Influence of Recycled Waste Compost on Soil Food Webs, Nutrient Cycling and Tree Growth in a Young Almond Orchard
Abstract
:1. Introduction
2. Materials and Methods
2.1. Orchard Establishment and Experimental Design
2.2. Soil Sampling and Plant Measurements
2.3. Nematode Communities
2.4. Phylogenetic and Taxonomic Analysis of Prokaryotic Communities
2.5. Statistics
3. Results
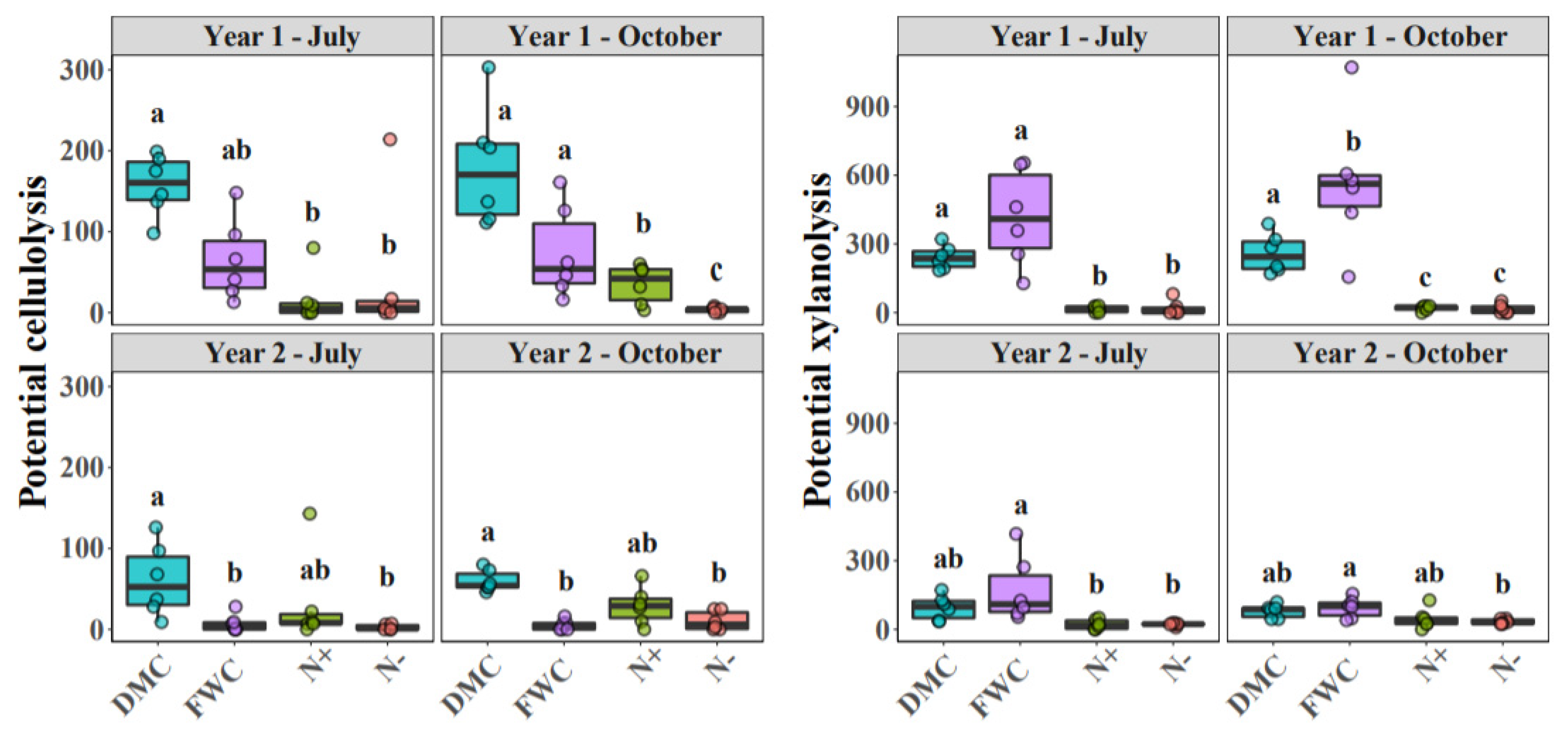
3.1. Soil Variables
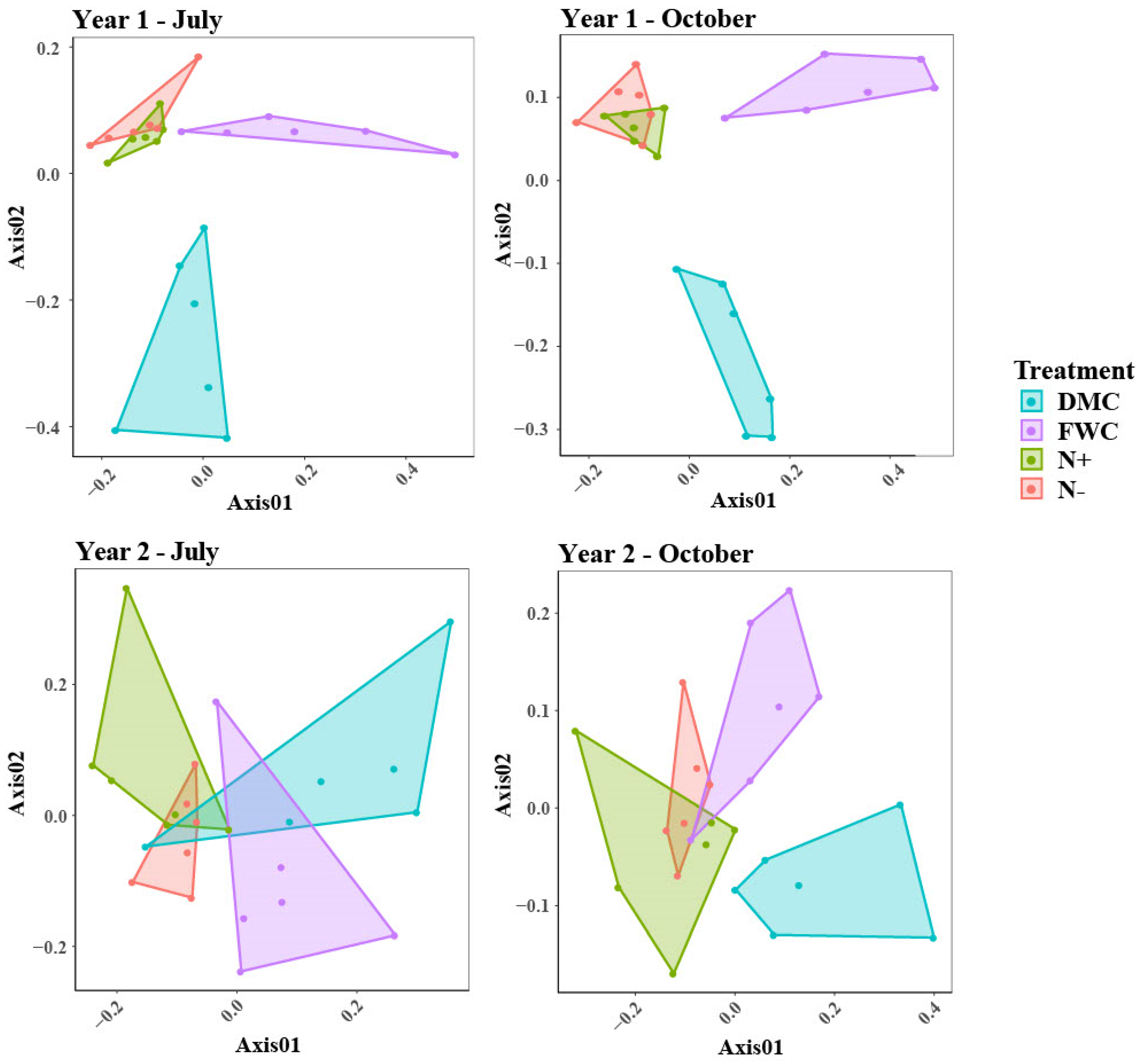
3.2. Bacterial and Archaeal Communities
3.3. Nematode Communities
3.4. Relationships between Microbes and Nematodes
3.5. Plant Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stott, D.E. Recommended Soil Health Indicators and Associated Laboratory Procedures; Soil Health Technical Note No. 450-03; United States Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2019. [Google Scholar]
- Van Bruggen, A.; Semenov, A. In search of biological indicators for soil health and disease suppression. Appl. Soil Ecol. 2000, 15, 13–24. [Google Scholar] [CrossRef]
- Pankhurst, C.; Doube, B.; Gupta, V. Biological Indicators of Soil Health; CAB Iternational: Wallingford, UK, 1997; Volume 17. [Google Scholar]
- Lal, R. Soil health and carbon management. Food Energy Secur. 2016, 5, 212–222. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef] [PubMed]
- Scotti, R.; Pane, C.; Spaccini, R.; Palese, A.M.; Piccolo, A.; Celano, G.; Zaccardelli, M. On-farm compost: A useful tool to improve soil quality under intensive farming systems. Appl. Soil Ecol. 2016, 107, 13–23. [Google Scholar] [CrossRef]
- Duong, T.T.T.; Penfold, C.; Marschner, P. Amending soils of different texture with six compost types: Impact on soil nutrient availability, plant growth and nutrient uptake. Plant Soil 2012, 354, 197–209. [Google Scholar] [CrossRef]
- Evanylo, G.; Sherony, C.; Spargo, J.; Starner, D.; Brosius, M.; Haering, K. Soil and water environmental effects of fertilizer-, manure-, and compost-based fertility practices in an organic vegetable cropping system. Agric. Ecosyst. Environ. 2008, 127, 50–58. [Google Scholar] [CrossRef]
- Owen, J.J.; Silver, W.L. Greenhouse gas emissions from dairy manure management: A review of field-based studies. Glob. Change Biol. 2015, 21, 550–565. [Google Scholar] [CrossRef] [Green Version]
- Levis, J.W.; Barlaz, M.A.; Themelis, N.J.; Ulloa, P. Assessment of the state of food waste treatment in the United States and Canada. Waste Manag. 2010, 30, 1486–1494. [Google Scholar] [CrossRef]
- Adhikari, B.; Barrington, S.; Martinez, J. Urban food waste generation: Challenges and opportunities. Int. J. Environ. Waste Manag. Indersci. 2011, 3, 4–21. [Google Scholar] [CrossRef] [Green Version]
- Butler, T.J.; Han, K.J.; Muir, J.P.; Weindorf, D.C.; Lastly, L. Dairy manure compost effects on corn silage production and soil properties. Agron. J. 2008, 100, 1541–1545. [Google Scholar] [CrossRef]
- Butler, T.J.; Muir, J.P. Dairy manure compost improves soil and increases tall wheatgrass yield. Agron. J. 2006, 98, 1090–1096. [Google Scholar] [CrossRef] [Green Version]
- Lepsch, H.C.; Brown, P.H.; Peterson, C.A.; Gaudin, A.C.M.; Khalsa, S.D.S. Impact of organic matter amendments on soil and tree water status in a California orchard. Agric. Water Manag. 2019, 222, 204–212. [Google Scholar] [CrossRef]
- Khalsa, S.D.S.; Brown, P.H. Grower analysis of organic matter amendments in California orchards. J. Environ. Qual. 2017, 46, 649–658. [Google Scholar] [CrossRef]
- Lee, J.J.; Park, R.D.; Kim, Y.W.; Shim, J.H.; Chae, D.H.; Rim, Y.S.; Sohn, B.K.; Kim, T.H.; Kim, K.Y. Effect of food waste compost on microbial population, soil enzyme activity and lettuce growth. Bioresour. Technol. 2004, 93, 21–28. [Google Scholar] [CrossRef]
- Bernard, E.; Larkin, R.P.; Tavantzis, S.; Erich, M.S.; Alyokhin, A.; Sewell, G.; Lannan, A.; Gross, S.D. Compost, rapeseed rotation, and biocontrol agents significantly impact soil microbial communities in organic and conventional potato production systems. Appl. Soil Ecol. 2012, 52, 29–41. [Google Scholar] [CrossRef]
- Zhen, Z.; Liu, H.; Wang, N.; Guo, L.; Meng, J.; Ding, N.; Wu, G.; Jiang, G. Effects of manure compost application on soil microbial community diversity and soil microenvironments in a temperate cropland in China. PLoS ONE 2014, 9, e108555. [Google Scholar] [CrossRef]
- Wagg, C.; Bender, S.F.; Widmer, F.; van der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Blanco, J.; Lazcano, C.; Christensen, T.H.; Muñoz, P.; Rieradevall, J.; Møller, J.; Antón, A.; Boldrin, A. Compost benefits for agriculture evaluated by life cycle assessment. A review. Agron. Sustain. Dev. 2013, 33, 721–732. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Guo, Y.; Wang, X.; Chen, C.; Hu, Y.; Cheng, L.; Gu, S.; Xu, X. Temporal variations of soil microbial community under compost addition in black soil of Northeast China. Appl. Soil Ecol. 2017, 121, 214–222. [Google Scholar] [CrossRef]
- O’Donnell, A.G.; Colvan, S.R. Biological Diversity and Function in Soils; Choice Reviews Online; Bardgett, R.D., Usher, M.B., Hopkins, D.W., Eds.; Cambridge University Press: Cambridge, UK, 2005; Volume 43, pp. 43–46. ISBN 0521609879. [Google Scholar]
- Van der Heijden, M.G.; Wagg, C. Soil microbial diversity and agro-ecosystem functioning. Plant Soil 2013, 363, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Saccá, M.L.; Caracciolo, A.B.; Di Lenola, M.; Grenni, P. Soil biological communities and ecosystem resilience. In Soil Biological Communities and Ecosystem Resilience; Springer: Cham, Switzerland, 2017; pp. 9–24. [Google Scholar] [CrossRef]
- Marx, V. Microbiology: The return of culture. Nat. Methods 2016, 14, 37–40. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Schielzeth, H.; Barnes, A.D.; Barry, K.E.; Bonn, A.; Brose, U.; Bruelheide, H.; Buchmann, N.; Buscot, F.; Ebeling, A.; et al. A multitrophic perspective on biodiversity-ecosystem functioning research. Adv. Ecol. Res. 2019, 61, 1–54. [Google Scholar] [CrossRef]
- Ruess, L.; Ferris, H. Decomposition pathways and successional changes. Nematol. Monogr. Perspect. 2004, 2, 547–556. [Google Scholar]
- Ferris, H. Form and function: Metabolic footprints of nematodes in the soil food web. Eur. J. Soil Biol. 2010, 46, 97–104. [Google Scholar] [CrossRef]
- Steel, H.; de la Peña, E.; Fonderie, P.; Willekens, K.; Borgonie, G.; Bert, W. Nematode succession during composting and the potential of the nematode community as an indicator of compost maturity. Pedobiologia 2010, 53, 181–190. [Google Scholar] [CrossRef]
- Ferris, H.; Matute, M.M. Structural and functional succession in the nematode fauna of a soil food web. Appl. Soil Ecol. 2003, 23, 93–110. [Google Scholar] [CrossRef]
- DuPont, S.T.; Culman, S.W.; Ferris, H.; Buckley, D.H.; Glover, J.D. No-tillage conversion of harvested perennial grassland to annual cropland reduces root biomass, decreases active carbon stocks, and impacts soil biota. Agric. Ecosyst. Environ. 2010, 137, 25–32. [Google Scholar] [CrossRef]
- Niles, R.K.; Wall Freckman, D. From the ground up: Nematode ecology in bioassessment and ecosystem health. Plant Nematode Interact. 2015, 65–85. [Google Scholar] [CrossRef]
- Kapp, C.; Storey, S.G.; Malan, A.P. Options for soil health measurement in vineyards and deciduous fruit orchards, with special reference to nematodes. S. Afr. J. Enol. Vitic. 2013, 34, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Bulluck, L.R.; Barker, K.R.; Ristaino, J.B. Influences of organic and synthetic soil fertility amendments on nematode trophic groups and community dynamics under tomatoes. Appl. Soil Ecol. 2002, 21, 233–250. [Google Scholar] [CrossRef]
- Hu, C.; Qi, Y. Effect of compost and chemical fertilizer on soil nematode community in a Chinese maize field. Eur. J. Soil Biol. 2010, 46, 230–236. [Google Scholar] [CrossRef]
- Herren, G.L.; Habraken, J.; Waeyenberge, L.; Haegeman, A.; Viaene, N.; Cougnon, M.; Reheul, D.; Steel, H.; Bert, W. Effects of synthetic fertilizer and farm compost on soil nematode community in long-term crop rotation plots: A morphological and metabarcoding approach. PLoS ONE 2020, 15, e0230153. [Google Scholar] [CrossRef] [PubMed]
- Abbott, L.K.; Manning, D.A.C. Soil health and related ecosystem services in organic agriculture. Sustain. Agric. Res. 2015, 4, 116. [Google Scholar] [CrossRef]
- Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture. Web Soil Survey. Available online: https://casoilresource.lawr.ucdavis.edu/gmap (accessed on 21 February 2019).
- Dufour, R.; Brown, S.; Troxell, D. Nutrient Management Plan (590) for Organic Systems Western State Implementation Guide; United States Department, North Carolina Agricultural and Technical State University: Greensboro, NC, USA, 2014; pp. 72–89. [Google Scholar] [CrossRef]
- Meyer, R.D. Nitrogen on drip irrigated almonds. In Years of Discovery. A Compendium of Production and Environmental Research Projects 1972–2003; Almond Board of California: Modesto, CA, USA, 2004; pp. 284–285. [Google Scholar]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Eshel, G.; Levy, G.J.; Mingelgrin, U.; Singer, M.J. Critical evaluation of the use of laser diffraction for particle-size distribution analysis. Soil Sci. Soc. Am. J. 2004, 68, 736–743. [Google Scholar] [CrossRef]
- Culman, S.W.; Snapp, S.S.; Freeman, M.A.; Schipanski, M.E.; Beniston, J.; Lal, R.; Drinkwater, L.E.; Franzluebbers, A.J.; Glover, J.D.; Grandy, A.S.; et al. Permanganate oxidizable carbon reflects a processed soil fraction that is sensitive to management. Soil Sci. Soc. Am. J. 2012, 76, 494. [Google Scholar] [CrossRef] [Green Version]
- Barker, K.R. Nematode extraction and bioassays. In An Advanced Treatise on Meloidogyne. Volume II: Methodology; Barker, K.R., Carter, C.C., Sasser, J.N., Eds.; Department of Plant Pathology, North Carolina State University: Raleigh, NC, USA, 1985; pp. 19–35. [Google Scholar]
- Bongers, T.; Ferris, H. Nematode community structure as a bioindicator for environmental monitoring. Trends Ecol. Evol. 1999, 14, 224–228. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T.; De Goede, R.G.M. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Sieriebriennikov, B.; Ferris, H.; de Goede, R.G.M. NINJA: An automated calculation system for nematode-based biological monitoring. Eur. J. Soil Biol. 2014, 61, 90–93. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsons, L.S.; Sayre, J.; Ender, C.; Rodrigues, J.L.M.; Barberán, A. Soil microbial communities in restored and unrestored coastal dune ecosystems in California. Restor. Ecol. 2020, 28, S311–S321. [Google Scholar] [CrossRef] [Green Version]
- Callahan, B.J.; McMurdie, P.J.; Holmes, S.P. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 2017, 11, 2639–2643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Science: Malden, MA, USA, 2004. [Google Scholar]
- Louca, S.; Wegener Parfrey, L.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting linear mixed-effects models using lme4. arXiv 2015, arXiv:1406.5823v1. [Google Scholar] [CrossRef]
- Oksanen, A.J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Minchin, P.R.; Hara, R.B.O.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. 2019. R Package Version 2.2-0. Available online: http://CRAN.Rproject.org/package=vegan (accessed on 21 August 2019).
- Wickham, H. Ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Mathews, C.R.; Bottrell, D.G.; Brown, M.W. A comparison of conventional and alternative understory management practices for apple production: Multi-trophic effects. Appl. Soil Ecol. 2002, 21, 221–231. [Google Scholar] [CrossRef]
- Forge, T.; Neilsen, G.; Neilsen, D.; Hogue, E.; Faubion, D. Composted dairy manure and alfalfa hay mulch affect soil ecology and early production of “Braeburn” apple on M.9 Rootstock. HortScience 2013, 48, 645–651. [Google Scholar] [CrossRef] [Green Version]
- Sharaf, H.; Thompson, A.A.; Williams, M.A.; Peck, G.M. Compost applications increase bacterial community diversity in the apple rhizosphere. Soil Sci. Soc. Am. J. 2021, 85, 1–17. [Google Scholar] [CrossRef]
- Lazcano, C.; Gómez-Brandón, M.; Revilla, P.; Domínguez, J. Short-term effects of organic and inorganic fertilizers on soil microbial community structure and function: A field study with sweet corn. Biol. Fertil. Soils 2013, 49, 723–733. [Google Scholar] [CrossRef]
- Wittebolle, L.; Marzorati, M.; Clement, L.; Balloi, A.; Daffonchio, D.; Heylen, K.; De Vos, P.; Verstraete, W.; Boon, N. Initial community evenness favours functionality under selective stress. Nature 2009, 458, 623–626. [Google Scholar] [CrossRef]
- Lian, T.; Jin, J.; Wang, G.; Tang, C.; Yu, Z.; Li, Y.; Liu, J.; Zhang, S.; Liu, X. The fate of soybean residue-carbon links to changes of bacterial community composition in Mollisols differing in soil organic carbon. Soil Biol. Biochem. 2017, 109, 50–58. [Google Scholar] [CrossRef]
- Fahrbach, M.; Kuever, J.; Remesch, M.; Huber, B.E.; Kämpfer, P.; Dott, W.; Hollender, J. Steroidobacter denitrificans gen. nov., sp. nov., a steroidal hormone-degrading gammaproteobacterium. Int. J. Syst. Evol. Microbiol. 2008, 58, 2215–2223. [Google Scholar] [CrossRef] [Green Version]
- Martínez, S.A.; Dussán, J. Lysinibacillus sphaericus plant growth promoter bacteria and lead phytoremediation enhancer with Canavalia ensiformis. Environ. Prog. Sustain. Energy 2018, 37, 276–282. [Google Scholar] [CrossRef]
- Aguirre-Monroy, A.M.; Santana-Martínez, J.C.; Dussán, J. Lysinibacillus sphaericus as a nutrient enhancer during fire-impacted soil replantation. Appl. Environ. Soil Sci. 2019, 2019, 3075153. [Google Scholar] [CrossRef] [Green Version]
- Naureen, Z.; Ur Rehman, N.; Hussain, H.; Hussain, J.; Gilani, S.A.; Al Housni, S.K.; Mabood, F.; Khan, A.L.; Farooq, S.; Abbas, G.; et al. Exploring the potentials of Lysinibacillus sphaericus ZA9 for plant growth promotion and biocontrol activities against phytopathogenic fungi. Front. Microbiol. 2017, 8, 1477. [Google Scholar] [CrossRef]
- Lazicki, P.; Geisseler, D.; Lloyd, M. Nitrogen mineralization from organic amendments is variable but predictable. J. Environ. Qual. 2020, 49, 483–495. [Google Scholar] [CrossRef]
- Horrocks, A.; Curtin, D.; Tregurtha, C.; Meenken, E. Municipal compost as a nutrient source for organic crop production in New Zealand. Agronomy 2016, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Milkereit, J.; Geisseler, D.; Lazicki, P.; Settles, M.L.; Durbin-Johnson, B.P.; Hodson, A. Interactions between nitrogen availability, bacterial communities, and nematode indicators of soil food web function in response to organic amendments. Appl. Soil Ecol. 2021, 157, 103767. [Google Scholar] [CrossRef]
- Ito, T.; Araki, M.; Komatsuzaki, M.; Kaneko, N.; Ohta, H. Soil nematode community structure affected by tillage systems and cover crop managements in organic soybean production. Appl. Soil Ecol. 2015, 86, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Treonis, A.M.; Austin, E.E.; Buyer, J.S.; Maul, J.E.; Spicer, L.; Zasada, I.A. Effects of organic amendment and tillage on soil microorganisms and microfauna. Appl. Soil Ecol. 2010, 46, 103–110. [Google Scholar] [CrossRef]
- Liu, T.; Yu, L.; Xu, J.; Yan, X.; Li, H.; Whalen, J.K.; Hu, F. Bacterial traits and quality contribute to the diet choice and survival of bacterial-feeding nematodes. Soil Biol. Biochem. 2017, 115, 467–474. [Google Scholar] [CrossRef]
- Venette, R.C.; Ferris, H. Influence of bacterial type and density on population growth of bacterial-feeding nematodes. Soil Biol. Biochem. 1998, 30, 949–960. [Google Scholar] [CrossRef]
- Djigal, D.; Brauman, A.; Diop, T.A.; Chotte, J.L.; Villenave, C. Influence of bacterial-feeding nematodes (Cephalobidae) on soil microbial communities during maize growth. Soil Biol. Biochem. 2004, 36, 323–331. [Google Scholar] [CrossRef]
- Xiao, H.F.; Li, G.; Li, D.M.; Hu, F.; Li, H.X. Effect of different bacterial-feeding nematode species on soil bacterial numbers, activity, and community composition. Pedosphere 2014, 24, 116–124. [Google Scholar] [CrossRef]
- Gebremikael, M.T.; Steel, H.; Buchan, D.; Bert, W.; De Neve, S. Nematodes enhance plant growth and nutrient uptake under C and N-rich conditions. Sci. Rep. 2016, 6, 32862. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, B.S. Microbial-feeding nematodes and protozoa in soil: Their effectson microbial activity and nitrogen mineralization in decomposition hotspots and the rhizosphere. Plant Soil 1994, 164, 25–33. [Google Scholar] [CrossRef]
- Wang, S.; Li, T.; Zheng, Z. Response of soil aggregate-associated microbial and nematode communities to tea plantation age. Catena 2018, 171, 475–484. [Google Scholar] [CrossRef]
- Blanc, C.; Sy, M.; Djigal, D.; Brauman, A.; Normand, P.; Villenave, C. Nutrition on bacteria by bacterial-feeding nematodes and consequences on the structure of soil bacterial community. Eur. J. Soil Biol. 2006, 42, 70–78. [Google Scholar] [CrossRef]

| NH4+ N (µg/g) | NO3− N (µg/g) | %N | %C | POXC (mg/kg) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| May 2016 | ||||||||||||||||||||
| DMC | 6.49 | ± | 1.93 | abd | 40.52 | ± | 3.85 | 0.12 | ± | 0.01 | 1.25 | ± | 0.2 | |||||||
| FWC | 10.45 | ± | 1.51 | d | 39 | ± | 4.41 | 0.14 | ± | 0.03 | 1.57 | ± | 0.4 | |||||||
| N+ | 1.31 | ± | 0.36 | ac | 37.19 | ± | 6.07 | 0.16 | ± | 0.02 | 1.69 | ± | 0.27 | |||||||
| N− | 1.79 | ± | 0.63 | a | 37.22 | ± | 10.5 | 0.11 | ± | 0.01 | 1.1 | ± | 0.12 | |||||||
| July 2016 | ||||||||||||||||||||
| DMC | 2.86 | ± | 0.39 | ab | 15.28 | ± | 3.15 | ab | 0.17 | ± | 0.02 | a | 1.64 | ± | 0.17 | a | ||||
| FWC | 2.97 | ± | 0.53 | ab | 4.87 | ± | 2.38 | a | 0.18 | ± | 0.03 | a | 2.08 | ± | 0.45 | a | ||||
| N+ | 8.04 | ± | 2.5 | b | 22.19 | ± | 1.34 | b | 0.1 | ± | 0 | b | 0.94 | ± | 0.02 | b | ||||
| N− | 2.09 | ± | 0.25 | a | 6.45 | ± | 2.45 | a | 0.1 | ± | 0.01 | b | 0.99 | ± | 0.05 | b | ||||
| October 2016 | ||||||||||||||||||||
| DMC | 2.69 | ± | 0.39 | b | 39.38 | ± | 11.87 | a | 0.19 | ± | 0.02 | a | 1.7 | ± | 0.17 | a | 700.63 | ± | 43.96 | b |
| FWC | 1.52 | ± | 0.64 | ac | 24.93 | ± | 6.34 | ac | 0.21 | ± | 0.03 | a | 2.17 | ± | 0.33 | a | 855.34 | ± | 104.51 | b |
| N+ | 0.58 | ± | 0.06 | a | 5.28 | ± | 0.73 | b | 0.11 | ± | 0.01 | b | 1.05 | ± | 0.1 | b | 263.45 | ± | 33.42 | a |
| N− | 0.73 | ± | 0.23 | a | 12.55 | ± | 5.59 | bc | 0.1 | ± | 0 | b | 0.97 | ± | 0.03 | b | 291.77 | ± | 33.15 | a |
| May 2017 | ||||||||||||||||||||
| DMC | 0.48 | ± | 0.23 | 3.98 | ± | 1.06 | 0.17 | ± | 0.03 | a | 1.56 | ± | 0.25 | ac | ||||||
| FWC | 0.75 | ± | 0.26 | 3.55 | ± | 1.48 | 0.35 | ± | 0.07 | a | 3.9 | ± | 0.8 | a | ||||||
| N+ | 0.26 | ± | 0.12 | 1.88 | ± | 0.54 | 0.1 | ± | 0 | b | 0.96 | ± | 0.03 | bc | ||||||
| N− | 0.2 | ± | 0.1 | 2.68 | ± | 0.41 | 0.1 | ± | 0 | b | 0.9 | ± | 0.02 | bc | ||||||
| July 2017 | ||||||||||||||||||||
| DMC | 1.18 | ± | 0.54 | 3.38 | ± | 1.78 | a | 0.18 | ± | 0.02 | a | 1.72 | ± | 0.22 | a | |||||
| FWC | 0.46 | ± | 0.4 | 4.71 | ± | 2.76 | a | 0.17 | ± | 0.03 | a | 1.82 | ± | 0.33 | a | |||||
| N+ | 12.23 | ± | 7.94 | 43.4 | ± | 12.77 | b | 0.11 | ± | 0 | b | 0.96 | ± | 0.04 | b | |||||
| N− | 0.26 | ± | 0.17 | 3 | ± | 3.44 | a | 0.1 | ± | 0 | b | 0.93 | ± | 0.03 | b | |||||
| October 2017 | ||||||||||||||||||||
| DMC | 0.76 | ± | 0.32 | 4.74 | ± | 2.35 | 0.15 | ± | 0 | a | 1.32 | ± | 0.05 | a | 551.01 | ± | 43.71 | b | ||
| FWC | 0.49 | ± | 0.14 | 3 | ± | 1.25 | 0.14 | ± | 0.01 | a | 1.35 | ± | 0.11 | a | 600.92 | ± | 61.12 | b | ||
| N+ | 1.03 | ± | 0.7 | 2.92 | ± | 0.97 | 0.11 | ± | 0.01 | b | 0.99 | ± | 0.08 | b | 377.45 | ± | 17.88 | a | ||
| N− | 0.27 | ± | 0.24 | 2.81 | ± | 0.64 | 0.1 | ± | 0 | b | 0.9 | ± | 0.02 | b | 281.56 | ± | 45.21 | a |
| Richness | Evenness | Shannon Diversity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| July 2016 | |||||||||
| DMC | 1071.00 | ± | 64.64 | 0.87 | ± | 0.01 | 6.07 | ± | 0.08 |
| FWC | 1067.33 | ± | 25.81 | 0.88 | ± | 0.00 | 6.14 | ± | 0.03 |
| N+ | 998.00 | ± | 82.25 | 0.87 | ± | 0.01 | 5.96 | ± | 0.11 |
| N− | 1011.00 | ± | 68.66 | 0.87 | ± | 0.00 | 5.99 | ± | 0.05 |
| October 2016 | |||||||||
| DMC | 944.67 | ± | 38.31 | 0.88 | ± | 0.00 a | 6.06 | ± | 0.05 |
| FWC | 881.33 | ± | 61.41 | 0.89 | ± | 0.00 a | 5.99 | ± | 0.07 |
| N+ | 1009.33 | ± | 25.92 | 0.87 | ± | 0.00 b | 6.01 | ± | 0.03 |
| N− | 1044.00 | ± | 52.56 | 0.87 | ± | 0.00 b | 6.02 | ± | 0.06 |
| July 2017 | |||||||||
| DMC | 781.33 | ± | 65.28 | 0.89 | ± | 0.01 a | 5.94 | ± | 0.08 a |
| FWC | 789.67 | ± | 55.18 | 0.90 | ± | 0.00 a | 5.97 | ± | 0.05 a |
| N+ | 659.67 | ± | 55.71 | 0.87 | ± | 0.00 b | 5.65 | ± | 0.08 b |
| N− | 779.00 | ± | 45.29 | 0.87 | ± | 0.00 b | 5.79 | ± | 0.05 ab |
| October 2017 | |||||||||
| DMC | 798.17 | ± | 38.12 | 0.89 | ± | 0.00 a | 5.94 | ± | 0.04 |
| FWC | 716.00 | ± | 68.84 | 0.88 | ± | 0.00 ab | 5.77 | ± | 0.10 |
| N+ | 739.00 | ± | 66.23 | 0.87 | ± | 0.00 b | 5.71 | ± | 0.09 |
| N− | 772.50 | ± | 53.37 | 0.86 | ± | 0.01 b | 5.72 | ± | 0.08 |
| May 2016 | |||||
| Nematode taxa | DMC | FWC | N+ | N− | |
| Panagrolaimus | bact. | 0.40 | 0.48 | 0.36 | 0.39 |
| Mesorhabditis | bact. | 0.12 | 0.09 | 0.05 | 0.08 |
| Cephalobus | bact. | 0.06 ab | 0.10 b | 0.01 c | 0.02 a |
| Acrobeloides | bact. | 0.07 | 0.00 | 0.08 | 0.07 |
| Acrobeles | bact. | 0.00 | 0.00 | 0.00 | 0.00 |
| Prismatolaimus | bact. | 0.01 | 0.00 | 0.02 | 0.00 |
| Aphelenchoides | fung. | 0.12 | 0.15 | 0.16 | 0.17 |
| Aphelenchus | fung. | 0.09 | 0.06 | 0.12 | 0.09 |
| Discolaimus | pred. | 0.00 | 0.00 | 0.00 | 0.00 |
| Qudsianematidae | omn. | 0.01 | 0.00 | 0.04 | 0.02 |
| Mesodorylaimus | omn. | 0.00 | 0.00 | 0.00 | 0.00 |
| Tylenchidae | herb. | 0.11 | 0.09 | 0.14 | 0.15 |
| Pratylenchus | herb. | 0.02 | 0.01 | 0.02 | 0.00 |
| Total bacterial feeders | 0.65 | 0.68 | 0.52 | 0.57 | |
| Total fungal feeders | 0.21 | 0.22 | 0.28 | 0.26 | |
| Total herbivores | 0.13 | 0.10 | 0.16 | 0.15 | |
| Total omnivores | 0.01 | 0.00 | 0.04 | 0.02 | |
| July 2016 | |||||
| Nematode taxa | DMC | FWC | N+ | N− | |
| Panagrolaimus | bact. | 0.09 | 0.14 | 0.12 | 0.15 |
| Mesorhabditis | bact. | 0.22 | 0.19 | 0.18 | 0.23 |
| Acrobeloides | bact. | 0.10 | 0.10 | 0.11 | 0.10 |
| Acrobeles | bact. | 0.01 | 0.01 | 0.00 | 0.00 |
| Prismatolaimus | bact. | 0.00 | 0.00 | 0.00 | 0.00 |
| Aphelenchoides | fung. | 0.22 | 0.19 | 0.18 | 0.18 |
| Aphelenchus | fung. | 0.12 | 0.11 | 0.10 | 0.14 |
| Discolaimus | pred. | 0.00 | 0.00 | 0.00 | 0.00 |
| Qudsianematidae | omn. | 0.01 | 0.01 | 0.02 | 0.02 |
| Mesodorylaimus | omn. | 0.01 | 0.00 | 0.00 | 0.00 |
| Tylenchidae | herb. | 0.19 | 0.22 | 0.22 | 0.17 |
| Pratylenchus | herb. | 0.03 ab | 0.03 ab | 0.07 b | 0.00 a |
| Total bacterial feeders | 0.48 | 0.43 | 0.40 | 0.43 | |
| Total fungal feeders | 0.28 | 0.30 | 0.29 | 0.37 | |
| Total herbivores | 0.22 | 0.25 | 0.29 | 0.17 | |
| Total omnivores | 0.02 | 0.01 | 0.02 | 0.02 | |
| October 2016 | |||||
| Nematode tax | DMC | FWC | N+ | N− | |
| Panagrolaimus | bact. | 0.10 | 0.11 | 0.14 | 0.11 |
| Mesorhabditis | bact. | 0.11 | 0.09 | 0.11 | 0.09 |
| Acrobeloides | bact. | 0.07 | 0.09 | 0.08 | 0.08 |
| Acrobeles | bact. | 0.09 | 0.02 | 0.00 | 0.00 |
| Prismatolaimus | bact. | 0.05 a | 0.04 ab | 0.01 b | 0.04 ab |
| Aphelenchoides | fung. | 0.10 | 0.12 | 0.10 | 0.13 |
| Aphelenchus | fung. | 0.07 | 0.07 | 0.10 | 0.07 |
| Discolaimus | pred. | 0.00 | 0.00 | 0.01 | 0.00 |
| Qudsianematidae | omn. | 0.00 | 0.00 | 0.00 | 0.00 |
| Mesodorylaimus | omn. | 0.03 | 0.02 | 0.03 | 0.04 |
| Tylenchidae | herb. | 0.37 | 0.44 | 0.40 | 0.42 |
| Pratylenchus | herb. | 0.01 | 0.01 | 0.03 | 0.02 |
| Total bacterial feeders | 0.41 | 0.34 | 0.34 | 0.32 | |
| Total fungal feeders | 0.17 | 0.19 | 0.20 | 0.19 | |
| Total herbivores | 0.38 | 0.45 | 0.43 | 0.44 | |
| Total omnivores | 0.03 | 0.02 | 0.03 | 0.04 | |
| May 2017 | |||||
| Nematode taxa | DMC | FWC | N+ | N− | |
| Panagrolaimus | bact. | 0.01 | 0.00 | 0.01 | 0.01 |
| Mesorhabditis | bact. | 0.01 | 0.02 | 0.02 | 0.01 |
| Cephalobus | bact. | 0.00 | 0.00 | 0.00 | 0.01 |
| Eucephalobus | bact. | 0.00 | 0.00 | 0.00 | 0 |
| Acrobeloides | bact. | 0.22 | 0.16 | 0.11 | 0.2 |
| Acrobeles | bact. | 0.00 | 0.03 | 0.00 | 0 |
| Prismatolaimus | bact. | 0.23 | 0.18 | 0.14 | 0.16 |
| Aphelenchoides | fung. | 0.12 | 0.12 | 0.09 | 0.11 |
| Aphelenchus | fung. | 0.07 b | 0.07 b | 0.20 a | 0.2 a |
| Discolaimus | pred. | 0.00 | 0.00 | 0.01 | 0.00 |
| Qudsianematidae | omn. | 0.03 | 0.05 | 0.11 | 0.08 |
| Dorylaimus | omn. | 0.00 | 0.01 | 0.01 | 0.01 |
| Tylenchidae | herb. | 0.29 | 0.33 | 0.28 | 0.2 |
| Meloidogyne | herb. | 0.01 | 0.03 | 0.01 | 0.00 |
| Total bacterial feeders | 0.48 a | 0.38 ab | 0.28 b | 0.39 ab | |
| Total fungal feeders | 0.19 b | 0.19 b | 0.30 a | 0.31 a | |
| Total herbivores | 0.16 ab | 0.19 b | 0.15 ab | 0.1 a | |
| Total omnivores | 0.03 | 0.06 | 0.12 | 0.09 | |
| July 2017 | |||||
| Nematode taxa | DMC | FWC | N+ | N− | |
| Panagrolaimus | bact. | 0.02 | 0.01 | 0.06 | 0.06 |
| Mesorhabditis | bact. | 0.01 | 0.01 | 0.01 | 0.01 |
| Eucephalobus | bact. | 0.01 | 0.00 | 0.02 | 0.01 |
| Acrobeloides | bact. | 0.25 | 0.13 | 0.19 | 0.17 |
| Prismatolaimus | bact. | 0.09 | 0.13 | 0.03 | 0.10 |
| Aphelenchoides | bact. | 0.01 | 0.01 | 0.03 | 0.03 |
| Aphelenchus | fung. | 0.13 a | 0.16 ab | 0.30 b | 0.29 ab |
| Qudsianematidae | omn. | 0.01 | 0.02 | 0.01 | 0.02 |
| Dorylaimus | omn. | 0.01 | 0.02 | 0.01 | 0.03 |
| Tylenchidae | herb. | 0.46 ab | 0.49 b | 0.33 ab | 0.27 a |
| Total bacterial feeders | 0.38 | 0.29 | 0.31 | 0.34 | |
| Total fungal feeders | 0.13 b | 0.18 ab | 0.33 c | 0.32 a | |
| Total herbivores | 0.23 ab | 0.25 b | 0.17 ab | 0.14 a | |
| Total omnivores | 0.02 | 0.04 | 0.03 | 0.05 | |
| October 2017 | |||||
| Nematode taxa | DMC | FWC | N+ | N− | |
| Panagrolaimus | bact. | 0.14 | 0.15 | 0.09 | 0.13 |
| Mesorhabditis | bact. | 0.09 | 0.10 | 0.11 | 0.06 |
| Cephalobus | bact. | 0.17 | 0.14 | 0.18 | 0.15 |
| Prismatolaimus | bact. | 0.08 | 0.11 | 0.06 | 0.15 |
| Aphelenchoides | fung. | 0.16 | 0.15 | 0.15 | 0.22 |
| Aphelenchus | fung. | 0.00 | 0.01 | 0.01 | 0.01 |
| Microdorylaimus | omn. | 0.01 | 0.01 | 0.02 | 0.00 |
| Tylenchidae | herb. | 0.34 | 0.33 | 0.31 | 0.25 |
| Paratylenchus | herb. | 0.00 | 0.00 | 0.00 | 0.01 |
| Tylenchorhynchus | herb. | 0.00 | 0.00 | 0.01 | 0.01 |
| Pratylenchus | herb. | 0.00 | 0.01 | 0.05 | 0.01 |
| Total bacterial feeders | 0.48 | 0.49 | 0.44 | 0.49 | |
| Total fungal feeders | 0.16 | 0.16 | 0.16 | 0.23 | |
| Total herbivores | 0.17 | 0.18 | 0.22 | 0.15 | |
| Total omnivores | 0.01 | 0.01 | 0.02 | 0.00 | |
| % N | % C | Trunk Diameter (mm) | Diameter Increase (mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| May 2016 | ||||||||||||
| DMC | 3.14 | ± | 0.09 | 44.16 | ± | 0.20 | 9.40 | ± | 0.46 | |||
| FWC | 3.11 | ± | 0.15 | 43.85 | ± | 0.61 | 9.70 | ± | 0.22 | |||
| N+ | 2.97 | ± | 0.17 | 44.29 | ± | 0.13 | 10.22 | ± | 0.23 | |||
| N− | 3.09 | ± | 0.03 | 44.02 | ± | 0.14 | 10.16 | ± | 0.26 | |||
| July 2016 | ||||||||||||
| DMC | 2.92 | ± | 0.15 | 43.85 | ± | 0.23 | ||||||
| FWC | 3.08 | ± | 0.21 | 43.80 | ± | 0.17 | ||||||
| N+ | 3.07 | ± | 0.13 | 43.97 | ± | 0.18 | ||||||
| N− | 2.82 | ± | 0.13 | 43.62 | ± | 0.17 | ||||||
| October 2016 | ||||||||||||
| DMC | 3.22 | ± | 0.06 ab | 45.22 | ± | 0.47 | 23.69 | ± | 0.41 | 14.30 | ± | 0.57 ab |
| FWC | 3.24 | ± | 0.14 ab | 44.98 | ± | 0.38 | 25.34 | ± | 1.19 | 15.64 | ± | 1.15 a |
| N+ | 3.46 | ± | 0.19 a | 45.11 | ± | 0.21 | 22.61 | ± | 0.68 | 12.39 | ± | 0.70 ab |
| N− | 2.86 | ± | 0.10 b | 44.14 | ± | 0.16 | 21.48 | ± | 1.05 | 11.32 | ± | 0.88 b |
| May 2017 | ||||||||||||
| DMC | 2.66 | ± | 0.09 | 46.07 | ± | 0.17 | ||||||
| FWC | 2.51 | ± | 0.08 | 45.54 | ± | 0.27 | ||||||
| N+ | 2.47 | ± | 0.05 | 45.23 | ± | 0.48 | ||||||
| N− | 2.58 | ± | 0.08 | 46.00 | ± | 0.36 | ||||||
| July 2017 | ||||||||||||
| DMC | 2.33 | ± | 0.07 b | 45.96 | ± | 0.26 | ||||||
| FWC | 2.19 | ± | 0.05 b | 45.76 | ± | 0.42 | ||||||
| N+ | 2.84 | ± | 0.06 a | 45.41 | ± | 0.27 | ||||||
| N− | 2.11 | ± | 0.08 b | 45.28 | ± | 0.27 | ||||||
| October 2017 | ||||||||||||
| DMC | 47.05 | ± | 2.10 | 37.65 | ± | 1.98 | ||||||
| FWC | 48.56 | ± | 2.22 | 38.86 | ± | 2.29 | ||||||
| N+ | 48.27 | ± | 3.48 | 38.05 | ± | 3.53 | ||||||
| N− | 42.68 | ± | 2.01 | 32.52 | ± | 2.02 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodson, A.K.; Sayre, J.M.; Lyra, M.C.C.P.; Rodrigues, J.L.M. Influence of Recycled Waste Compost on Soil Food Webs, Nutrient Cycling and Tree Growth in a Young Almond Orchard. Agronomy 2021, 11, 1745. https://doi.org/10.3390/agronomy11091745
Hodson AK, Sayre JM, Lyra MCCP, Rodrigues JLM. Influence of Recycled Waste Compost on Soil Food Webs, Nutrient Cycling and Tree Growth in a Young Almond Orchard. Agronomy. 2021; 11(9):1745. https://doi.org/10.3390/agronomy11091745
Chicago/Turabian StyleHodson, Amanda K., Jordan M. Sayre, Maria C. C. P. Lyra, and Jorge L. Mazza Rodrigues. 2021. "Influence of Recycled Waste Compost on Soil Food Webs, Nutrient Cycling and Tree Growth in a Young Almond Orchard" Agronomy 11, no. 9: 1745. https://doi.org/10.3390/agronomy11091745
APA StyleHodson, A. K., Sayre, J. M., Lyra, M. C. C. P., & Rodrigues, J. L. M. (2021). Influence of Recycled Waste Compost on Soil Food Webs, Nutrient Cycling and Tree Growth in a Young Almond Orchard. Agronomy, 11(9), 1745. https://doi.org/10.3390/agronomy11091745






