Allelopathic Activity and Characterization of Allelopathic Substances from Elaeocarpus floribundus Blume Leaves for the Development of Bioherbicides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Extraction and Growth Bioassay
2.3. Isolation and Purification of the Substances
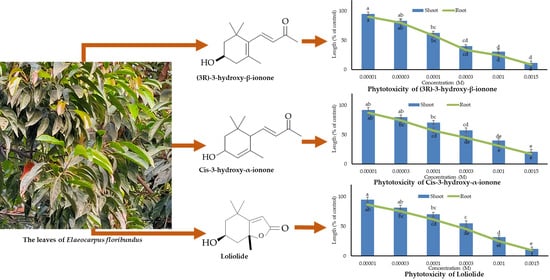
2.4. Bioassay of the Identified Compounds
2.5. Analysis
3. Results
3.1. Phytotoxic Activity of the Elaeocarpus floribundus Extracts
3.2. Characterization of the Allelopathic Compounds
3.3. Biological Potential of the Identified Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, N. The effect of seed sources variation and presowing treatments on the seed germination of Acacia catechu and Elaeocarpus floribundus species in Bangladesh. Int. J. Financ. Res. 2014, 2014, 984194. [Google Scholar]
- Ogundele, A.V.; Das, A.M. Chemical constituents from the leaves of Elaeocarpus floribundus. Nat. Prod. Res. 2019, 35, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Pullaiah, T. Encyclopedia of World Medicinal Plants; Regency Publication: New Delhi, India, 2006; Volume 2, pp. 852–853. [Google Scholar]
- Singh, U.V.; Ahlawat, S.P.; Bisht, N.S. Nursery Technique of Local Tree Species II; SFRI Information Bulletin No. 11; State Forest Research Institute: Jabalpur, India, 2003. [Google Scholar]
- Joshi, H.B.; Rashid, M.A.; Venkataramany, P. The Silviculture of Indian Trees; Controller Publication: New Delhi, India, 1981. [Google Scholar]
- Mahomoodally, M.F.; Sookhy, V. Ethnobotany and pharmacological uses of Elaeocarpus floribundus Blume (Elaeocarpaceae). Plant Hum. Health 2018, 1, 125–137. [Google Scholar]
- Dadhich, A.; Rishi, A.; Sharma, G.; Chandra, S. Phytochemicals of Elaeocarpus with their therapeutic value: A review. Int. J. Phar. Bio Sci. 2011, 4, 591–598. [Google Scholar]
- Utami, R.; Khalid, N.; Sukari, M.A.; Rahmani, M.; Abdul, A.B. Phenolic contents, antioxidant and cytotoxic activities of Elaeocarpus floribundus Blume. Pak. J. Pharm. Sci. 2013, 26, 245–250. [Google Scholar]
- Zaman, S. Exploring the antibacterial and antioxidant activities of Elaeocarpus floribundus leaves. Indo Am. J. Pharmaceut. Sci. 2016, 3, 92–97. [Google Scholar]
- Sircar, B.; Mandal, S. Indian olive, Elaeocarpus floribundus fruit: Perspective to the antioxidative capacity and antibacterial activity. Ec Microb. 2017, 12, 273–282. [Google Scholar]
- Saric-Krsmanovic, M.; Gajic Umiljendic, J.; Radivojevic, L.; Šantric, L.; Potocnik, I.; Ðurovic-Pejcev, R. Bio-herbicidal effects of five essential oils on germination and early seedling growth of velvetleaf (Abutilon theophrasti Medik.). J. Environ. Sci. Health B 2019, 54, 247–251. [Google Scholar] [CrossRef]
- Macías, A.F.; Mejías, F.J.R.; Molinillo, J.M.G. Recent advances in allelopathy for weed control: From knowledge to applications. Pest. Manag. Sci. 2019, 75, 2413–2436. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.H.; Peerzada, A.M.; Hanif, Z.; Hashim, S.; Chauhan, B.S. Weed management using crop competition in Pakistan: A review. Crop. Prot. 2017, 95, 22–30. [Google Scholar] [CrossRef]
- Farooq, N.; Abbas, T.; Tanveer, A.; Jabran, K. Allelopathy for Weed Management. In Coevolution of Secondary Metabolites; Springer: Berlin/Heidelberg, Germany, 2020; pp. 505–519. [Google Scholar]
- Gharde, Y.; Singh, P.K.; Dubey, R.P.; Gupta, P.K. Assessment of yield and economic losses in agriculture due to weeds in India. Crop Prot. 2018, 107, 12–18. [Google Scholar] [CrossRef]
- Chauhan, B.S. Grand challenges in weed management. Front. Agron. 2020, 1, 3. [Google Scholar] [CrossRef]
- Weed Science Society of America. Available online: http://wssa.net/wssa/weed/biological-control/ (accessed on 15 November 2021).
- Vencill, W.; Grey, T.; Culpepper, S. Resistance of Weeds to Herbicides. In Herbicides and Environment; Kortekamp, A., Ed.; Intech Open: London, UK, 2011; pp. 585–594. [Google Scholar]
- Hicks, H.L.; Comont, D.; Coutts, S.R. The factors driving evolved herbicide resistance at a national scale. Nat. Ecol. Evol. 2018, 2, 529–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bàrberi, P. Ecological weed management in sub-Saharan Africa: Prospects and implications on other agroecosystem services. Adv. Agron. 2019, 156, 219–264. [Google Scholar]
- Bo, A.B.; Khaitov, B.; Umurzokov, M.; Cho, K.M.; Park, K.W.; Choi, J.S. Biological control using plant pathogens in weed management. Weed Turfgrass Sci. 2020, 9, 11–19. [Google Scholar]
- Reganold, J.P.; Wachter, J.M. Organic agriculture in the twenty-first century. Nat. Plants 2016, 2, 15221. [Google Scholar] [CrossRef]
- Farooq, M.; Jabran, K.; Cheema, Z.A.; Wahid, A.; Siddique, K.H.M. The role of allelopathy in agricultural pest management. Pest. Manag. Sci. 2011, 67, 493–506. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Adeola, R.G. Perceptions of environmental effects of pesticides use in vegetable production by farmers in Ogbomoso, Nigeria. Glob. J. Sci. Front. Res. Agric. Biol. 2012, 12, 73–78. [Google Scholar]
- Naeem, M.; Cheema, Z.A.; Ihsan, M.Z.; Hussain, Y.; Mazari, A.; Abbas, H.T. Allelopathic effects of different plant aqueous extracts on yield and weeds of wheat. Planta Daninha 2018, 36, e018177840. [Google Scholar] [CrossRef] [Green Version]
- Soltys, D.; Krasuska, U.; Bogatek, R.; Gniazdow, A. Allelochemicals as Bio-Herbicides Present and Perspectives. In Herbicides-Current Research and Case Studies in Use; InTech: Rijeka, Croatia, 2013; pp. 517–542. [Google Scholar]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural products in crop protection. Bioorg. Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef]
- Bailey, K.L. The Bioherbicide Approach to Weed Control Using Plant Pathogens. In Integrated Pest Management; Academic Press: Cambridge, MA, USA, 2014; pp. 245–266. [Google Scholar]
- Lamberth, C. Naturally occurring amino acid derivatives with herbicidal, fungicidal or insecticidal activity. Amino Acids 2016, 48, 929–940. [Google Scholar] [CrossRef] [PubMed]
- El-Darier, S.M.; Abdelaziz, H.A.; ZeinEl-Dien, M.H. Effect of soil type on the allelotoxic activity of Medicago sativa L. residues in Vicia faba L. agroecosystems. J. Taibah Univ. Sci. 2014, 8, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Hosni, K.; Hassen, I.; Sebei, H.; Casabianca, H. Secondary metabolites from Chrysanthemum coronarium (Garland) flowerheads: Chemical composition and biological activities. Ind. Crop. Prod. 2013, 44, 263–271. [Google Scholar] [CrossRef]
- Khachik, F.; Chang, A.N. Total synthesis of (3R,3′R,6′R)-lutein and its stereoisomers. J. Org. Chem. 2009, 74, 3875–3885. [Google Scholar] [CrossRef]
- Khachik, F.; Chang, A.N. Synthesis of (3S)- and (3R)-3-hydroxy-β-ionone and their transformation into (3S)-and (3R)-β-cryptoxanthin. Synthesis 2011, 3, 509–516. [Google Scholar] [CrossRef]
- Kim, M.R.; Lee, S.K.; Kim, C.S.; Kim, K.S.; Moon, D.C. Phytochemical constituents of Carpesium macrocephalum FR- et SAV-. Arch Pharm Res 2004, 27, 1029–1033. [Google Scholar] [CrossRef]
- Islam, M.S.; Zaman, F.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Isolation and identification of three potential phytotoxic compounds from Chrysopogon aciculatus (Retz.) Trin. Acta Physiol. Plant. 2021, 43, 56. [Google Scholar] [CrossRef]
- Rob, M.M.; Iwasaki, A.; Suenaga, K.; Ozaki, K.; Teruya, T.; Kato-Noguchi, H. Potential use of Schumannianthus dichotomus waste: The phytotoxic activity of the waste and its identified compounds. J. Environ. Sci. Health B 2020, 55, 1099–1105. [Google Scholar] [CrossRef]
- Islam, M.S.; Zaman, F.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic potential of Chrysopogon aciculatus (Retz.) Trin. (Poaceae). Weed Biol. Manag. 2019, 19, 51–58. [Google Scholar] [CrossRef]
- Hossen, K.; Das, K.R.; Okada, S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Allelopathic potential and active substances from Wedelia chinensis (Osbeck). Foods 2020, 9, 1591. [Google Scholar] [CrossRef]
- Hossen, K.; Kato-Noguchi, H. Determination of allelopathic properties of Acacia catechu (L.f.) Willd. Not. Bot. Horti Agrobot. Cluj Napoca 2020, 48, 2050–2059. [Google Scholar] [CrossRef]
- Rob, M.; Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic activity and identification of phytotoxic substances from Schumannianthus dichotomus. Plants 2020, 9, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rob, M.M.; Hossen, K.; Khatun, M.R.; Iwasaki, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Identification and application of bioactive compounds from Garcinia xanthochymus Hook. for weed management. Appl. Sci. 2021, 11, 2264. [Google Scholar] [CrossRef]
- Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic activity and growth inhibitory substances from Albizia richardiana (Voigt.) King & Prain. Appl. Sci. 2021, 11, 1455. [Google Scholar] [CrossRef]
- Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxicity of the novel compound 3-hydroxy-4-oxo-β-dehydroionol and compound 3-oxo-α-ionone from Albizia richardiana (Voigt.) King & Prain. Environ. Technol. Innov. 2021, 23, 101779. [Google Scholar]
- Hossen, K.; Ozaki, K.; Teruya, T.; Kato-Noguchi, H. Three active phytotoxic compounds from the leaves of Albizia richardiana (Voigt.) King and Prain for the development of bioherbicides to control weeds. Cells 2021, 10, 2385. [Google Scholar] [CrossRef] [PubMed]
- Ladhari, A.; Omezzine, F.; DellaGreca, M.; Zarrelli, A.; Zuppolini, S.; Haouala, R. Phytotoxic activity of Cleome arabica L. and its principal discovered active compounds. S. Afr. J. Bot. 2013, 88, 341–351. [Google Scholar] [CrossRef] [Green Version]
- Amb, M.K.; Ahluwalia, A.S. Allelopathy: Potential role to achieve new milestones in rice cultivation. Rice Sci. 2016, 23, 165–183. [Google Scholar] [CrossRef] [Green Version]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef]
- Yoshimura, H.; Sawa, Y.; Tamotsu, S.; Sakai, A. 1,8-Cineole inhibits both proliferation and elongation of BY-2 cultured tobacco cells. J. Chem. Ecol. 2011, 37, 320–328. [Google Scholar] [CrossRef] [Green Version]
- Grana, E.; Sotelo, T.; Diaz-Tielas, C.; Araniti, F.; Krasuska, U.; Bogatek, R.; Reigosa, M.J.; Sanchez-Moreiras, A.M. Citral induces auxin and ethylene-mediated malformations and arrests cell division in Arabidopsis thaliana roots. J. Chem. Ecol. 2013, 39, 271–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anese, S.; Jatoba, L.J.; Grisi, P.U.; Gualtieri, S.C.J.; Santos, M.F.C.; Berlinck, R.G.S. Bioherbicidal activity of drimane sesquiterpenes from Drimys brasiliensis Miers roots. Ind. Crops Prod. 2015, 74, 28–35. [Google Scholar] [CrossRef]
- Rahman, A.; Ahamed, A.; Amakawa, T.; Goto, N.; Tsurumi, S. Chromosaponin I specifically interacts with AUX1 protein in regulating the gravitropic response of Arabidopsis roots. Plant Physiol. 2001, 125, 990–1000. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.M.; Radhakrishnan, R.; Kang, S.M.; Kim, J.H.; Lee, I.Y.; Moon, B.Y.; Yoon, B.W.; Lee, I.J. Phytotoxic mechanisms of bur cucumber seed extracts on lettuce with special reference to analysis of chloroplast proteins, phytohormones, and nutritional elements. Ecotoxicol. Environ. Saf. 2015, 122, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Heide, H.; Kalisz, H.M.; Follmann, H. The oxygen evolving enhancer protein1 (OEE) of photosystem II in green algae exhibits thioredoxin activity. J. Plant Physiol. 2004, 161, 139–149. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Lee, I.J. Spermine promotes acclimation to osmotic stress by modifying antioxidant, abscisic acid, and jasmonic acid signals in soybean. J. Plant Growth Regul. 2015, 32, 22–30. [Google Scholar] [CrossRef]
- Grossmann, K. Mediation of herbicide effects by hormone interactions. J. Plant Growth. 2003, 2, 109–122. [Google Scholar] [CrossRef]
- Duke, S.O.; Romagni, J.G.; Dayan, F.E. Natural products as sources for new mechanisms of herbicidal action. Crop Prot. 2000, 19, 583–589. [Google Scholar] [CrossRef]
- Tigre, R.C.; Silva, N.H.; Santos, M.G.; Honda, N.K.; Falcao, E.P.S.; Pereira, E.C. Allelopathic and bioherbicidal potential of Cladonia verticillaris on the germination and growth of Lactuca sativa. Ecotoxicol. Environ. Saf. 2012, 84, 125–132. [Google Scholar] [CrossRef]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef]
- Travaini, M.L.; Sosa, G.M.; Ceccarelli, E.A.; Walter, H.; Cantrell, C.L.; Carrillo, N.J.; Dayan, F.E.; Meepagala, K.M.; Duke, S.O. Khellin and visnagin, furanochromones from Ammi visnaga (L.) Lam., as potential bioherbicides. J. Agric. Food Chem. 2016, 64, 9475–9487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosheh, H.Z. Constraints in implementing biological weed control: A review. Weed Biol. Manag. 2005, 5, 83–92. [Google Scholar] [CrossRef]
- Mathieu, S.; Terrier, N.; Procureur, J.; Bigey, F.; Gunata, Z. A carotenoid cleavage dioxygenase from Vitis vinifera L., functional characterization and expression during grape berry development in relation to C13-norisoprenoid accumulation. J. Exp. Bot. 2005, 56, 2721–2731. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. An endogenous growth inhibitor, 3-hydroxy-β-ionone. I. Its role in light induced growth inhibition of hypocotyls of Phaseolus vulgaris. Physiol. Plant. 1992, 86, 583–586. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Yamamoto, M.; Tamura, K.; Teruya, T.; Suenaga, K.; Fujii, Y. Isolation and identification of potent allelopathic substances in rattail fescue. Plant Grow. Regul. 2010, 60, 127–131. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Hamada, N.; Clements, D.R. Phytotoxicities of the invasive species Plantago major and non-invasive species Plantago asiatica. Acta Physiol. Plant. 2015, 37, 60. [Google Scholar] [CrossRef]
- Masum, S.M.; Hossain, M.A.; Akamine, H.; Sakagami, J.I.; Ishii, T.; Gima, S.; Kensaku, T.; Bhowmik, P.C. Isolation and characterization of allelopathic compounds from the indigenous rice variety ‘Boterswar’ and their biological activity against Echinochloa crus-galli L. Allelopath. J. 2018, 43, 31–42. [Google Scholar] [CrossRef]
- Ida, N.; Iwasaki, A.; Teruya, T.; Suenaga, K.; Kato-Noguchi, H. Tree fern Cyathea lepifera may survive by its phytotoxic property. Plants 2020, 9, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato-Noguchi, H.; Seki, T.; Shigemori, H. Allelopathy and allelopathic substance in the moss Rhynchostegium pallidifolium. J. Plant Physiol. 2010, 167, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Khatri, Y.; Girhard, M.; Romankiewicz, A.; Ringle, M.; Hannemann, F.; Urlacher, V.B.; Hutter, M.C.; Bernhardt, R. Regioselective hydroxylation of norisoprenoids by CYP109D1 from Sorangium cellulosum So ce56. Appl. Microbiol. Biotechnol. 2010, 88, 485–495. [Google Scholar] [CrossRef]
- Putkaradze, N.; Litzenburger, M.; Abdulmughni, A.; Milhim, M.; Brill, E.; Hannemann, F.; Bernhardt, R. CYP109E1 is a novel versatile statin and terpene oxidase from Bacillus megaterium. Appl. Microbiol. Biotechnol. 2017, 101, 8379–8393. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Enomoto, H.; Nishida, R. New attractants for males of the solanaceous fruit fly Bactrocera latifrons. J. Chem. Ecol. 2008, 34, 1532–1535. [Google Scholar] [CrossRef]
- Mottaghipisheh, J.; Nové, M.; Spengler, G.; Kúsz, N.; Hohmann, J.; Csupor, D. Antiproliferative and cytotoxic activities of furocoumarins of Ducrosia anethifolia. Pharmaceut. Biol. 2018, 56, 658–664. [Google Scholar] [CrossRef] [Green Version]
- Girhard, M.; Klaus, T.; Khatri, Y.; Bernhardt, R.; Urlacher, V.B. Characterization of the versatile monooxygenase CYP109B1 from Bacillus subtilis. Appl. Microbiol. Biotechnol. 2010, 87, 595–607. [Google Scholar] [CrossRef]
- Bari, I.N.; Kato-Noguchi, H.; Iwasaki, A.; Suenaga, K. Allelopathic potency and an active substance from Anredera cordifolia (Tenore) Steenis. Plants 2019, 8, 134. [Google Scholar] [CrossRef] [Green Version]
- Grabarczyk, M.; Winska, K.; Maczka, W.; Potaniec, B.; Anioł, M. Loliolide-The most ubiquitous lactone. Folia Biol. Oecol. 2015, 11, 1–8. [Google Scholar] [CrossRef]
- Mayer, H. Synthesis of optically active carotenoids and related compounds. Pure Appl. Chem. 1979, 51, 535–564. [Google Scholar] [CrossRef] [Green Version]
- Zaman, F.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Two allelopathic substances from Paspalum commersonii Lam. Acta Agric. Scand. Sect. B Plant. Soil Sci. 2017, 68, 342–348. [Google Scholar]
- Islam, M.S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Isolation and identification of two potential phytotoxic substances from the aquatic fern Marsilea crenata. J. Plant Biol. 2017, 60, 75–81. [Google Scholar] [CrossRef]
- Macías, F.A.; Marín, D.; Oliveros-Bastidas, A.; Molinillo, J.M.G. Optimization of benzoxazinones as natural herbicide models by lipophilicity enhancement. J. Agric. Food Chem. 2006, 54, 9357–9365. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Test Plant Species | I50 Value (g DW Equivalent Extract/mL) | ||
|---|---|---|---|
| Shoot | Root | ||
| Dicot | Cress | 0.00622 | 0.00553 |
| Monocot | Barnyard grass | 0.0289 | 0.0076 |
| Test Plant | (3R)-3-Hydroxy-β-ionone | cis-3-Hydroxy-α-ionone | Loliolide | |
|---|---|---|---|---|
| (M) | ||||
| Cress | Shoot | 0.0002 | 0.0005 | 0.0004 |
| Root | 0.0001 | 0.0002 | 0.0002 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossen, K.; Das, K.R.; Asato, Y.; Teruya, T.; Kato-Noguchi, H. Allelopathic Activity and Characterization of Allelopathic Substances from Elaeocarpus floribundus Blume Leaves for the Development of Bioherbicides. Agronomy 2022, 12, 57. https://doi.org/10.3390/agronomy12010057
Hossen K, Das KR, Asato Y, Teruya T, Kato-Noguchi H. Allelopathic Activity and Characterization of Allelopathic Substances from Elaeocarpus floribundus Blume Leaves for the Development of Bioherbicides. Agronomy. 2022; 12(1):57. https://doi.org/10.3390/agronomy12010057
Chicago/Turabian StyleHossen, Kawsar, Krishna Rany Das, Yuka Asato, Toshiaki Teruya, and Hisashi Kato-Noguchi. 2022. "Allelopathic Activity and Characterization of Allelopathic Substances from Elaeocarpus floribundus Blume Leaves for the Development of Bioherbicides" Agronomy 12, no. 1: 57. https://doi.org/10.3390/agronomy12010057
APA StyleHossen, K., Das, K. R., Asato, Y., Teruya, T., & Kato-Noguchi, H. (2022). Allelopathic Activity and Characterization of Allelopathic Substances from Elaeocarpus floribundus Blume Leaves for the Development of Bioherbicides. Agronomy, 12(1), 57. https://doi.org/10.3390/agronomy12010057