Pre-Symptomatic Disease Detection in the Vine, Chrysanthemum, and Rose Leaves with a Low-Cost Infrared Sensor
Abstract
:1. Introduction
2. Materials and Methods
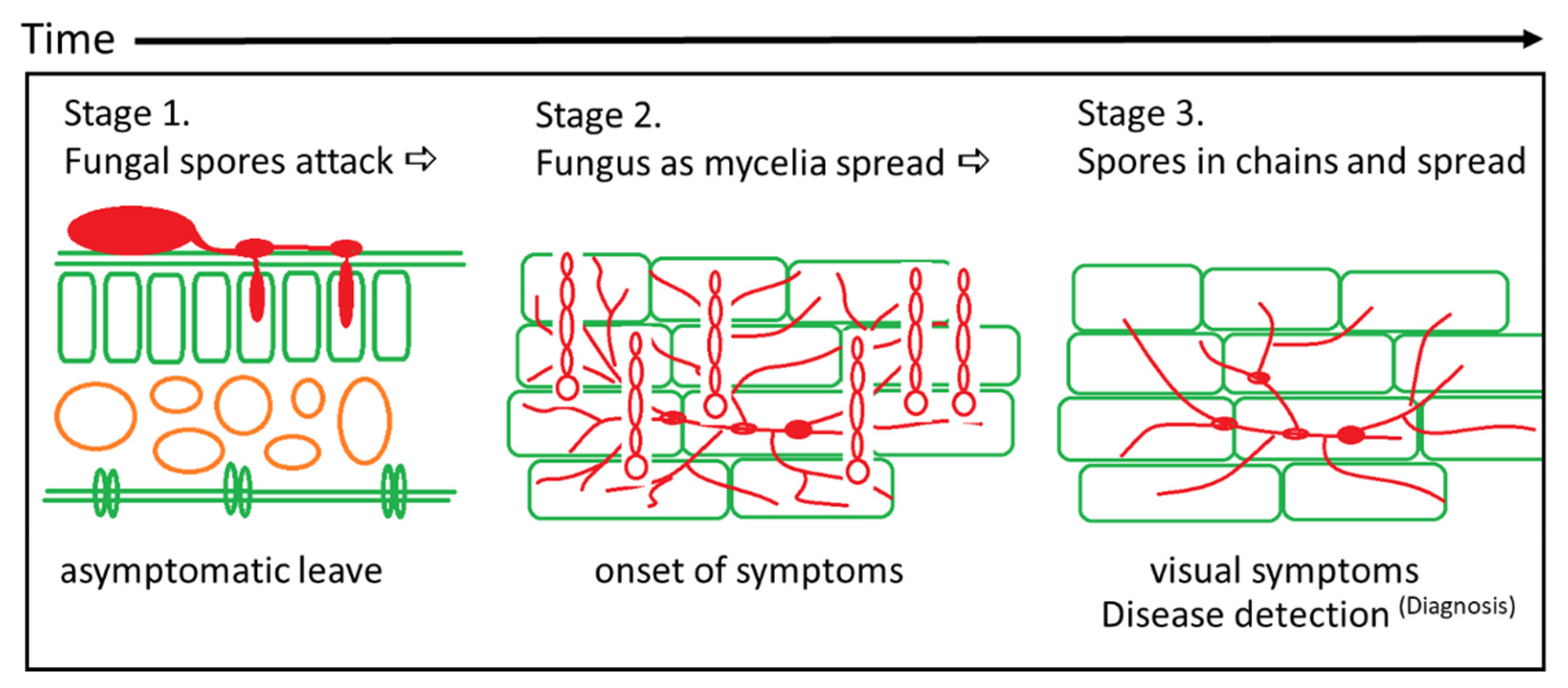
2.1. Early Disease Detection—The Methodology
2.2. Development of Low-Cost Thermal Imaging System as a Screening Instrument
2.3. Sensor Calibration and Software Development
2.4. Fungal Mycelium TRGB Imaging In Vitro
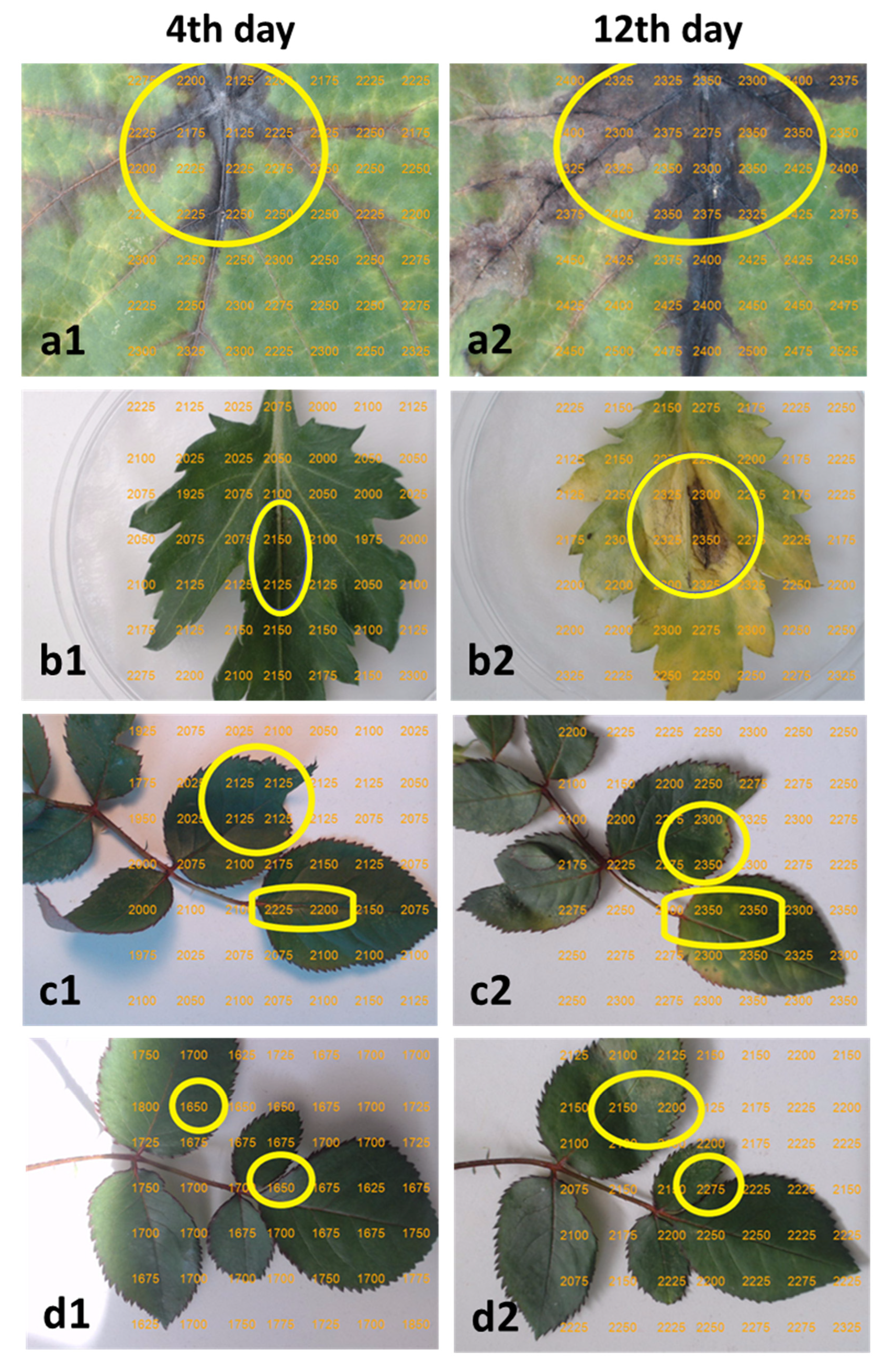
2.5. TRGB Imaging of Infected Leaves
2.6. Statistical Analysis
3. Results and Discussion
3.1. Fungal Mycelium TRGB Imaging In Vitro
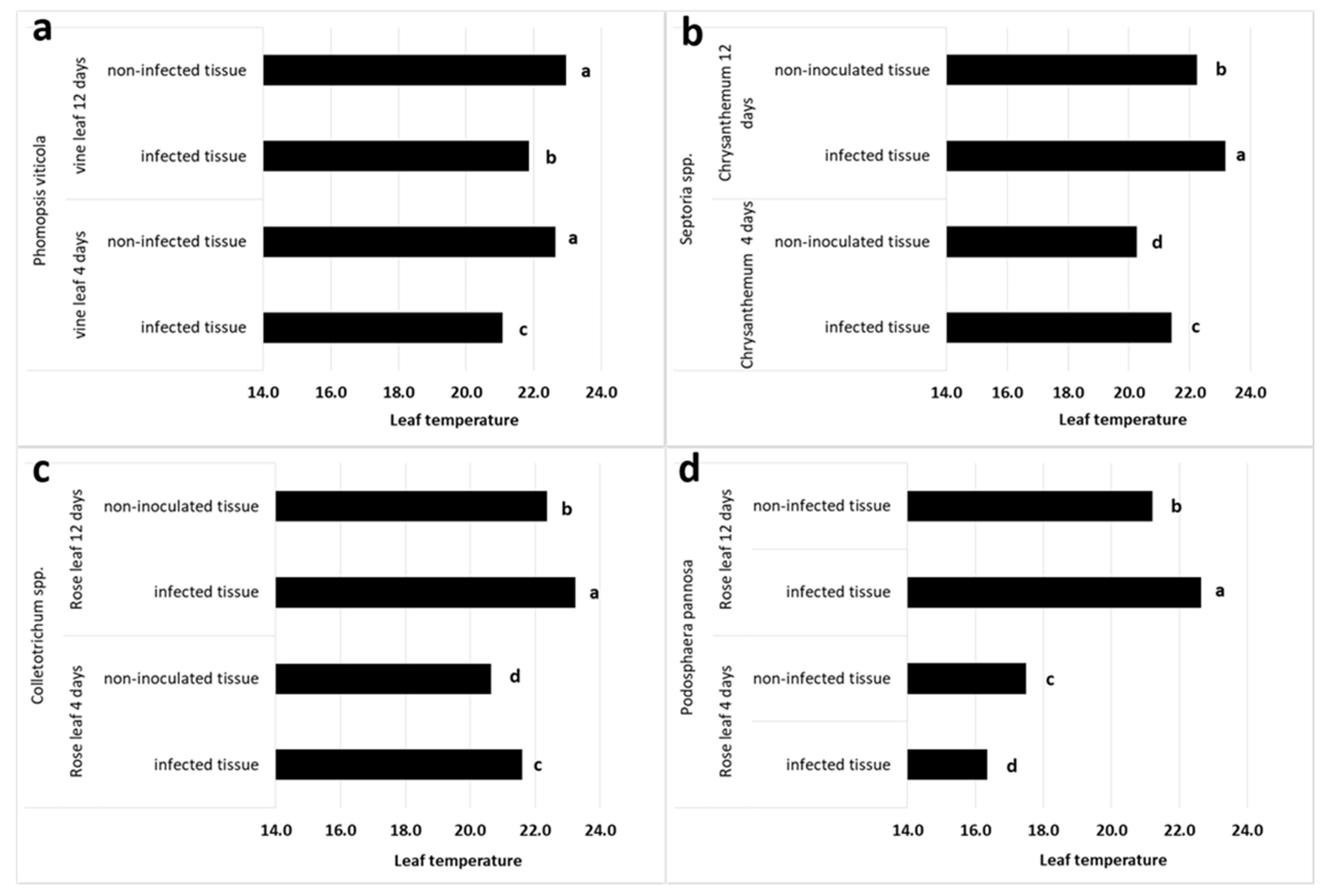
3.2. TRGB Imaging of Infected Leaves
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chaerle, L.; Van der Straeten, D. Imaging techniques and the early detection of plant stress. Trends Plant Sci. 2000, 5, 495–501. [Google Scholar] [CrossRef]
- Bauriegel, E.; Giebel, A.; Geyer, M.; Schmidt, U.; Herppich, W.B. Early detection of Fusarium infection in wheat using hyper-spectral imaging. Comput. Electron. Agric. 2011, 75, 304–312. [Google Scholar] [CrossRef]
- Lowe, A.; Harrison, N.; French, A.P. Hyperspectral image analysis techniques for the detection and classification of the early onset of plant disease and stress. Plant Methods 2017, 13, 80. [Google Scholar] [CrossRef]
- Takamatsu, S. Origin and evolution of the powdery mildews (Ascomycota 1660, Erysiphales). Mycoscience 2013, 54, 75–86. [Google Scholar] [CrossRef]
- Coyier, D.L. Control of Rose Powdery Mildew in the Greenhouse and Field. Plant Dis. 1983, 67, 919. [Google Scholar] [CrossRef]
- Linde, M.; Shishkoff, N. DISEASE | Powdery Mildew. Encycl. Rose Sci. 2003, 158–165. [Google Scholar] [CrossRef]
- Marchetti, M.A.; Melching, J.S.; Bromfield, K.R. The effects of temperature and dew period on germination and infection by urediospores of Phakopsora pachyrhizi. Phytopathology 1976, 66, 461–463. [Google Scholar] [CrossRef]
- Tomerlin, J.R.; Jones, A.L. Effect of temperature and relative humidity on the latent period of Venturia inaequalis in apple leaves. Phytopathology 1983, 73, 51–54. [Google Scholar] [CrossRef]
- Fraser, C.; Riley, S.; Anderson, R.M.; Ferguson, N.M. Factors that make an infectious disease outbreak controllable. Proc. Natl. Acad. Sci. USA 2004, 101, 6146–6151. [Google Scholar] [CrossRef] [Green Version]
- Roger, C.; Tivoli, B.; Huber, L. Effects of temperature and moisture on disease and fruit body development of Mycosphaerella pinodes on pea (Pisum sativum). Plant Pathol. 1999, 48, 1–9. [Google Scholar] [CrossRef]
- Bock, C.H.; Poole, G.H.; Parker, P.E.; Gottwald, T.R. Plant disease severity estimated visually, by digital photography and image analysis, and by hyperspectral imaging. Crit. Rev. Plant Sci. 2010, 29, 59–107. [Google Scholar] [CrossRef]
- Hansen, M.A.; Wick, R.L. Plant disease diagnosis: Present and future prospects. Adv. Plant Pathol. 1993, 10, 65–126. [Google Scholar]
- Nutter, F.W., Jr. Disease assessment terms and concepts. In Encyclopedia of Plant Pathology; Maloy, O.C., Murray, T.D., Eds.; John Wiley and Sons, Inc.: New York, NY, USA, 2001; pp. 312–323. [Google Scholar]
- Xu, H.; Zhu, S.; Ying, Y.; Jiang, H. Early detection of plant disease using infrared thermal imaging. Opt. Nat. Resour. Agric. Foods 2006, 6381, 638110. [Google Scholar] [CrossRef]
- Mahlein, A.-K. Plant Disease Detection by Imaging Sensors—Parallels and Specific Demands for Precision Agriculture and Plant Phenotyping. Plant Dis. 2016, 100, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Mahlein, A.-K.; Oerke, E.-C.; Steiner, U.; Dehne, H.-W. Recent advances in sensing plant diseases for precision crop protection. Eur. J. Plant Pathol. 2012, 133, 197–209. [Google Scholar] [CrossRef]
- Yao, Z.; Lei, Y.; He, D. Early Visual Detection of Wheat Stripe Rust Using Visible/Near-Infrared Hyperspectral Imaging. Sensors 2019, 19, 952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagasubramanian, K.; Jones, S.; Singh, A.K.; Sarkar, S.; Singh, A.; Ganapathysubramanian, B. Plant disease identification using explainable 3D deep learning on hyperspectral images. Plant Methods 2019, 15, 98. [Google Scholar] [CrossRef] [PubMed]
- Kwan, C. Methods and Challenges Using Multispectral and Hyperspectral Images for Practical Change Detection Applications. Information 2019, 10, 353. [Google Scholar] [CrossRef] [Green Version]
- Abildso, C.G.; Haas, V.; Daily, S.M.; Bias, T.K. Field Test of a Passive Infrared Camera for Measuring Trail-Based Physical Activity. Front. Public Health 2021, 9, 225. [Google Scholar] [CrossRef]
- Jones, H.G. Use of thermography for quantitative studies of spatial and temporal variation of stomatal conductance over leaf surfaces. Plant Cell Environ. 1999, 22, 1043–1055. [Google Scholar] [CrossRef] [Green Version]
- Jones, H.G. Application of thermal imaging and infrared sensing in plant physiology and ecophysiology. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2004; Volume 41, pp. 107–163. [Google Scholar]
- Belfiore, N.; Vinti, R.; Lovat, L.; Chitarra, W.; Tomasi, D.; de Bei, R.; Meggio, F.; Gaiotti, F. Infrared Thermography to Estimate Vine Water Status: Optimizing Canopy Measurements and Thermal Indices for the Varieties Merlot and Moscato in Northern Italy. Agronomy 2019, 9, 821. [Google Scholar] [CrossRef] [Green Version]
- Oerke, E.C.; Steiner, U.; Dehne, H.W.; Lindenthal, M. Thermal imaging of cucumber leaves affected by downy mildew and environmental conditions. J. Exp. Bot. 2006, 57, 2121–2132. [Google Scholar] [CrossRef] [PubMed]
- Mendgen, K.; Hahn, M.; Deising, H. Morphogenesis and mechanisms of penetration by plant pathogenic fungi. Annu. Rev. Phytopathol. 1996, 34, 364–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fradin, E.F.; Thomma, B.P.H.J. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006, 7, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, Y.; Sun, G.; Liu, X.; Zhai, L.; Shen, Q.; Guo, S. Water balance altered in cucumber plants infected with Fusarium oxysporum f. sp. cucumerinum. Sci. Rep. 2015, 5, 7722. [Google Scholar] [CrossRef] [Green Version]
- Chaerle, L.; Hagenbeek, D.; De Bruyne, E.; Valcke, R.; Van Der Straeten, D. Thermal and Chlorophyll-Fluorescence Imaging Distinguish Plant-Pathogen Interactions at an Early Stage. Plant Cell Physiol. 2004, 45, 887–896. [Google Scholar] [CrossRef] [Green Version]
- Chaerle, L.; Leinonen, I.; Jones, H.G.; Van Der Straeten, D. Monitoring and screening plant populations with combined thermal and chlorophyll fluorescence imaging. J. Exp. Bot. 2006, 58, 773–784. [Google Scholar] [CrossRef] [Green Version]
- Cordero, R.J.; Mattoon, E.R.; Casadevall, A. Fungi are colder than their surroundings. BioRxiv 2020. [Google Scholar] [CrossRef]
- Lee, H.J.; Tucker, E.B.; Crain, R.C.; Lee, Y. Stomatal opening is induced in epidermal peels of Commelina communis L. by GTP analogs or pertussis toxin. Plant Physiol. 1993, 102, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Pineda, M.; Barón, M.; Pérez-Bueno, M.L. Thermal Imaging for Plant Stress Detection and Phenotyping. Remote. Sens. 2020, 13, 68. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; Huang, D. A review of imaging techniques for plant phenotyping. Sensors 2014, 14, 20078–20111. [Google Scholar] [CrossRef]
- Chaerle, L.; Van der Straeten, D. Seeing is believing: Imaging techniques to monitor plant health. Biochim. Biophys. Acta 2001, 1519, 153–166. [Google Scholar] [CrossRef]
- Prashar, A.; Yildiz, J.; McNicol, J.W.; Bryan, G.J.; Jones, H.G. Infra-red thermography for high throughput field phenotyping in Solanum tuberosum. PLoS ONE 2013, 8, e65816. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.; Fulton, J.; Shearer, S. An overview of current and potential applications of thermal remote sensing in precision agriculture. Comput. Electron. Agric. 2017, 139, 22–32. [Google Scholar] [CrossRef]
- Haripriya, A.B.; Sunitha, K.A.; Mahima, B. Development of Low-cost Thermal Imaging System as a Preliminary Screening Instrument. Procedia Comput. Sci. 2020, 172, 283–288. [Google Scholar] [CrossRef]
- Moghaddam, S.; Lawler, J.; Currano, J.; Kim, J. Novel Method for Measurement of Total Hemispherical Emissivity. J. Heat Transf. 2007, 21, 128–133. [Google Scholar] [CrossRef] [Green Version]
- Suthaparan, A.; Stensvand, A.; Solhaug, K.A.; Torre, S.; Mortensen, L.M.; Gadoury, D.M.; Seem, R.C.; Gislerød, H.R. Suppression of Powdery Mildew (Podosphaera pannosa) in Greenhouse Roses by Brief Exposure to Supplemental UV-B radiation. Plant Dis. 2012, 96, 1653–1660. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Chen, H.; Ciechanowska, I.; Spaner, D. Application of infrared thermal imaging for the rapid diagnosis of crop disease. IFAC-PapersOnLine 2018, 51, 424–430. [Google Scholar] [CrossRef]
- Lee, J.H.; Oh, S.I.; Lee, A.K. Measurement and prediction of Cladosporium cucumerinum and Collectotrichum orbiculare using infrared thermography. In Proceedings of the Quantitative InfraRed Thermography Asia 2017, Daejeon, Korea, 2–6 July 2017. [Google Scholar] [CrossRef]
- Lindenthal, M.; Steiner, U.; Dehne, H.-W.; Oerke, E.-C. Effect of Downy Mildew Development on Transpiration of Cucumber Leaves Visualized by Digital Infrared Thermography. Phytopathology 2005, 95, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.C.G.; Heritage, A.D.; Stapper, M.; Barrs, H.D. Effect of stripe rust (Puccinia striiformis West.) and irrigation on the yield and foliage temperature of wheat. Field Crop. Res. 1986, 14, 39–51. [Google Scholar] [CrossRef]
- Rosyara, R.U.; Subedi, S.; Duveiller, E.; Sharma, R.C. The effect of spot blotch and heat stress on variation of canopy temperature depression, chlorophyll fluorescence and chlorophyll content of hexaploid wheat genotypes. Euphytica 2010, 174, 377–390. [Google Scholar] [CrossRef]
- Jones, H.G. Plant and Microclimate, 2nd ed.; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Raza, S.E.A.; Prince, G.; Clarkson, J.P.; Rajpoot, N.M. Automatic Detection of Diseased Tomato Plants Using Thermal and Stereo Visible Light Images. PLoS ONE 2015, 10, e012. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zia-Khan, S.; Owusu-Adu, S.; Miedaner, T.; Müller, J. Early Detection of Zymoseptoria tritici in Winter Wheat by Infrared Thermography. Agriculture 2019, 9, 139. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Xu, H.; Yi, H.; Yang, L.; Kong, Z.; Zhang, L.; Xue, S.; Jia, H.; Ma, Z. Resistance to hemi-biotrophic F. graminearum infection is associated with coordinated and ordered expression of diverse defense signaling pathways. PLoS ONE 2011, 6, e19008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaman, F.; Lee, S.; Rahim, M.K.A.; Khan, S. Smart Antennas and Intelligent Sensors Based Systems: Enabling Technologies and Applications. Wirel. Commun. Mob. Comput. 2019, 2019, 1–3. [Google Scholar] [CrossRef] [Green Version]


| 4 Days | 12 Days | ||||
|---|---|---|---|---|---|
| Tested Plant | Foliar Pathogen | MTD (°C) | MTTD (°C) | MTD (°C) | MTTD (°C) |
| Vine | P. viticola | −0.5 | −1.6 ± 0.4 | −1.0 | −1.1 ± 0.4 |
| Chrysanthemum | Septoria ssp. | −0.6 | 1.1 ± 0.6 | 1.1 | 0.9 ± 0.6 |
| Rose | Colletotrichum spp. | 0.8 | 1.0 ± 0.4 | 0.5 | 0.9 ± 0.3 |
| Rose | P. pannosa | −1.5 | −1.1 ± 0.4 | 2.0 | 1.4 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vagelas, I.; Papadimos, A.; Lykas, C. Pre-Symptomatic Disease Detection in the Vine, Chrysanthemum, and Rose Leaves with a Low-Cost Infrared Sensor. Agronomy 2021, 11, 1682. https://doi.org/10.3390/agronomy11091682
Vagelas I, Papadimos A, Lykas C. Pre-Symptomatic Disease Detection in the Vine, Chrysanthemum, and Rose Leaves with a Low-Cost Infrared Sensor. Agronomy. 2021; 11(9):1682. https://doi.org/10.3390/agronomy11091682
Chicago/Turabian StyleVagelas, Ioannis, Athanasios Papadimos, and Christos Lykas. 2021. "Pre-Symptomatic Disease Detection in the Vine, Chrysanthemum, and Rose Leaves with a Low-Cost Infrared Sensor" Agronomy 11, no. 9: 1682. https://doi.org/10.3390/agronomy11091682
APA StyleVagelas, I., Papadimos, A., & Lykas, C. (2021). Pre-Symptomatic Disease Detection in the Vine, Chrysanthemum, and Rose Leaves with a Low-Cost Infrared Sensor. Agronomy, 11(9), 1682. https://doi.org/10.3390/agronomy11091682







