Potential of the Biomass of Plants Grown in Trace Element-Contaminated Soils under Mediterranean Climatic Conditions for Bioenergy Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Sampling and Chemical Analyses
2.2. Anaerobic Degradation
2.3. Statistical Analysis
3. Results and Discussion
3.1. Plant Composition and Calorific Value
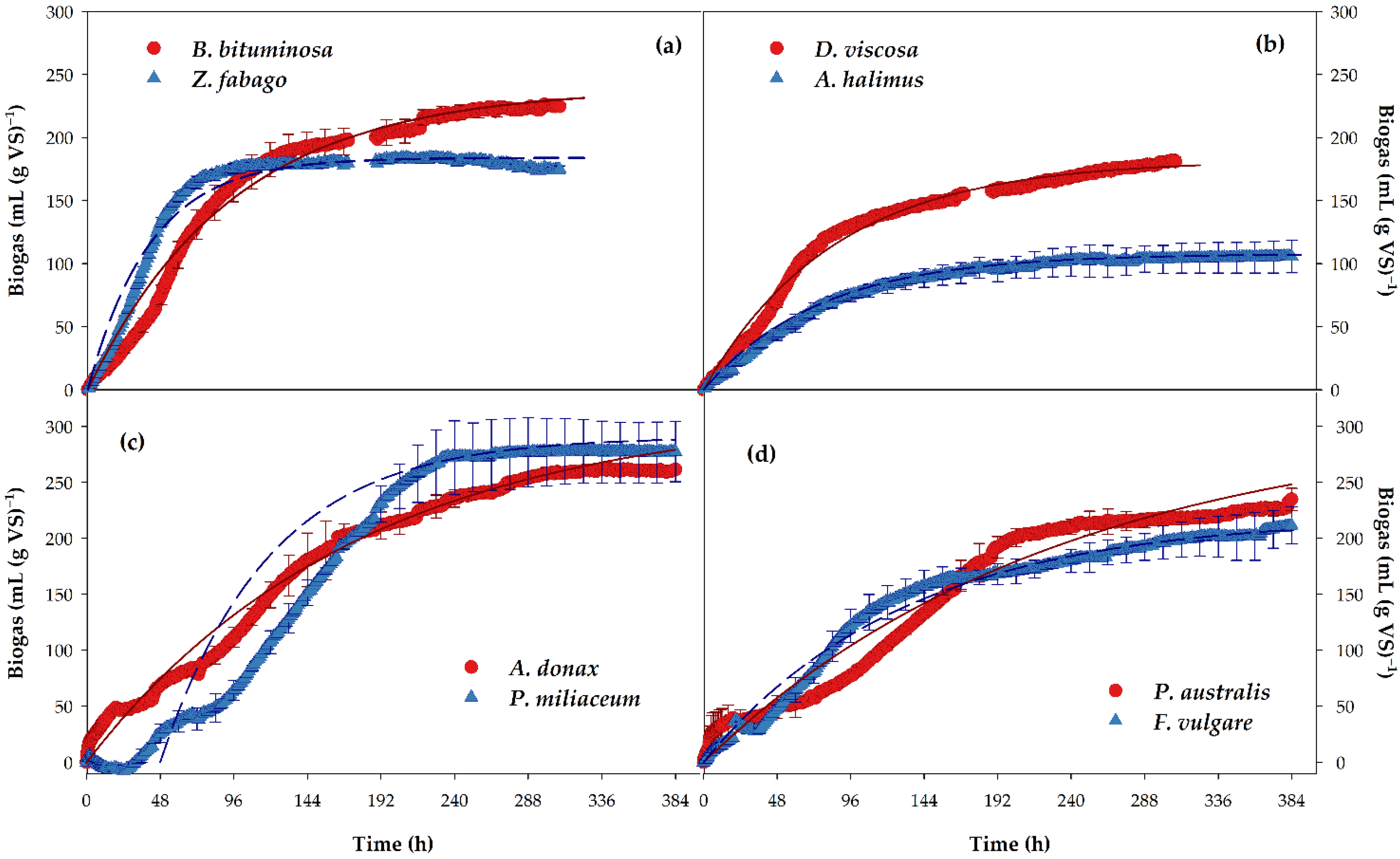
3.2. Anaerobic Digestion
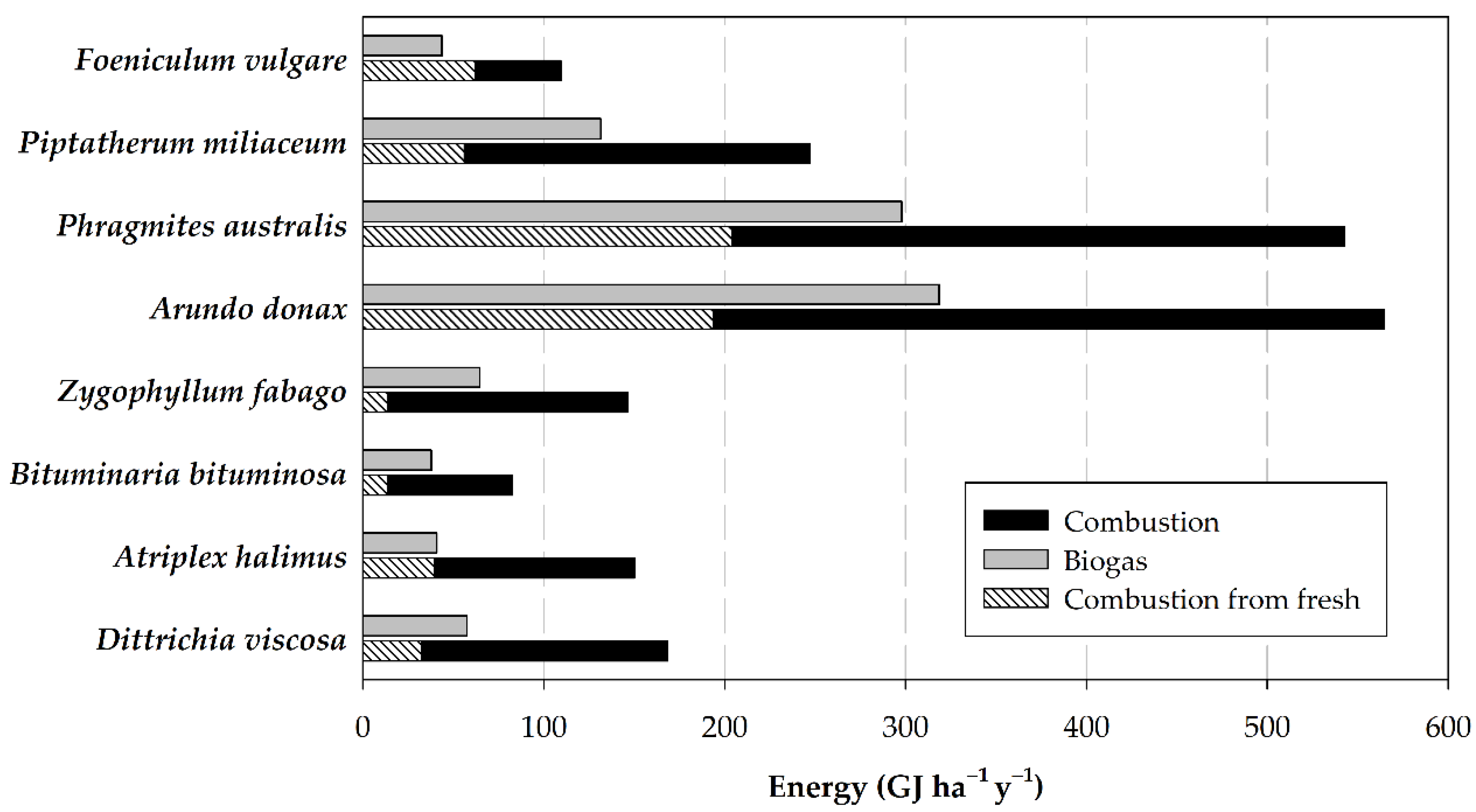
3.3. Potential Energy Yield
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez-Eugenio, N.; McLaughlin, M.; Pennock, D. Soil Pollution: A Hidden Reality; FAO: Rome, Italy, 2018. [Google Scholar]
- Conesa, H.M.; Schulin, R. The Cartagena–La Unión mining district (SE spain): A review of environmental problems and emerging phytoremediation solutions after fifteen years research. J. Environ. Monit. 2010, 12, 1225–1233. [Google Scholar] [CrossRef]
- Conesa Alcaraz, H.M.; Faz Cano, A. Metal uptake by naturally occurring vegetation in a metal polluted salt marsh in southeast Spain. In Land Degradation and Rehabilitation; Faz-Cano, A., Mermut, A.R., Arocena, J.M., Ortiz, R., Eds.; Catena Verlag: Stuttgart, Germany, 2009; Volume 40, pp. 287–294. [Google Scholar]
- Mendez, M.O.; Maier, R.M. Phytostabilization of Mine Tailings in Arid and Semiarid Environments—An Emerging Remediation Technology. Environ. Health Perspect. 2008, 116, 278–283. [Google Scholar] [CrossRef] [Green Version]
- Conesa, H.M.; Schulin, R.; Nowack, B. Mining landscape: A cultural tourist opportunity or an environmental problem: The study case of the Cartagena–La Unión Mining District (SE Spain). Ecol. Econ. 2008, 64, 690–700. [Google Scholar] [CrossRef]
- Pardo, T.; Clemente, R.; Epelde, L.; Garbisu, C.; Bernal, M.P. Evaluation of the phytostabilisation efficiency in a trace elements contaminated soil using soil health indicators. J. Hazard. Mater. 2014, 268, 68–76. [Google Scholar] [CrossRef]
- Gomes, H.I. Phytoremediation for bioenergy: Challenges and opportunities. Environ. Technol. Rev. 2012, 1, 59–66. [Google Scholar] [CrossRef]
- Robinson, B.; Bañuelos, G.; Conesa, H.M.; Evangelou, M.W.H.; Schulin, R. The Phytomanagement of Trace Elements in Soil. Crit. Rev. Plant Sci. 2009, 28, 240–266. [Google Scholar] [CrossRef]
- Pandey, V.C.; Bajpai, O.; Singh, N. Energy crops in sustainable phytoremediation. Renew. Sustain. Energy Rev. 2016, 54, 58–73. [Google Scholar] [CrossRef]
- Cundy, A.; Bardos, R.; Puschenreiter, M.; Mench, M.; Bert, V.; Friesl-Hanl, W.; Müller, I.; Li, X.; Weyens, N.; Witters, N.; et al. Brownfields to green fields: Realising wider benefits from practical contaminant phytomanagement strategies. J. Environ. Manag. 2016, 184, 67–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Rowe, R.; Street, N.; Taylor, G. Identifying potential environmental impacts of large-scale deployment of dedicated bioenergy crops in the UK. Renew. Sustain. Energy Rev. 2009, 13, 271–290. [Google Scholar] [CrossRef]
- Naik, S.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Grippi, D.; Clemente, R.; Bernal, M.P. Chemical and Bioenergetic Characterization of Biofuels from Plant Biomass: Perspectives for Southern Europe. Appl. Sci. 2020, 10, 3571. [Google Scholar] [CrossRef]
- Saxena, R.; Adhikari, D.; Goyal, H. Biomass-based energy fuel through biochemical routes: A review. Renew. Sustain. Energy Rev. 2009, 13, 167–178. [Google Scholar] [CrossRef]
- Feliu Jofre, A.; Flotats Ripoll, X. Renewable Gases. An Emerging Energy Vector. Naturgy Foundation. 2020. Available online: http://www.fundacionnaturgy.org (accessed on 15 June 2021).
- Thijs, S.; Witters, N.; Janssen, J.; Ruttens, A.; Weyens, N.; Herzig, R.; Mench, M.; Van Slycken, S.; Meers, E.; Meiresonne, L.; et al. Tobacco, Sunflower and High Biomass SRC Clones Show Potential for Trace Metal Phytoextraction on a Moderately Contaminated Field Site in Belgium. Front. Plant Sci. 2018, 9, 1879. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, M.T.; Madejón, P.; Madejón, E.; Diaz, M.J. Novel energy crops for Mediterranean contaminated lands: Valorization of Dittrichia viscosa and Silybum marianum biomass by pyrolysis. Chemosphere 2017, 186, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, M.T.; Montiel-Rozas, M.M.; Madejón, P.; Diaz, M.J.; Madejon, E. The potential of native species as bioenergy crops on trace-element contaminated Mediterranean lands. Sci. Total Environ. 2017, 590–591, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Bernal, M.P.; Gómez, X.; Chang, R.; Arco-Lázaro, E.; Clemente, R. Strategies for the use of plant biomass obtained in the phytostabilisation of trace-element-contaminated soils. Biomass Bioenergy 2019, 126, 220–230. [Google Scholar] [CrossRef]
- Hunce, S.Y.; Clemente, R.; Bernal, M.P. Energy production potential of phytoremediation plant biomass: Helianthus annuus and Silybum marianum. Ind. Crop. Prod. 2019, 135, 206–216. [Google Scholar] [CrossRef]
- Álvarez-Robles, M.J.; Bernal, M.P.; Clemente, R. Differential response of Oryza sativa L. and Phragmites australis L. plants in trace elements contaminated soils under flooded and unflooded conditions. Environ. Geochem. Health 2021, 1–17. [Google Scholar] [CrossRef]
- EPA. Method 1684. Total, Fixed and Volatile Solids in Water, Solids, and Biosolids; US Environmental Protection Agency: Washington, DC, USA, 2001. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi/P1002CZ8.PDF?Dockey=P1002CZ8.PDF (accessed on 27 August 2021).
- ANSI and ASTM D 1106. Standard Test Method for Lignin Wood; American National Standard Institute: Washington, DC, USA, 1977.
- ANSI and ASTM D 1103-60. Standard Test Method for Alpha-Cellulose in Wood; American National Standard Institute: Washington DC, USA, 1977.
- Hedge, J.E.; Hofreiter, B.T. Estimation of carbohydrate. In Carbohydrate Chemistry; Whistler, R.L., BeMiller, J.N., Eds.; Academic Press: Cambridge, MA, USA, 1962; pp. 17–22. [Google Scholar]
- Sheng, C.; Azevedo, J. Estimating the higher heating value of biomass fuels from basic analysis data. Biomass Bioenergy 2005, 28, 499–507. [Google Scholar] [CrossRef]
- Hansen, T.L.; Schmidt, J.E.; Angelidaki, I.; Marca, E.; Jansen, J.L.C.; Mosbæk, H.; Christensen, T.H. Method for determination of methane potentials of solid organic waste. Waste Manag. 2004, 24, 393–400. [Google Scholar] [CrossRef]
- Triolo, J.M.; Pedersen, L.; Qu, H.; Sommer, S.G. Biochemical methane potential and anaerobic biodegradability of non-herbaceous and herbaceous phytomass in biogas production. Bioresour. Technol. 2012, 125, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.; Lutts, S.; Sanchez-Garcia, M.; Correal, E. Atriplex halimus L.: Its biology and uses. J. Arid Environ. 2014, 100–101, 111–121. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 3th ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Martínez-Fernández, D.; Arco-Lázaro, E.; Bernal, M.P.; Clemente, R. Comparison of compost and humic fertiliser effects on growth and trace elements accumulation of native plant species in a mine soil phytorestoration experiment. Ecol. Eng. 2014, 73, 588–597. [Google Scholar] [CrossRef]
- Barbafieri, M.; Dadea, C.; Tassi, E.; Bretzel, F.; Fanfani, L. Uptake of Heavy Metals by Native Species Growing in a Mining Area in Sardinia, Italy: Discovering Native Flora for Phytoremediation. Int. J. Phytoremediat. 2011, 13, 985–997. [Google Scholar] [CrossRef]
- Marchiol, L.; Fellet, G.; Boscutti, F.; Montella, C.; Mozzi, R.; Guarino, C. Gentle remediation at the former “Pertusola Sud” zinc smelter: Evaluation of native species for phytoremediation purposes. Ecol. Eng. 2013, 53, 343–353. [Google Scholar] [CrossRef]
- Melendo, M.; Benítez, E.; Nogales, R. Assessment of the feasibility of endogeneous Mediterranean species for phytoremedia-tion of Pb-contaminated areas. Fresenius Environ. Bull. 2002, 11, 1105–1109. [Google Scholar]
- Baroni, F.; Boscagli, A.; Di Lella, L.; Protano, G.; Riccobono, F. Arsenic in soil and vegetation of contaminated areas in southern Tuscany (Italy). J. Geochem. Explor. 2004, 81, 1–14. [Google Scholar] [CrossRef]
- Parraga-Aguado, I.; González-Alcaraz, M.N.; Schulin, R.; Conesa, H.M. The potential use of Piptatherum miliaceum for the phytomanagement of mine tailings in semiarid areas: Role of soil fertility and plant competition. J. Environ. Manag. 2015, 158, 74–84. [Google Scholar] [CrossRef]
- Arco-Lázaro, E.; Martínez-Fernández, D.; Bernal, M.P.; Clemente, R. Response of Piptatherum miliaceum to co-culture with a legume species for the phytostabilisation of trace elements contaminated soils. J. Soils Sediments 2015, 17, 1349–1357. [Google Scholar] [CrossRef]
- Clemente, R.; Arco-Lázaro, E.; Pardo, T.; Martín, I.; Sánchez-Guerrero, A.; Sevilla, F.; Bernal, M. Combination of soil organic and inorganic amendments helps plants overcome trace element induced oxidative stress and allows phytostabilisation. Chemosphere 2019, 223, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Kabas, S.; Faz, A.; Acosta, J.; Zornoza, R.; Martínez-Martínez, S.; Carmona, D.; Bech, J. Effect of marble waste and pig slurry on the growth of native vegetation and heavy metal mobility in a mine tailing pond. J. Geochem. Explor. 2012, 123, 69–76. [Google Scholar] [CrossRef]
- Pardo, T.; Bernal, M.P.; Clemente, R. Phytostabilisation of severely contaminated mine tailings using halophytes and field addition of organic and inorganic amendments. Chemosphere 2017, 178, 556–564. [Google Scholar] [CrossRef]
- Párraga-Aguado, I.; González-Alcaraz, M.N.; López-Orenes, A.; Ferrer-Ayala, M.; Conesa, H. Evaluation of the environmental plasticity in the xerohalophyte Zygophyllum fabago L. for the phytomanagement of mine tailings in semiarid areas. Chemosphere 2016, 161, 259–265. [Google Scholar] [CrossRef]
- Pardo, T.; Bes, C.; Bernal, M.P.; Clemente, R.; Bernal, M.P. Alleviation of environmental risks associated with severely contaminated mine tailings using amendments: Modeling of trace element speciation, solubility, and plant accumulation. Environ. Toxicol. Chem. 2016, 35, 2874–2884. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, S.L.; Sanzone, E.; Bellomia, L.; Sortino, O.; Belligno, A. Chemical composition and energetic value of perennial ligno-cellulosic species (Miscantus x Giganteus Greef and Deu. and Arundo donax L.) grown in Mediterranean region. In Proceedings of the 15th European Biomass Conference & Exhibition, Berlin, Germany, 7–11 May 2007; pp. 820–823. [Google Scholar]
- Palumbo, A.; Mastro, G. Produzione di “bioetanolo di seconda generazione” dal cardo (Cynara cardunculus L. var. altilis DC.). In Proceedings of the SIA—Società Italiana di Agronomia, XLIII Convegno Nazionale, Pisa, Italy, 17–19 September 2014. [Google Scholar]
- Pimentel, L.D.; Batista, V.A.P.; de Barros, A.F.; Teófilo, R.F.; dos Santos Dias, L.A. Caracterização química e bioenergética de grupos agronômicos de sorgo. Pesqui. Agropecuária Trop. 2017, 47, 424–431. [Google Scholar] [CrossRef]
- Corno, L.; Pilu, S.R.; Adani, F. Arundo donax L.: A non-food crop for bioenergy and bio-compound production. Biotechnol. Adv. 2014, 32, 1535–1549. [Google Scholar] [CrossRef]
- Cencič, V.; Jodice, R.; Migliardi, D.; Picco, D.; Pin, M.; Tomasinsig, E.; Zanotto, G.; Radu, R.; Taccani, R.; Chindaruska, S.; et al. I Biocarburanti. Le Filiere Produttive, le Tecnologie, i Vantaggi Ambientalie le Prospettive di Diffusione; Area Science Park: Padriciano, Italy, 2007; Volume 27, pp. 26–158. [Google Scholar]
- Boundy, B.; Diegel, S.W.; Wright, L.; Davis, S.C. Biomass Energy Data Book, 4th ed.; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2011; Available online: http://ledsgp.org/wp-content/uploads/2015/10/bio-mass-energy-data-book.pdf (accessed on 15 June 2021).
- Saidur, R.; Abdelaziz, E.; Demirbas, A.; Hossain, M.; Mekhilef, S. A review on biomass as a fuel for boilers. Renew. Sustain. Energy Rev. 2011, 15, 2262–2289. [Google Scholar] [CrossRef]
- Amon, T.; Amon, B.; Kryvoruchko, V.; Machmüller, A.; Hopfner-Sixt, K.; Bodiroza, V.; Hrbek, R.; Friedel, J.; Pötsch, E.; Wagentristl, H.; et al. Methane production through anaerobic digestion of various energy crops grown in sustainable crop rotations. Bioresour. Technol. 2007, 98, 3204–3212. [Google Scholar] [CrossRef] [PubMed]
- Braun, R. Anaerobic digestion: A multi-faceted process for energy, environmental management and rural development. In Improvement of Crop Plants for Industrial End Users; Ranalli, P., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 335–416. [Google Scholar]
- Raposo, F.; DE LA Rubia, M.A.; Fernández-Cegrí, V.; Borja, R. Anaerobic digestion of solid organic substrates in batch mode: An overview relating to methane yields and experimental procedures. Renew. Sustain. Energy Rev. 2012, 16, 861–877. [Google Scholar] [CrossRef]
- Gunaseelan, V.N. Regression models of ultimate methane yields of fruits and vegetable solid wastes, sorghum and napiergrass on chemical composition. Bioresour. Technol. 2007, 98, 1270–1277. [Google Scholar] [CrossRef]
- Nizami, A.-S.; Korres, N.E.; Murphy, J.D. Review of the Integrated Process for the Production of Grass Biomethane. Environ. Sci. Technol. 2009, 43, 8496–8508. [Google Scholar] [CrossRef] [PubMed]
- Raposo, F.; Borja, R.; Rincon, B.; Jiménez-Rodríguez, A.-M. Assessment of process control parameters in the biochemical methane potential of sunflower oil cake. Biomass Bioenergy 2008, 32, 1235–1244. [Google Scholar] [CrossRef]
- Kalamaras, S.; Kotsopoulos, T. Anaerobic co-digestion of cattle manure and alternative crops for the substitution of maize in South Europe. Bioresour. Technol. 2014, 172, 68–75. [Google Scholar] [CrossRef]
- Gunaseelan, V.N. Predicting ultimate methane yields of Jatropha curcus and Morus indica from their chemical composition. Bioresour. Technol. 2009, 100, 3426–3429. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y. Anaerobic digestion of giant reed for methane production. Bioresour. Technol. 2014, 171, 233–239. [Google Scholar] [CrossRef]
- Buffiere, P.; Loisel, D.; Bernet, N.; Delgenes, J.-P. Towards new indicators for the prediction of solid waste anaerobic digestion properties. Water Sci. Technol. 2006, 53, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Walker, D.J.; Lutts, S. The tolerance of Atriplex halimus L. to environmental stresses. Emir. J. Food Agric. 2014, 26, 1081. [Google Scholar] [CrossRef] [Green Version]
- Kida, K.; Shigematsu, T.; Kijima, J.; Numaguchi, M.; Mochinaga, Y.; Abe, N.; Morimura, S. Influence of Ni2+ and Co2+ on Methanogenic Activity and the Amounts of Coenzymes Involved in Methanogenesis. J. Biosci. Bioeng. 2001, 91, 590–595. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. Trace element requirements of agricultural biogas digesters during biological conversion of renewable biomass to methane. Biomass Bioenergy 2011, 35, 992–998. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Suzuki, K.; Maekawa, T. Uptake and mass balance of trace metals for methane producing bacteria. Biomass Bioenergy 2003, 25, 427–433. [Google Scholar] [CrossRef]
- Bożym, M.; Florczak, I.; Zdanowska-Skowrońska, P.; Wojdalski, J.; Klimkiewicz, M. An analysis of metal concentrations in food wastes for biogas production. Renew. Energy 2015, 77, 467–472. [Google Scholar] [CrossRef]
- Mudhoo, A.; Kumar, S. Effects of heavy metals as stress factors on anaerobic digestion processes and biogas production from biomass. Int. J. Environ. Sci. Technol. 2013, 10, 1383–1398. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Xia, X.; Yang, Z. Growth and nutrient accumulation of Phragmites australis in relation to water level variation and nutrient loadings in a shallow lake. J. Environ. Sci. 2013, 25, 16–25. [Google Scholar] [CrossRef]
- Suriyagoda, L.D.B.; Real, D.; Renton, M.; Lambers, H.; Ryan, M.H. Establishment, survival, and herbage production of novel, summer-active perennial pasture legumes in the low-rainfall cropping zone of Western Australia as affected by plant density and cutting frequency. Crop. Pasture Sci. 2013, 64, 71–85. [Google Scholar] [CrossRef]
- Cucci, G.; LaColla, G.; Boari, F.; Cantore, V. Yield response of fennel (Foeniculum vulgareMill.) to irrigation with saline water. Acta Agric. Scand. Sect. B Plant Soil Sci. 2014, 64, 129–134. [Google Scholar] [CrossRef]
- Serra Riera, J. Producció de Biomassa de Diferents Genotips de Gramínies Perennes Mediterrànies per a Producció de Biocom-Bustibles de Segona Generació, Honor Project; University of Balearic Islands: Palma, Spain, 2018; Available online: https://dspace.uib.es/xmlui/bitstream/handle/11201/151639/Memoria_EPSU1168.pdf?sequence=1 (accessed on 16 June 2021).
| Plants | Al | As | Cd | Cu | Fe | Mn | Pb | Zn |
|---|---|---|---|---|---|---|---|---|
| D. viscosa | 563.4 ± 47.0 a | 5.6 ± 0.08 a | 8.0 ± 0.31 a | 13.70 ± 0.12 b | 1099.4 ± 29.3 a | 70.9 ± 0.2 c | 108.5 ± 3.4 a | 347.5 ± 2.9 b |
| A. halimus | 76.4 ± 4.7 d | 0.7 ± 0.15 c | 2.8 ± 0.03 d | 5.81 ± 0.17 e | 106.1 ± 6.1 fg | 73.5 ± 0.9 c | 11.1 ± 0.8 e | 254.9 ± 6.1 c |
| B. bituminosa | 200.0 ± 1.5 c | 1.4 ± 0.10 bc | 2.2 ± 0.07 d | 7.46 ± 0.05 d | 282.4 ± 4.1 d | 70.6 ± 2.0 c | 45.4 ± 1.7 c | 197.7 ± 3.1 d |
| Z. fabago | 88.6 ± 14.6 d | 1.9 ± 0.26 b | 0.5 ± 0.1 e | 8.00 ± 0.18 d | 101.6 ± 1.4 g | 52.5 ± 0.1 e | 5.1 ± 0.3 g | 158.2 ± 1.4 e |
| A. donax | 364.2 ± 6.9 b | 0.6 ± 0.12 c | 4.1 ± 0.06 c | 11.19 ± 0.21 c | 230.3 ± 4.0 e | 84.2 ± 1.4 b | 17.8 ± 0.7 d | 314.8 ± 3.8 b |
| P. australis | 336.9 ± 4.2 b | 3.0 ± 0.27 ab | 5.7 ± 0.16 b | 17.74 ± 0.24 a | 446.2 ± 9.9 c | 241.4 ± 0.4 a | 77.1 ± 3.4 b | 646.0 ± 19.3 a |
| P. miliaceum | 571.7 ± 22.5 a | 2.1 ± 0.74 b | 5.4 ± 0.05 b | 14.46 ± 0.27 b | 670.0 ± 8.2 b | 59.2 ± 0.5 d | 94.1 ± 1.9 ab | 276.8 ± 1.7 c |
| F. vulgare | 95.7 ± 3. 8 cd | 1.3 ± 0.03 bc | 0.56 ± 0.02 e | 5.56 ± 0.1 e | 121.2 ± 3.0 f | 34.4 ± 0.4 f | 8.3 ± 0.3 f | 76.3 ± 2.5 f |
| ANOVA | *** | *** | *** | *** | *** | *** | *** | *** |
| Plants | Ash (%) | VS (%) | Lignin (%) | Holocellulose (%) | Soluble Carbohydrates (g kg−1 dw) |
|---|---|---|---|---|---|
| D. viscosa | 7.03 ± 0.02 c | 90.82 ± 0.16 b | 26.96 ± 0.72 a | 50.94 ± 7.21 bc | 54.85 ± 2.95 bc |
| A. halimus | 14.25 ± 0.14 b | 84.14 ± 0.05 c | 22.40 ± 0.23 b | 62.11 ± 5.73 abc | 12.40 ± 0.40 f |
| B. bituminosa | 5.31 ± 0.06 c | 93.19 ± 0.02 a | 20.09 ± 0.45 bc | 65.19 ± 0.57 abc | 26.5 ± 0.80 ef |
| Z. fabago | 16.28 ± 0.31 a | 80.57 ± 0.57 d | 17.08 ± 0.92 c | 48.58 ± 6.07 c | 35.15 ± 0.95 de |
| A. donax | 6.26 ± 0.19 c | 93.74 ± 0.19 a | 20.92 ± 1.67 bc | 68.23 ± 0.59 abc | 83.85 ± 3.15 a |
| P. australis | 6.81 ± 0.45 c | 93.19 ± 0.45 a | 20.87 ± 0.55 bc | 72.32 ± 056 a | 49.5 ± 0.01 cd |
| P. miliaceum | 7.09 ± 0.61 c | 92.92 ± 0.60 a | 18.42 ± 0.33 bc | 72.42 ± 0.65 a | 44.15 ± 3.05 cde |
| F. vulgare | 6.92 ± 0.33 c | 93.08 ± 0.33 a | 19.59 ± 0.33 bc | 70.98 ± 0.38 ab | 69.35 ± 7.35 ab |
| ANOVA | *** | *** | ** | ** | *** |
| Plants | C (%) | N (%) | S (%) | H (%) | O (%) | HHV (MJ kg−1) | LHV (MJ kg−1) | C/N |
|---|---|---|---|---|---|---|---|---|
| D. viscosa | 46.24 ± 0.09 a | 0.57 ± 0.01 e | 0.10 ± 0.02 d | 5.12 ± 0.08 d | 38.78 ±0.03 c | 17.96 ± 0.03 c | 16.83 ± 0.01 b | 80.85 ± 1.44 a |
| A. halimus | 41.33 ± 0.01 e | 1.21 ± 0.00 b | 0.14 ± 0.02 cd | 4.66 ± 0.07 e | 36.81 ± 0.12 d | 16.03 ± 0.04 e | 15.01 ± 0.04 d | 34.27 ± 0.07 d |
| B. bituminosa | 45.01 ± 0.09 b | 1.48 ± 0.01 ab | 0.07 ± 0.01 d | 5.07 ± 0.02 d | 41.57 ± 0.14 b | 17.62 ± 0.04 d | 16.51 ± 0.05 c | 30.49 ± 0.26 d |
| Z. fabago | 40.33 ± 0.02 f | 1.99 ± 0.08 a | 0.52 ± 0.07 a | 4.75 ± 0.02 e | 32.99 ± 0.22 e | 15.66 ± 0.01 f | 14.62 ± 0.01 e | 20.35 ± 0.86 e |
| A. donax | 43.13 ± 0.02 c | 0.72 ± 0.02 cd | 0.32 ± 0.02 b | 7.50 ± 0.01 a | 42.07 ± 0.05 b | 18.75 ± 0.00 a | 17.11 ± 0.01 a | 59.93 ± 1.22 bc |
| P. australis | 43.38 ± 0.05 c | 0.59 ± 0.00 e | 0.29 ± 0.02 bc | 7.02 ± 0.01 b | 41.91 ± 0.09 b | 18.49 ± 0.01 b | 16.95 ± 0.01 ab | 73.84 ± 0.27 a |
| P. miliaceum | 42.70 ± 0.04 d | 0.66 ± 0.03 d | 0.19 ± 0.00 bcd | 6.38 ± 0.03 c | 42.98 ± 0.14 a | 17.87 ± 0.03 c | 16.46 ± 0.03 c | 64.43 ± 2.75 b |
| F. vulgare | 43.44 ± 0.11 c | 0.77 ± 0.02 c | 0.08 ± 0.02 d | 6.11 ± 0.07 c | 42.68 ± 0.25 a | 17.89 ± 0.08 c | 16.55 ± 0.09 c | 56.38 ± 1.39 c |
| ANOVA | *** | *** | *** | *** | *** | *** | *** | *** |
| Plants | Bm (mL (g SV)−1) | B0 (mL (g SV)−1) | K (h−1) | BMP (mL CH4 (g VS)−1) | TBMP (mL CH4 (g VS)−1) | Anaerobic Biodegradability (%) |
|---|---|---|---|---|---|---|
| D. viscosa | 180.9 ± 3.6 bc | 182.2 ± 5.0 bc | 0.012 ± 0.001 bc | 131.2 ± 3.6 bc | 497.9 ± 26.6 | 26.4 ± 0.7 bcd |
| A. halimus | 116.6 ± 10.7 c | 119.9 ± 14.8 c | 0.012 ± 0.004 bc | 86.0 ± 10.4 c | 576.5 ± 30.6 | 15.1 ± 2.9 c |
| B. bituminosa | 224.4 ± 3.2 ab | 239.2 ± 0.6 ab | 0.011 ± 0.001 bc | 177.6 ± 1.1 ab | 525.3 ± 6.2 | 33.8 ± 0.2 abc |
| Z. fabago | 173.8 ± 2.5 bc | 184.0 ± 0.1 bc | 0.025 ± 0.001 a | 130.6 ± 0.04 bc | 514.7 ± 39.9 | 25.5 ± 2.0 cd |
| A. donax | 261.4 ± 1.5 a | 315.9 ± 25.5 a | 0.006 ± 0.001 bc | 227.4 ± 18.4 a | 517.1 ± 9.8 | 43.9 ± 2.7 a |
| P. australis | 234.5 ± 9.6 ab | 307.0 ± 23.6 a | 0.004 ± 0.001 c | 218.0 ± 16.8 a | 533.5 ± 9.3 | 40.8 ± 2.4 ab |
| P. miliaceum | 277.5 ± 26.9 a | 292.3 ± 38.8 ab | 0.015 ± 0.003 ab | 204.6 ± 27.2 ab | 518.9 ± 2.7 | 39.5 ± 5.5 abc |
| F. vulgare | 211.5 ± 16.1 ab | 219.4 ± 15.3 abc | 0.008 ± 0.0002 bc | 154.6 ± 108 abc | 524.3 ± 2.0 | 29.5 ± 2.2 abcd |
| ANOVA | *** | ** | ** | ** | NS | ** |
| Ashes | VS | N | H | HHV | Bm | B0 | K | BMP | |
|---|---|---|---|---|---|---|---|---|---|
| Bm | −0.721 * | 0.759 * | −0.448 | 0.789 * | 0.760 * | ||||
| B0 | −0.675 | 0.730 * | −0.471 | 0.896 ** | 0.792 * | 0.947 *** | |||
| K | 0.721 * | −0.777 * | 0.739 * | −0.658 | −0.792 * | −0.336 | −0.476 | ||
| BMP | −0.693 | 0.740 * | −0.451 | 0.882 ** | 0.798 * | 0.943 *** | 0.998 *** | −0.488 | |
| Anaerobic biodegradability | −0.700 | 0.738 * | −0.451 | 0.869 ** | 0.016 | 0.955 *** | 0.994 *** | −0.451 | 0.996 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernal, M.P.; Grippi, D.; Clemente, R. Potential of the Biomass of Plants Grown in Trace Element-Contaminated Soils under Mediterranean Climatic Conditions for Bioenergy Production. Agronomy 2021, 11, 1750. https://doi.org/10.3390/agronomy11091750
Bernal MP, Grippi D, Clemente R. Potential of the Biomass of Plants Grown in Trace Element-Contaminated Soils under Mediterranean Climatic Conditions for Bioenergy Production. Agronomy. 2021; 11(9):1750. https://doi.org/10.3390/agronomy11091750
Chicago/Turabian StyleBernal, María Pilar, Donatella Grippi, and Rafael Clemente. 2021. "Potential of the Biomass of Plants Grown in Trace Element-Contaminated Soils under Mediterranean Climatic Conditions for Bioenergy Production" Agronomy 11, no. 9: 1750. https://doi.org/10.3390/agronomy11091750
APA StyleBernal, M. P., Grippi, D., & Clemente, R. (2021). Potential of the Biomass of Plants Grown in Trace Element-Contaminated Soils under Mediterranean Climatic Conditions for Bioenergy Production. Agronomy, 11(9), 1750. https://doi.org/10.3390/agronomy11091750







