 95, 911–921. [Google Scholar] [CrossRef]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Sedgley, M. Light-microscope study of pollen-tube growth, fertilization and early embryo and endosperm development in the avocado varieties Fuerte and Hass. Ann. Bot. 1979, 44, 353–359. [Google Scholar] [CrossRef]
- Shore, J.S.; Barrett, S.C.H. The effect of pollination intensity and incompatible pollen on seed set in Turnera ulmifolia (Turneraceae). Can. J. Bot. 1984, 62, 1298–1303. [Google Scholar] [CrossRef] [Green Version]
- Snow, A.A. Effects of pollen-load size and number of donors on sporophyte fitness in wild radish (Raphanus raphanistrum). Am. Nat. 1990, 136, 742–758. [Google Scholar] [CrossRef]
- Mitchell, R.J. Effects of pollen quantity on progeny vigor: Evidence from the desert mustard Lesquerella fendleri. Evolution 1997, 51, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Kalla, S.E.; Ashman, T.L. The effects of pollen competition on progeny vigor in Fragaria virginiana (Rosaceae) depend on progeny growth environment. Int. J. Plant Sci. 2002, 163, 335–340. [Google Scholar] [CrossRef]
- Aizen, M.A.; Harder, L.D. Expanding the limits of the pollen limitation concept: Effects of pollen quantity and quality. Ecology 2007, 88, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Field, D.L.; Pickup, M.; Barrett, S.C.H. The influence of pollination intensity on fertilization success, progeny sex ratio, and fitness in a wind-pollinated, dioecious plant. Int. J. Plant Sci. 2012, 173, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Leach, G.J. Variation in lucerne seed yields in relation to genotype and intensity of pollination. Aust. J. Exp. Agric. Anim. Husb. 1972, 12, 420–427. [Google Scholar] [CrossRef]
- Bertin, R.I. Floral biology, hummingbird pollination and fruit production of trumpet creeper (Campsis radicans, Bignoniaceae). Am. J. Bot. 1982, 69, 122–134. [Google Scholar] [CrossRef]
- Snow, A.A. Pollination intensity and potential seed set in Passiflora vitifolia. Oecologia 1982, 55, 231–237. [Google Scholar] [CrossRef]
- McDade, L.A. Pollination intensity and seed set in Trichanthera gigantea (Acanthaceae). Biotropica 1983, 15, 122–124. [Google Scholar] [CrossRef]
- McDade, L.A.; Davidar, P. Determinants of fruit and seed set in Pavonia dasypetala (Malvaceae). Oecologia 1984, 64, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.D.; Hartgerink, A.P. Pollination intensity, fruit maturation pattern, and offspring quality in Cassia fasciculata (Leguminosae). In Biotechnology and Ecology of Pollen; Mulcahy, D.L., Mulcahy, G.B., Ottaviano, E., Eds.; Springer: New York, NY, USA, 1986; pp. 417–422. [Google Scholar]
- Stephenson, A.G.; Winsor, J.A.; Davis, L.E. Effects of pollen load size on fruit maturation and sporophyte quality in zucchini. In Biotechnology and Ecology of Pollen; Mulcahy, D.L., Mulcahy, G.B., Ottaviano, E., Eds.; Springer: New York, NY, USA; Berlin/Heidelberg, Germany, 1986; pp. 429–434. [Google Scholar]
- Björkman, T. The effect of pollen load and pollen grain competition on fertilization success and progeny performance in Fagopyrum esculentum. Euphytica 1995, 83, 47–52. [Google Scholar] [CrossRef]
- Freihat, N.M.; Al-Ghzawi, A.; Zaitoun, S.; Alqudah, A. Fruit set and quality of loquats (Eriobotrya japonica) as effected by pollinations under sub-humid Mediterranean. Sci. Hortic. 2008, 117, 58–62. [Google Scholar] [CrossRef]
- Davenport, T.L. A new look at avocado pollination. Trop. Fruit World 1991, 2, 3–4. [Google Scholar]
- Davenport, T.L. A view from Florida on avocado pollination. In Proceedings of Avocado Brainstorming ’99, Riverside, CA, USA, 27–28 October 1999; Arpaia, M.L., Hofshi, R., Eds.; California Avocado Commission and College of Natural and Agricultural Sciences, University of California: Riverside, CA, USA, 1999; pp. 101–104. [Google Scholar]
- Davenport, T.L. Cross- vs. self-pollination in ‘Hass’ avocados growing in coastal and inland orchards of Southern California. Sci. Hortic. 2019, 246, 307–316. [Google Scholar] [CrossRef]
- Davenport, T.L.; Parnitzki, P.; Fricke, S.; Hughes, M.S. Evidence and significance of self-pollination of avocado in Florida. J. Am. Soc. Hortic. Sci. 1994, 119, 1200–1207. [Google Scholar] [CrossRef]
- Aizen, M.A. Flower sex ratio, pollinator abundance, and the seasonal pollination dynamics of a protandrous plant. Ecology 2001, 82, 127–144. [Google Scholar] [CrossRef]
- Ohashi, K.; Yahara, T. Behavioral responses of pollinators to variation in floral display size and their influences on the evolution of floral traits. In Cognitive Ecology of Pollination; Chittka, L., Thomson, J.D., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 274–296. [Google Scholar]
- Harder, L.D.; Jordan, C.Y.; Gross, W.E.; Routley, M.B. Beyond floricentrism: The pollination function of inflorescences. Plant Spec. Biol. 2004, 19, 137–148. [Google Scholar] [CrossRef]
- Degani, C.; Gazit, S. Selfed and crossed proportions of avocado progenies produced by caged pairs of complementary cultivars. HortScience 1984, 19, 258–260. [Google Scholar]
- Degani, C.; Goldring, A.; Gazit, S.; Lavi, U. Pollen parent effect on outcrossing rate in ‘Hass’ and ‘Fuerte’ avocado plots during fruit development. J. Am. Soc. Hortic. Sci. 1989, 114, 106–111. [Google Scholar]
- Degani, C.; Goldring, A.; Adato, I.; El-Batri, R.; Gazit, S. Pollen parent effect on outcrossing rate, yield and fruit characteristics of ‘Fuerte’ avocado. HortScience 1990, 25, 471–473. [Google Scholar] [CrossRef] [Green Version]
- Degani, C.; El-Batsri, R.; Gazit, S. Outcrossing rate, yield and selective fruit abscission in ‘Ettinger’ and ‘Ardith’ avocado plots. J. Am. Soc. Hortic. Sci. 1997, 122, 813–817. [Google Scholar] [CrossRef]
- Ashworth, V.E.T.M.; Chen, H.; Clegg, M.T. Chapter 17: Avocado. In Genome Mapping and Molecular Breeding in Plants; Fruits and Nuts; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 4, pp. 325–329. [Google Scholar]
- Chen, H.; Ashworth, V.E.T.M.; Xu, S.; Clegg, M.T. Quantitative genetic analysis of growth rate in avocado. J. Am. Soc. Hortic. Sci. 2007, 132, 691–696. [Google Scholar] [CrossRef]
- Borrone, J.W.; Tondo, C.T.; Kuhn, D.N.; Brown, J.S.; Schnell, R.J.; Violi, H.A. Outcrossing in Florida avocados as measured using microsatellite markers. J. Am. Soc. Hortic. Sci. 2008, 133, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Stahl, P.; Mirom, Y.L.; Stern, R.A.; Goldway, M. Comparing ‘Iriet’ and ‘Ettinger’ avocado cultivars as pollinators of ‘Hass’ using SNPs for paternal identification. Sci. Hortic. 2019, 248, 50–57. [Google Scholar] [CrossRef]
- Degani, C.; Stern, R.A.; El-Batsri, R.; Gazit, S. Pollen parent effect on the selective abscission of ‘Mauritius’ and ‘Floridian’ lychee fruitlets. J. Am. Soc. Hortic. Sci. 1995, 120, 523–526. [Google Scholar] [CrossRef]
- Dag, A.; Eisenstein, D.; Gazit, S.; El-Batsri, R. Effect of pollenizer distance and selective fruitlet abscission on outcrossing rate and yield in ‘Tommy Atkins’ mango. J. Am. Soc. Hortic. Sci. 1998, 123, 618–622. [Google Scholar] [CrossRef] [Green Version]
- Pérez, V.; Herrero, M.; Hormaza, J.I. Self-fertility and preferential cross-fertilization in mango (Mangifera indica). Sci. Hortic. 2016, 213, 373–378. [Google Scholar] [CrossRef] [Green Version]
- Richards, T.E.; Kämper, W.; Trueman, S.J.; Wallace, H.M.; Ogbourne, S.M.; Brooks, P.R.; Nichols, J.; Baiv, S.H. Relationships between nut size, kernel quality, nutritional composition and levels of outcrossing in three macadamia cultivars. Plants 2020, 9, 228. [Google Scholar] [CrossRef] [Green Version]
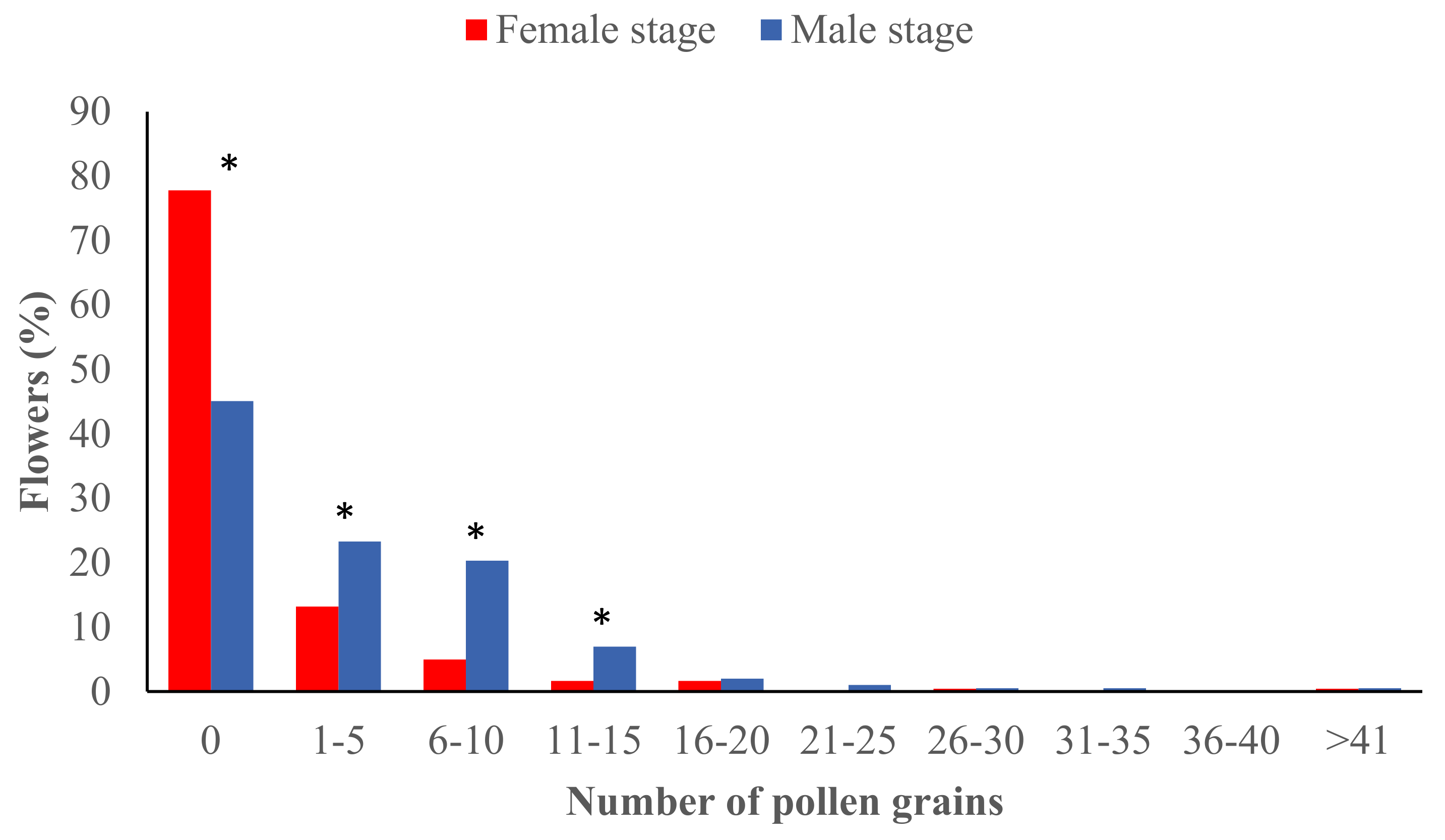





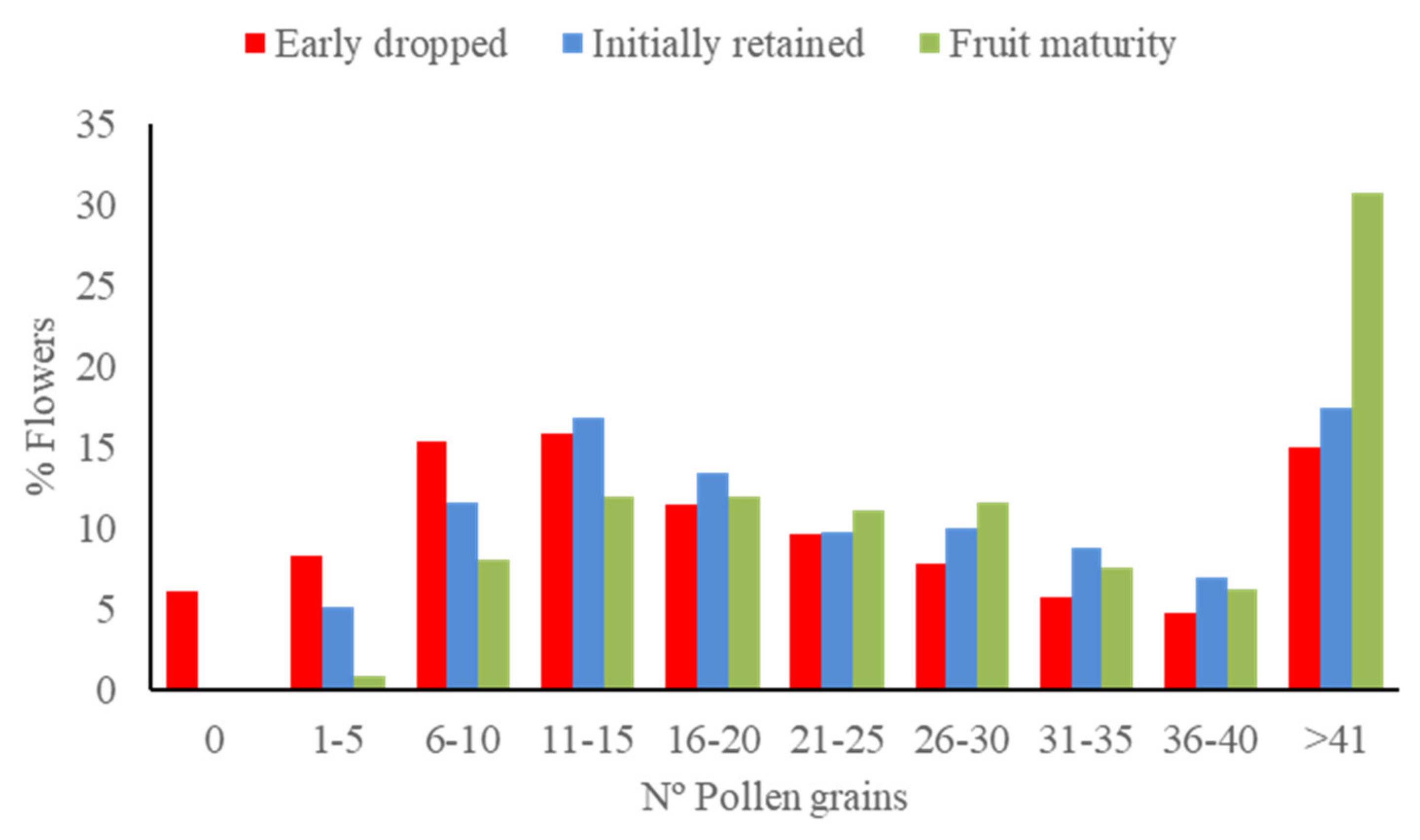


| Period | Female Stage | Pollen Adhesion | Male Stage | Pollen Adhesion | Comparison between Stages | ||
|---|---|---|---|---|---|---|---|
| N | Mean ± SE | N | Mean ± SE | t | d.f. | p | |
| 1st week | 72 | 2.96 ± 0.87 a | 70 | 7.01 ± 1.03 a | 3.013 | 140 | 0.003 |
| 2nd week | 46 | 0.23 ± 0.11 ab | 50 | 1.3 ± 0.30 b | 3.27 | 94 | 0.01 |
| 3rd week | 82 | 1.36 ± 0.43 ab | 45 | 2.87 ± 0.244 b | 2.27 | 125 | 0.025 |
| 4th week | 43 | 0.51 ± 0.27 b | 37 | 3.02 ± 0.64 b | 3.772 | 78 | 0 |
| Period | Female Stage | Germination (%) | Male Stage | Germination (%) | Comparison between Stages | ||
| N | Mean ± SE | N | Mean ± SE | t | d.f. | p | |
| 1st week | 72 | 2.33 ± 0.81 a | 70 | 5.2 ± 0.86 a | 2.43 | 140 | 0.016 |
| 2nd week | 46 | 0.73 ± 0.28 b | 50 | 1.04 ± 0.52 b | 3.14 | 94 | 0.02 |
| 3rd week | 82 | 0.96 ± 0.38 ab | 45 | 1.02 ± 0.20 b | 0.111 | 125 | 0.911 |
| 4th week | 43 | 0 b | 37 | 1.56 ± 0.38 b | 4.39 | 78 | 0.006 |
| Pollen | Germinated | Adhered | % Germination | Tubes 1/4 Style | Tubes 1/2 Style |
|---|---|---|---|---|---|
| Germinated | 1.00 | 0.886 ** | 0.593 ** | 0.788 ** | 0.708 ** |
| Adhered | 0.886 ** | 1.00 | 0.437 ** | 0.739 ** | 0.660 ** |
| % Germination | 0.593 ** | 0.437 ** | 1.00 | 0.643 ** | 0.646 ** |
| Tubes 1/4 Style | 0.788 ** | 0.739 ** | 0.643 ** | 1.00 | 0.907 ** |
| Tubes 1/2 Style | 0.708 ** | 0.660 ** | 0.646 ** | 0.907 ** | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcaraz, M.L.; Hormaza, J.I. Fruit Set in Avocado: Pollen Limitation, Pollen Load Size, and Selective Fruit Abortion. Agronomy 2021, 11, 1603. https://doi.org/10.3390/agronomy11081603
Alcaraz ML, Hormaza JI. Fruit Set in Avocado: Pollen Limitation, Pollen Load Size, and Selective Fruit Abortion. Agronomy. 2021; 11(8):1603. https://doi.org/10.3390/agronomy11081603
Chicago/Turabian StyleAlcaraz, María L., and José I. Hormaza. 2021. "Fruit Set in Avocado: Pollen Limitation, Pollen Load Size, and Selective Fruit Abortion" Agronomy 11, no. 8: 1603. https://doi.org/10.3390/agronomy11081603
APA StyleAlcaraz, M. L., & Hormaza, J. I. (2021). Fruit Set in Avocado: Pollen Limitation, Pollen Load Size, and Selective Fruit Abortion. Agronomy, 11(8), 1603. https://doi.org/10.3390/agronomy11081603






