Phenotypic Plasticity in Morphological Traits of Abelmoschus esculentus L. Induced by Histone Deacetylase Inhibitor, Trichostatin A
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Site
2.2. Preparation of Mutagenic Solution
2.3. Recording Field Observations
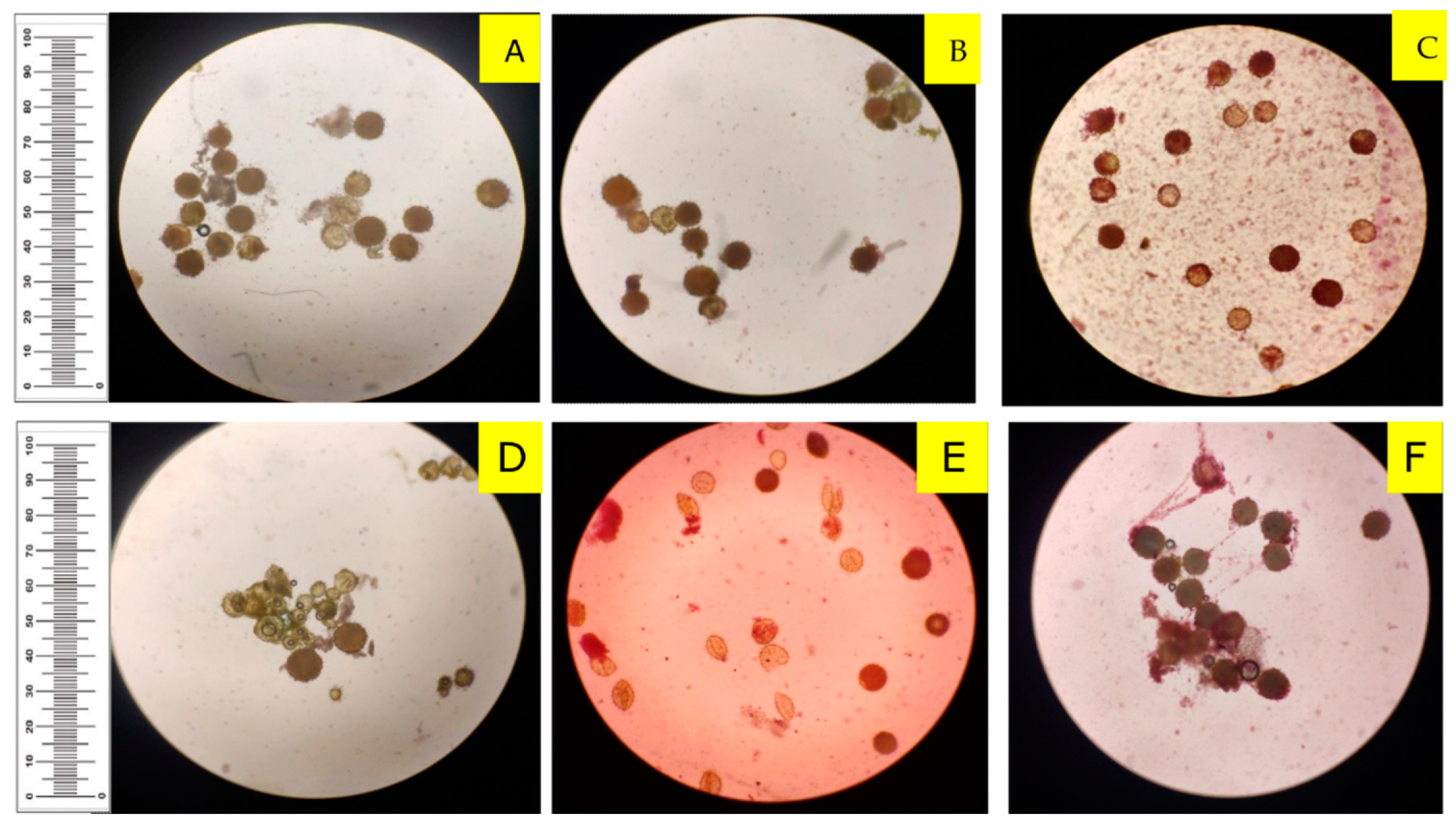
2.4. Assessment of Pollen Sterility
3. Results
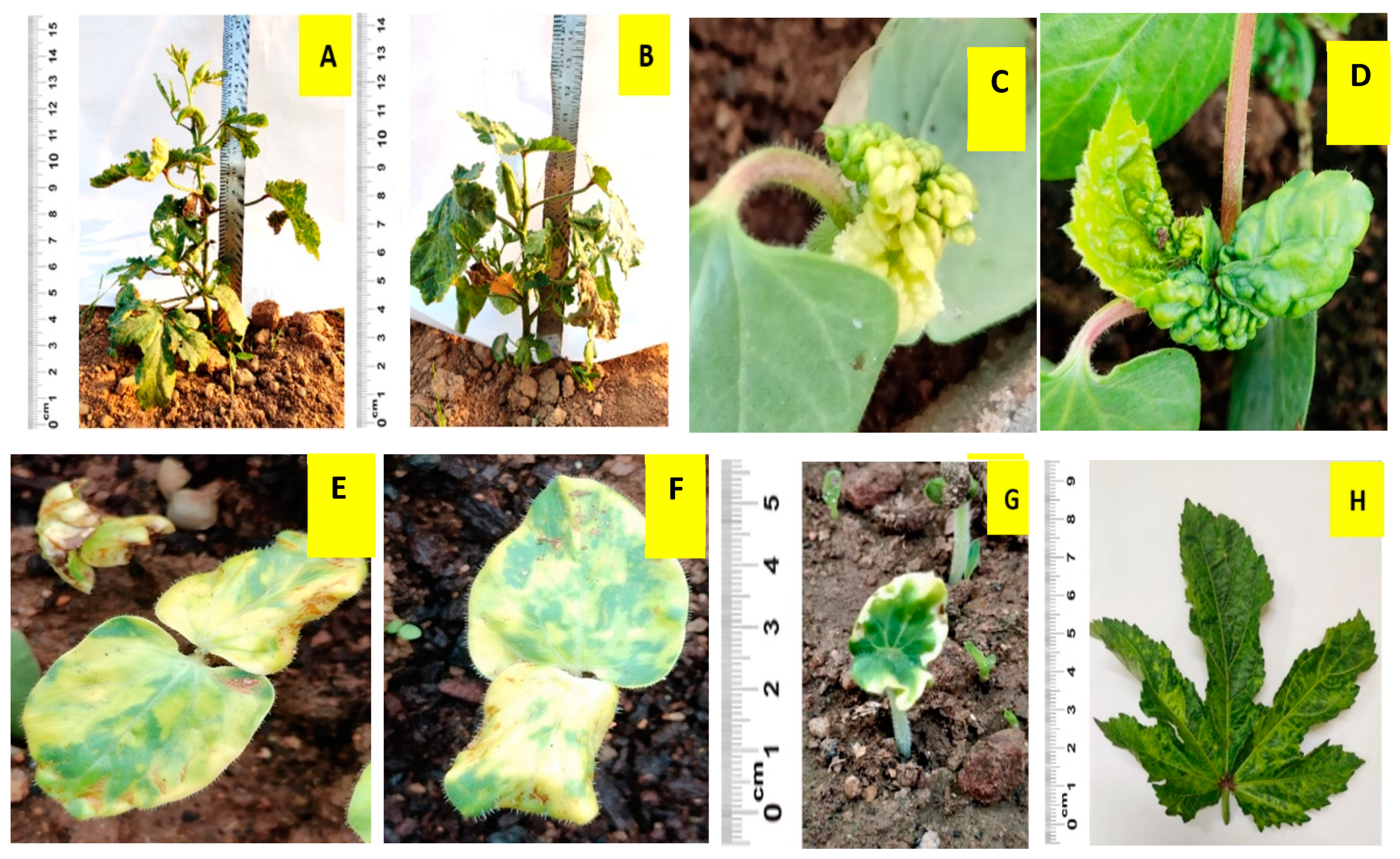
3.1. Seedling Traits among TSA Treatments
3.2. Plant Stature and Leaf Morphology
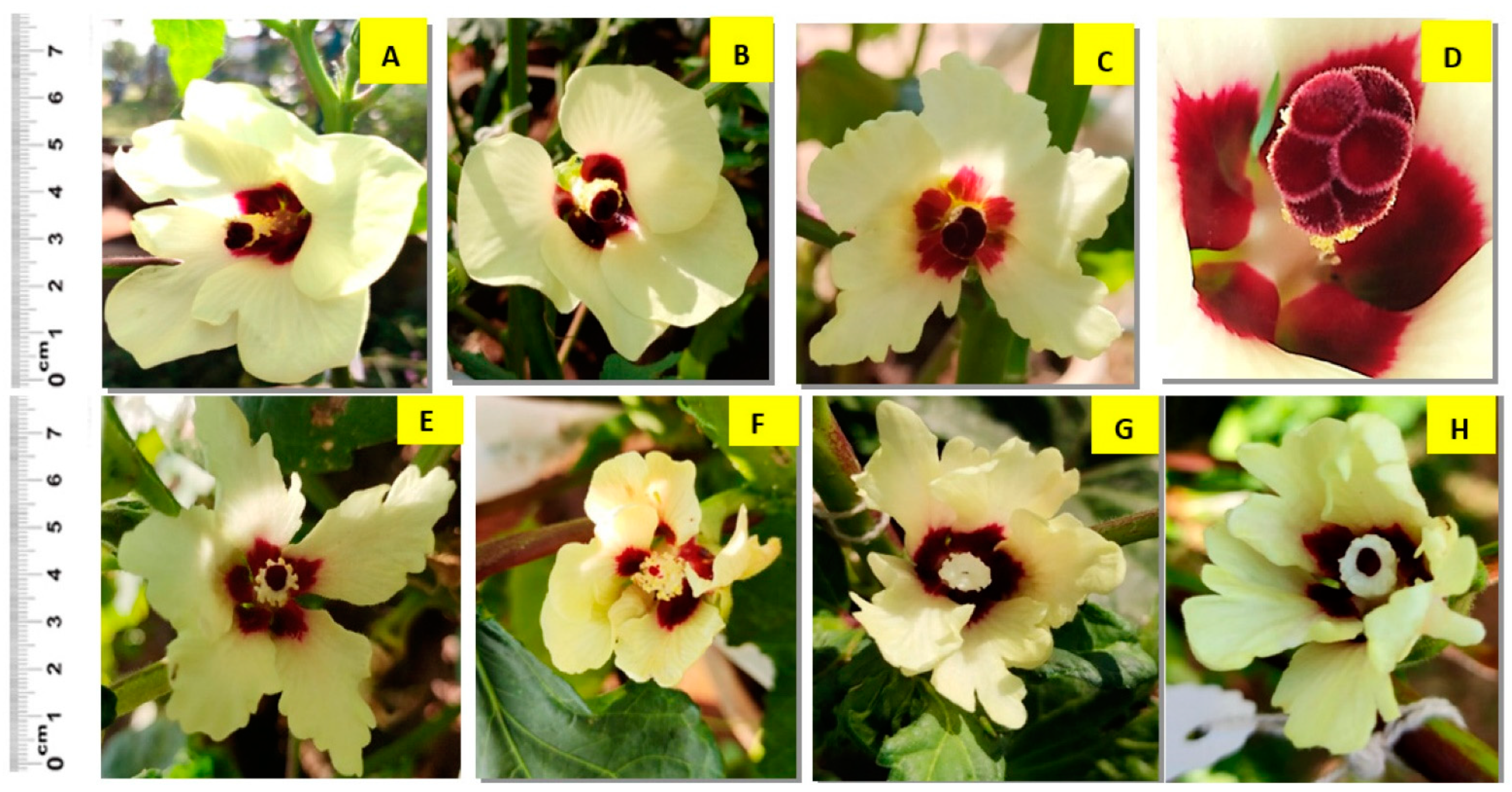
3.3. Flower Morphology and Delay in Flowering
3.4. Pollen Production and Sterility
3.5. Fruit and Seed Morphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wammanda, D.T.; Kadams, A.M.; Jonah, P.M. Combining ability analysis and heterosis in a diallel cross of okra (Abelmoschus esculentus (L.) Moench). Afr. J. Agric. Res. 2010, 5, 2108–2115. [Google Scholar]
- Benchasri, S. Okra (Abelmoschus esculentus (L.) Moench) as a valuable vegetable of the world. Ratarstvo Povrtarstvo 2012, 49, 105–112. [Google Scholar]
- Kumar, S.; Yadav, Y.C. Correlation coefficient and path analysis studies in okra [Abelmoschus esculentus (L.) Moench]. Ann. Hortic. 2009, 2, 166–170. [Google Scholar]
- Sheikh, M.; Safiuddin, S.; Khan, Z.; Mahmood, I. Effect of Bhendi Yellow Vein Mosaic Virus on Yield Components of Okra Plants. Eur. J. Plant Pathol. 2013, 95, 391–393. [Google Scholar] [CrossRef]
- Lengsfeld, C.; Titgemeyer, F.; Faller, G.; Hensel, A. Glycosylated Compounds from Okra Inhibit Adhesion of Helicobacter pylori to Human Gastric Mucosa. J. Agric. Food Chem. 2004, 52, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Jagajanantham, N.; Dhanavel, D.; Pavadai, P.; Chidambaram, A. Growth and yield parameters using gamma rays in bhendi (Abelmoschus esculentus (L.) Moench) var. Arka Anamika. Int. J. Res. Plant Sci. 2012, 2, 56–58. [Google Scholar]
- Kumar, S.; Choudhary, S. Okra Breeding: Recent Approaches and Constraint. Ann. Biol. 2019, 35, 55–60. [Google Scholar]
- Sahu, P.P.; Pandey, G.; Sharma, N.; Puranik, S.; Muthamilarasan, M.; Prasad, M. Epigenetic mechanisms of plant stress responses and adaptation. Plant Cell Rep. 2013, 32, 1151–1159. [Google Scholar] [CrossRef]
- Cubas, P.; Vincent, C.; Coen, E. An epigenetic mutation responsible for natural variation in floral symmetry. Nature 1999, 401, 157–161. [Google Scholar] [CrossRef]
- Weigel, D.; Colot, V. Epialleles in plant evolution. Genome Biol. 2012, 13, 249. [Google Scholar] [CrossRef]
- Johannes, F.; Schmitz, R.J. Spontaneous epimutations in plants. New Phytol. 2018, 221, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.L.; Wendel, J.F. The hairy problem of epigenetics in evolution. New Phytol. 2011, 191, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A.; Takai, D. The Role of DNA Methylation in Mammalian Epigenetics. Science 2001, 293, 1068–1070. [Google Scholar] [CrossRef] [PubMed]
- Bie, X.M.; Dong, L.; Li, X.H.; Wang, H.; Gao, X.-Q.; Li, X.G. Trichostatin A and sodium butyrate promotes plant regeneration in common wheat. Plant Signal. Behav. 2020, 15, 1820681. [Google Scholar] [CrossRef]
- Sako, K.; Kim, J.M.; Matsui, A.; Nakamura, K.; Tanaka, M.; Kobayashi, M.; Saito, K.; Nishino, N.; Kusano, N.; Taji, T.; et al. Ky-2, a Histone deacetylase inhibitor, enhances high-salinity stress tolerance in Arabidopsis thaliana. Plant Cell Physiol. 2016, 57, 776–783. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.M.; Sako, K.; Matsui, A.; Ueda, M.; Tanaka, M.; Ito, A.; Nishino, N.; Yoshida, M.; Seki, M. Transcriptomic analysis of Arabidopsis thaliana plants treated with the Ky-9 and Ky-72 histone deacetylase inhibitors. Plant Signal. Behav. 2018, 13, e1448333. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, J.; Jie, E.Y.; Choi, S.H.; Jiang, L.; Ahn, W.S.; Kim, C.Y.; Kim, S.W. Temporal and Spatial Expression Analysis of Shoot-Regeneration Regulatory Genes during the Adventitious Shoot Formation in Hypocotyl and Cotyledon Explants of Tomato (CV. Micro-Tom). Int. J. Mol. Sci. 2020, 21, 5309. [Google Scholar] [CrossRef]
- Li, H.; Soriano, M.; Cordewener, J.; Muino, J.M.; Riksen, T.; Fukuoka, H.; Angenent, G.C.; Boutilier, K. The Histone Deacetylase Inhibitor Trichostatin A Promotes Totipotency in the Male Gametophyte. Plant Cell 2014, 26, 195–209. [Google Scholar] [CrossRef]
- Tanaka, M.; Kikuchi, A.; Kamada, H. The Arabidopsis Histone Deacetylases HDA6 and HDA19 Contribute to the Repression of Embryonic Properties after Germination. Plant Physiol. 2007, 146, 149–161. [Google Scholar] [CrossRef]
- Pagano, A.; de Sousa Araújo, S.; Macovei, A.; Dondi, D.; Lazzaroni, S.; Balestrazzi, A. Metabolic and gene expression hallmarks of seed germination uncovered by sodium butyrate in Medicago truncatula. Plant Cell Environ. 2019, 42, 259–269. [Google Scholar] [CrossRef]
- Uddenberg, D.; Valladares, S.; Abrahamsson, M.; Sundström, J.F.; Sundås-Larsson, A.; Von Arnold, S. Embryogenic potential and expression of embryogenesis-related genes in conifers are affected by treatment with a histone deacetylase inhibitor. Planta 2011, 234, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Daghma, D.S.; Houben, A.; Kumlehn, J.; Melzer, M.; Rutten, T. Dynamics of post-translationally modified histones during barley pollen embryogenesis in the presence or absence of the epi-drug trichostatin A. Plant Reprod. 2017, 30, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Horinouchi, S.; Beppu, T. Trichostatin A and trapoxin: Novel chemical probes for the role of histone acetylation in chromatin structure and function. BioEssays 1995, 17, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, L.; Li, J.; Huang, J.; Wen, R.; Ma, L.; Zhou, D.; Li, L. Trichostatin A and 5-azacytidine both cause an increase in global histone H4 acetylation and a decrease in global DNA and H3K9 methylation during mitosis in maize. BMC Plant Biol. 2010, 10, 178. [Google Scholar] [CrossRef]
- Ma, X.; Lv, S.; Zhang, C.; Yang, C. Histone deacetylases and their functions in plants. Plant Cell Rep. 2013, 32, 465–478. [Google Scholar] [CrossRef]
- Perales, M.; Más, P. A Functional Link between Rhythmic Changes in Chromatin Structure and the Arabidopsis Biological Clock. Plant Cell 2007, 19, 2111–2123. [Google Scholar] [CrossRef]
- Lochmanová, G.; Ihnatová, I.; Kuchaříková, H.; Brabencová, S.; Zachová, D.; Fajkus, J.; Zdráhal, Z.; Fojtová, M. Different modes of action of genetic and chemical downregulation of histone deacetylases with respect to plant development and histone modifications. Int. J. Mol. Sci. 2019, 20, 5093. [Google Scholar] [CrossRef]
- Macovei, A.; Pagano, A.; Leonetti, P.; Carbonera, D.; Balestrazzi, A.; Araújo, S.S. Systems biology and genome-wide approaches to unveil the molecular players involved in the pre-germinative metabolism: Implications on seed technology traits. Plant Cell Rep. 2016, 36, 669–688. [Google Scholar] [CrossRef]
- Hou, H.; Wang, P.; Zhang, H.; Wen, H.; Gao, F.; Ma, N.; Wang, Q.; Li, L. Histone Acetylation Is Involved in Gibberellin-Regulated sodCp Gene Expression in Maize Aleurone Layers. Plant Cell Physiol. 2015, 56, 2139–2149. [Google Scholar] [CrossRef]
- Colville, A.; Alhattab, R.; Hu, M.; Labbé, H.; Xing, T.; Miki, B. Role of HD2 genes in seed germination and early seedling growth in Arabidopsis. Plant Cell Rep. 2011, 30, 1969–1979. [Google Scholar]
- Singh, R.P.; Chauhan, M.P.S. Seed Structure and Systematic Position of Hampea nutricia (Malvaceae). Plant Syst. Evol. 1984, 147, 55–61. [Google Scholar] [CrossRef]
- Steinbrecher, T.; Leubner-Metzger, G. The biomechanics of seed germination. J. Exp. Bot. 2017, 68, 765–783. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-R.; Liu, C.; Wang, Y.-L.; Li, L.-C.; Chen, W.-Q.; Xu, Z.-H.; Bai, S.-N. Histone acetylation affects expression of cellular patterning genes in the Arabidopsis root epidermis. Proc. Natl. Acad. Sci. USA 2005, 102, 14469–14474. [Google Scholar] [CrossRef] [PubMed]
- Pagano, A.; Andolina, A.; Sabatini, M.E.; Araújo, S.S.; Balestrazzi, A.; Macovei, A. Sodium butyrate induces genotoxic stress in function of photoperiod variations and differentially modulates the expression of genes involved in chromatin modification and DNA repair in Petunia hybrida seedlings. Planta 2020, 251, 102. [Google Scholar] [CrossRef]
- Pagano, A.; Araújo, S.; Macovei, A.; Leonetti, P.; Balestrazzi, A. The Seed Repair Response during Germination: Disclosing Correlations between DNA Repair, Antioxidant Response, and Chromatin Remodeling in Medicago truncatula. Front. Plant Sci. 2017, 8, 1972. [Google Scholar] [CrossRef]
- Benková, E.; Hejátko, J. Hormone interactions at the root apical meristem. Plant Mol. Biol. 2008, 69, 383–396. [Google Scholar] [CrossRef]
- West, G.; Inzé, D.; Beemster, G.T. Cell Cycle Modulation in the Response of the Primary Root of Arabidopsis to Salt Stress. Plant Physiol. 2004, 135, 1050–1058. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, P.; Hou, H.; Zhang, H.; Tan, J.; Huang, Y.; Li, Y.; Wu, J.; Qiu, Z.; Li, L. Histone acetylation and reactive oxygen species are involved in the preprophase arrest induced by sodium butyrate in maize roots. Protoplasma 2016, 254, 167–179. [Google Scholar] [CrossRef]
- Hu, Y.; Qin, F.; Huang, L.; Sun, Q.; Li, C.; Zhao, Y.; Zhou, D.-X. Rice histone deacetylase genes display specific expression patterns and developmental functions. Biochem. Biophys. Res. Commun. 2009, 388, 266–271. [Google Scholar] [CrossRef]
- Tian, L.; Chen, Z.J. Blocking histone deacetylation in Arabidopsis induces pleiotropic effects on plant gene regulation and development. Proc. Natl. Acad. Sci. USA 2000, 98, 200–205. [Google Scholar] [CrossRef]
- Jang, I.-C.; Pahk, Y.-M.; Song, S.I.; Kwon, H.J.; Nahm, B.H.; Kim, J.-K. Structure and expression of the rice class-I type histone deacetylase genes OsHDAC1-3: OsHDAC1 overexpression in transgenic plants leads to increased growth rate and altered architecture. Plant J. 2003, 33, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Forestan, C.; Farinati, S.; Rouster, J.; Lassagne, H.; Lauria, M.; Ferro, N.D.; Varotto, S. Control of Maize Vegetative and Reproductive Development, Fertility, and rRNAs Silencing by HISTONE DEACETYLASE 108. Genetics 2018, 208, 1443–1466. [Google Scholar] [CrossRef] [PubMed]
- Rossi, V.; Locatelli, S.; Varotto, S.; Donn, G.; Pirona, R.; Henderson, D.A.; Hartings, H.; Motto, M. Maize Histone Deacetylase hda101 Is Involved in Plant Development, Gene Transcription, and Sequence-Specific Modulation of Histone Modification of Genes and Repeats. Plant Cell 2007, 19, 1145–1162. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Ishikawa, T.; Watanabe, K.; Terakura, S.; Iwakawa, H.; Okada, K.; Machida, C.; Machida, Y. Histone Deacetylases and ASYMMETRIC LEAVES2 Are Involved in the Establishment of Polarity in Leaves of Arabidopsis. Plant Cell 2007, 19, 445–457. [Google Scholar] [CrossRef]
- He, Y.; Michaels, S.D.; Amasino, R.M. Regulation of Flowering Time by Histone Acetylation in Arabidopsis. Science 2003, 302, 1751–1754. [Google Scholar] [CrossRef]
- Gu, X.; Jiang, D.; Yang, W.; Jacob, Y.; Michaels, S.D.; He, Y. Arabidopsis homologs of retinoblastoma-associated protein 46/48 associate with a histone deacetylase to act redundantly in chromatin silencing. PLoS Genet. 2011, 7, e1002366. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Huang, L.; Xu, C.; Zhao, Y.; Zhou, D.-X. Altered Levels of Histone Deacetylase OsHDT1 Affect Differential Gene Expression Patterns in Hybrid Rice. PLoS ONE 2011, 6, e21789. [Google Scholar] [CrossRef]
- Yu, C.-W.; Liu, X.; Luo, M.; Chen, C.; Lin, X.; Tian, G.; Lu, Q.; Cui, Y.; Wu, K. HISTONE DEACETYLASE6 Interacts with FLOWERING LOCUS D and Regulates Flowering in Arabidopsis. Plant Physiol. 2011, 156, 173–184. [Google Scholar] [CrossRef]
- Futamura, N.; Mori, H.; Kouchi, H.; Shinohara, K. Male Flower-Specific Expression of Genes for Polygalacturonase, Pectin Methylesterase and -1,3-Glucanase in a Dioecious Willow (Salix gilgiana Seemen). Plant Cell Physiol. 2000, 41, 16–26. [Google Scholar] [CrossRef]
- Wilson, Z.A.; Song, J.; Taylor, B.; Yang, C. The final split: The regulation of anther dehiscence. J. Exp. Bot. 2011, 62, 1633–1649. [Google Scholar] [CrossRef]
- Wu, C.-T.; Morris, J.R. Genes, Genetics, and Epigenetics: A Correspondence. Science 2001, 293, 1103–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Treatment | Description of the Treatments |
|---|---|
| T1 (Control) | Control: pre-soaking and incubation in DMSO |
| T2 | Pre-soaking for 1 h followed by 3 days of incubation in 0.4 µM TSA. |
| T3 | Pre-soaking for 1 h followed by 5 days of incubation in 0.4 µM TSA. |
| T4 | Pre-soaking for 1 h followed by 3 days of incubation in 1.2 µM TSA. |
| T5 | Pre-soaking for 1 h followed by 5 days of incubation in 1.2 µM TSA. |
| Treatments | FG (%) | Mean Root Length (cm) | Mean Shoot Length (cm) | DFFs | % Increase in DFFs |
|---|---|---|---|---|---|
| Control | 88 | 12.77 | 19.15 | 49.22 | 0.00 |
| T1 | 61 | 9.51 | 13.23 | 51.58 | 4.79 |
| T2 | 43 | 8.12 | 10.06 | 55.77 | 13.30 |
| T3 | 51 | 10.85 | 17.25 | 51.83 | 5.30 |
| T4 | 37 | 5.66 | 7.02 | 58.27 | 18.38 |
| CD (p = 0.05) | 4.90 | 1.32 | 3.05 | 1.11 |
| Treatments | Average Number of Pollens/Microscopic Slide | % Decrease in Total Pollen Production | Average Number of Fertile Pollens | Average Number of Sterile Pollens | Average Pollen Sterility (%) |
|---|---|---|---|---|---|
| T1 | 80 | 14.89 | 60 | 20 | 25.00 |
| T2 | 69 | 26.59 | 40 | 19 | 27.53 |
| T3 | 54 | 42.55 | 33 | 21 | 61.11 |
| T4 | 48 | 48.93 | 15 | 33 | 68.75 |
| Control | 94 | 0.00 | 93 | 1 | 1.06 |
| CD | 2.52 | - | 1.48 | 0.87 | 0.08 |
| Treatment | PH | NR | NL | NS | TWT |
|---|---|---|---|---|---|
| T1 | 50.50 | 5.45 | 5.36 | 31.55 | 5.74 |
| T2 | 39.38 | 6.11 | 5.70 | 45.77 | 4.77 |
| T3 | 55.91 | 5.40 | 5.11 | 50.87 | 6.84 |
| T4 | 49.33 | 7.11 | 6.88 | 37.86 | 3.91 |
| Control | 767.92 | 5.00 | 5.00 | 56.0 | 8.15 |
| CD (p = 0.05) | 8.50 | 2.04 | 1.77 | 3.76 | 1.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasikumar, S.; Marulasiddappa Dushyanthakumar, B.; Sridhara, S.; Adivappar, N.; Babu Bheemanapalli Nagraja, H.; M. El-Shehawi, A.; Aloufi, S.; Alqurashi, M.; Elansary, H.O.; Elhindi, K.M.; et al. Phenotypic Plasticity in Morphological Traits of Abelmoschus esculentus L. Induced by Histone Deacetylase Inhibitor, Trichostatin A. Agronomy 2022, 12, 2247. https://doi.org/10.3390/agronomy12102247
Sasikumar S, Marulasiddappa Dushyanthakumar B, Sridhara S, Adivappar N, Babu Bheemanapalli Nagraja H, M. El-Shehawi A, Aloufi S, Alqurashi M, Elansary HO, Elhindi KM, et al. Phenotypic Plasticity in Morphological Traits of Abelmoschus esculentus L. Induced by Histone Deacetylase Inhibitor, Trichostatin A. Agronomy. 2022; 12(10):2247. https://doi.org/10.3390/agronomy12102247
Chicago/Turabian StyleSasikumar, Sasipriya, Banur Marulasiddappa Dushyanthakumar, Shankarappa Sridhara, Nagarajappa Adivappar, Harish Babu Bheemanapalli Nagraja, Ahmed M. El-Shehawi, Salman Aloufi, Mohammed Alqurashi, Hosam O. Elansary, Khalid M. Elhindi, and et al. 2022. "Phenotypic Plasticity in Morphological Traits of Abelmoschus esculentus L. Induced by Histone Deacetylase Inhibitor, Trichostatin A" Agronomy 12, no. 10: 2247. https://doi.org/10.3390/agronomy12102247







