Chickpeas’ Tolerance of Drought and Heat: Current Knowledge and Next Steps
Abstract
:1. Introduction
2. Genes Associated with Heat and Drought Tolerance in Chickpea
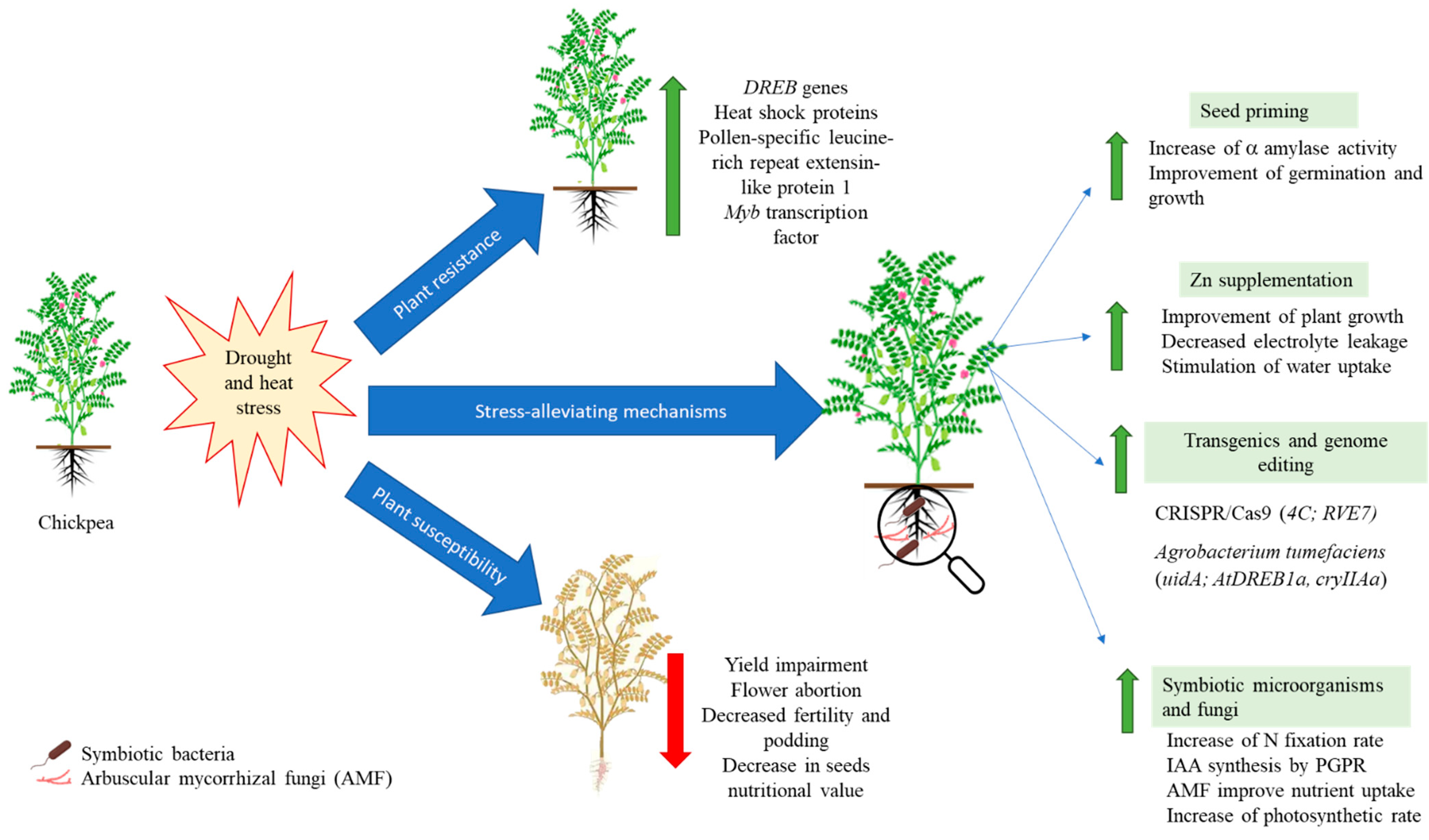
3. Chickpeas’ Fight against Heat and Drought Stress
3.1. Alleviation by Supplementation
3.2. Alleviation by Seed Priming
3.3. Role of Symbiotic Microorganisms and Fungi in Heat and Drought Alleviation
3.4. Transgenics and Genome Editing in Chickpea for Drought Tolerance
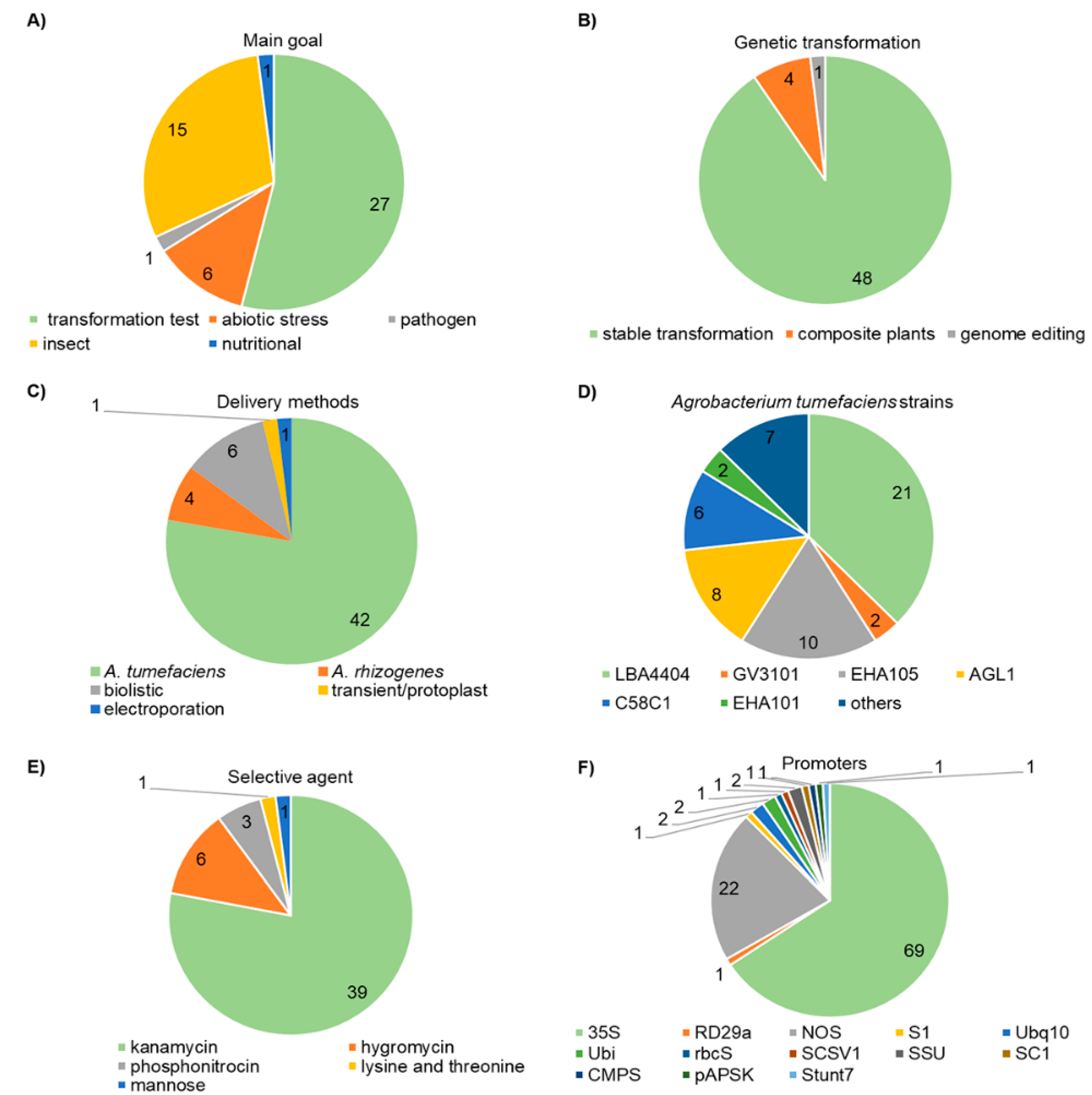
| Chickpea Cultivar | Delivery Method | Selectable Marker Gene | Promoter | Selective Agent | Reporter Protein | Target Gene | Plant Tissue | Improved Trait | TE (%) |
|---|---|---|---|---|---|---|---|---|---|
| ICCV2, ICCV10, ICCV92944, ICCV37, JAKI9218, and JG11 [122] | A. tumefaciens strain LBA4404 | nptII | 35S::uidA 35S::nptII | kanamycin | GUS | uidA | embryonic axes | genetic transformation test | 4.6 to 8.6 |
| DCP 92-3 [103] | A. tumefaciens strain GV3101 | nptII | rd29a::AtDREB1a 35S::nptII | kanamycin | unused | AtDREB1a | cotyledons with half embryonic axes | tolerance to water deficit | 0.1 |
| ICC283 and/or ICC8261 [102] | DNA-free CRISPR/Cas9 NHEJ | unused | Cas9::NLS gRNA | unused | unused | 4CL RVE7 | protoplast | genome editing test and drought tolerance improvement | non-informed |
| ICCV89314 [123] | A. tumefaciens strain EHA105 | nptII | NOS::nptII 35S::uidA | kanamycin | GUS | uidA | plumular meristem | genetic transformation test | 44 |
| HatTrick [105] | A. tumefaciens strain AGL1 | nptII | 35S::uidA S1::nptII 35S::GmFerritin NOS::CaNas2 NOS::OsNas2 | kanamycin | GUS | GmFerritin, AtBAG4, TlBAG, CaNas2, and OsNas2 | half-embryos | stress tolerance and grains biofortification | 0.66 to 2.1 |
| ICC4958, BDG2 56, ICC17258, ICC1885, ICC8261, and local varieties [124] | A. rhizogenes strain R1000, ARqua1, and MSU440 | nptII | 35S::DsRed Ubq10::DsRed 35S::uidA 35S::nptII Ubq10::mCherry others | kanamycin | GUS mCherry DsRed GFP | several genes | seedlings | genetic transformation test | 50 |
| Annigeri, C235, CPS 1, JG-62, K850, Vijay, and WR-315 [125] | A. rhizogenes strain K599 | unused | 35S:AtTT2::GFP | unused | GFP | AtTT2 | chickpea hairy roots | resistance to pathogen | 72.5 to 73.3 23.5 to 61.6 |
| Annigeri 1 [109] | A. tumefaciens strain EHA105 | nptII | 35S::cryIIAa NOS::nptII | kanamycin | unused | cryIIAa | embryonic axes | resistance to insect | 6.62 to 16.12 |
| C235 [126] | A. tumefaciens strain EHA105 | nptII | 35S::nptII 35S::uidA | kanamycin | GUS | uidA | apical meristem explants | genetic transformation test | 1.2 |
| non-informed [106] | A. tumefaciens strain LBA4404 | nptII | 35S:CaHDZ12 35S:CaWRKY70 35S::uidA NOS::nptII | kanamycin | GUS | CaHDZ12 CaWRKY70 | non-informed | abiotic stress tolerance | non-informed |
| DCP92-3 [103] | A. tumefaciens strain EHA105 | nptII | 35S::cry1Aabc NOS::nptII | kanamycin | unused | cry1Aabc | decoated seeds | resistance to insect | 0.076 |
| ICCV89314 [112] | A. tumefaciens strain AGL1 | nptII | Ubi::cry1Ac 35S::cry1Ac rbcS::cry1Ac 35S::nptII 35S::uidA | kanamycin | GUS | cry1Ac | non-informed | resistance to insect | 0.8 to 1.72 |
| ICCV-2 [127] | A. tumefaciens strain C58C1 | hptII | 35S::hptII 35S::uidA | hygromycin | GUS | uidA | cotyledonary node | genetic transformation test | 2.3 |
| C235 and HC1 [113] | A. tumefaciens strain LBA4404 | nptII | 35S::cry1Ac NOS::nptII | kanamycin | unused | cry1Ac | soaking sterilized seeds | resistance to insect | 13.4 to 41 |
| C235, BG 256, P362, and P372 [128] | A. tumefaciens strain LBA4404 | nptII | 35S::uidA NOS::nptII | kanamycin | GUS | uidA | immature cotyledon | genetic transformation test | 1.6 to 2.08 |
| Bch-4 and Bch-5 [129] | A. tumefaciens strain LBA4404 | nptII | NOS::nptII 35S::uidA | kanamycin | GUS | uidA | embryonic axes | genetic transformation test | non-informed |
| C235 [130] | A. tumefaciens strain EHA105 | unused | 35S::cryIAa3 | Not used | unused | cryIAa3 | soaking sterilized seeds | resistance to insect | non-informed |
| two kabuli and two desi [131] | biolistic | nptII | NOS::nptII | kanamycin | GUS | uidA | embryonic axes | genetic transformation test | non confirmed |
| P-362 [111] | A. tumefaciens strain LBA4404 | nptII | 35S::uidA 35S::cry1Ac | kanamycin | GUS | cry1Ac | callus derived from mature embryonic axes | resistance to insect | 3.6 |
| Jimbour [132] | A. tumefaciens strain AGL1 | PAT/bar nptII | 35S::uidA 35S::PAT/bar SCSV1::nptII SSU::cry1Ac | Phosphinothricin kanamycin | GUS | uidA nptII | embryonic axes | genetic transformation test | 0.37 to 4.3 |
| Annigerig [107] | A. tumefaciens strain LBA4404 | hptII | 35S::hptII 35S::P5CS 35S::uidA | hygromycin | GUS | P5CS | cotyledonary nodes | salt tolerance improvement | non-informed |
| Semsen and ICCV 89314 [114] | A. tumefaciens strain AGL1 | nptII | SSU::cry2Aa SC1::nptII | kanamycin | unused | cry2Aa | embryonic axes with half of the cotyledon | resistance to insect | 0.3 |
| Chaffa, PG12, ICCC37, and ICCC32 [115] | biolistic and A. tumefaciens strain LBA4404 | nptII | NOS::nptII 2x35S:AMV:: cryIAc::uidA | kanamycin | GUS | cryIAc | stems, epicotyls, and embryonal axes | resistance to caterpillar | 5 to 16 |
| Pusa-256, KWR-108, Pusa-1003, and non-informed local lines [133] | A. tumefaciens strain EHA105, AGL1, and LBA4404 | hptII | 35S::uidA 35S::hptII | hygromycin | GUS | uidA | cotyledonary node-derived calli and embryo axes | resistance to insect | 0.11 to 25.5 |
| C235 [134] | A. tumefaciens strain C58C1 | nptII | 35S::P5CSF129A 35S::nptII::uidA | kanamycin | GUS | P5CSF129A | axillary meristem | drought tolerance improvement | 70 |
| ICCV 89314 [116] | A. tumefaciens strain AGL1 | nptII | 35S::ASAL 35S::uidA 35S::nptII | kanamycin | GUS | ASAL | embryonic axes with half of the cotyledon | resistance to insect | 0.066 |
| C235 [134] | A. tumefaciens strain GV3101 | pmi | CMPS::pmi | mannose | unused | pmi | embryonic axes | genetic transformation test | 3 |
| Gökçe, Er, Akçin, Uzunlu, and Küsmen [135] | A. tumefaciens strain C58C1, EHA105, and KYRT1 | nptII | NOS::nptII 35S::uidA | kanamycin | GUS | uidA | embryonic axes | genetic transformation test | non-informed |
| ICC10943 and ICC10386 [136] | sonication and A. tumefaciens strain LBA4404 | hptII | 35S::CS::uidA 35S::hptII | hygromycin | GUS | uidA | embryonic axes | genetic transformation test | 9 to 26 |
| C235, BG 256, Pusa 362, and Pusa 372 [137] | A. tumefaciens strain GV2260, GV3850, LBA4404, and EHA105 | nptII | ?::uidA ?::uidA | kanamycin | GUS | uidA | cotyledonary nodes | genetic transformation test | 1.12 |
| C235 and HC1 [138] | A. tumefaciens strain LBA4404 | hptII | 35S::hptII Ubi::cry1Ab ?::cry1Ac | hygromycin | unused | cry1Ab and cry1Ac | embryonic axes | resistance to insect | 4.92 to 7.7 |
| K850 [119] | A. tumefaciens strain LBA4404 | nptII | pAPSK::αAI1 35S::nptII 35S::uidA | kanamycin | GUS | α-amylase inhibitor | embryonic axes | resistance to insect | 0.3 |
| C235 [139] | A. tumefaciens strain C58C1 | nptII | NOS::nptII 35S::uidA | kanamycin | unused | nptII | axillary meristem | genetic transformation test | 70 |
| C235, BG256, Pusa 362, and Pusa 372 [140] | A. tumefaciens strain LBA4404, EHA105, GV3850, and GV2260 | nptII | 35S::cry1Ac NOS::nptII 35S::uidA | kanamycin | GUS | cry1Ac | Cotyledonary nodes | resistance to insect | 0.32 to 1.12 |
| CDC Yuma [141] | A. tumefaciens strain EHA105 | nptII | 2x35S::uidA::nptII | kanamycin | GUS | uidA | embryonic axes | genetic transformation test | 1.3 |
| P-362, P-1043, and P-1042 [142] | biolistic and A. tumefaciens strain EHA101 | PAT/bar, nptII, and desensitized AK gene | 35S::PAT/bar 35S::TP::AK 35S::uidA NOS::nptII | kanamycin, lysine and threonine, phosphonitrocin | GUS | uidA AK | embryonic axes with half of the cotyledon | genetic transformation test | 0.5 to 1.3 |
| H208, ICCL87322, K850, Annigeri, and ICCV5 [143] | A. tumefaciens strain AGL1, C58C1, and LBA4404 | PAT/bar | 35S::PAT/bar 35S::uidA 35S::PGIP | phosphonitrocin | GUS | uidA PGIP | embryonic axes | genetic transformation test | 2 to 13.3 |
| Gökçe, Akçin 91, and Izmır 92 [144] | A. rhizogenes strain 15834 | nptII | ?::nptII | kanamycin | unused | nptII | growing tender shoots | genetic transformation test | 5 to 80 |
| Semsen [118] | A. tumefaciens strain AGL1 | nptII | Stunt7::nptII | kanamycin | unused | αAI1 | embryonic axes with half of the cotyledon | resistance to insect | 0.56 |
| C235, BG256, Pusa 362, and Pusa 372 [145] | biolistc and A. tumefaciens strain LBA4404 | nptII hptII | NOS::nptII 35S::uidA 35S::hptII | kanamycin hygromicyn | GUS | uidA | embryonic axes and cotyledonary axes | genetic transformation test | 0.05 to 0.8 |
| PG1, PG12, and Chafa [146] | A. tumefaciens strain C58C1, GV2260 and EHA101 | PAT/bar nptII | 35S::uidA 35S::PAT/bar NOS::nptII | kanamycin or phosphonitrocin | GUS | uidA | embryonic axes | genetic transformation test | 0.2 to 1.5 |
| 6153 and CM72 [147] | biolistic | nptII | 35S::uidA NOS::nptII | kanamycin | GUS | uidA | hypocotyl segments | genetic transformation test | non-informed |
| ICCV1 and ICCV6 [148] | biolistic | nptII | 35S::cry1Ac ?::nptII | kanamycin | unused | cry1Ac | embryonic axes | resistance to insect | non-informed |
| Red chickpea, Canitez 87, and MB10 [149] | A. tumefaciens strain LBA4404 and A. rhizogenes strain 9402 | nptII | NOS::nptII 35S::uidA | kanamycin | GUS | uidA | embryonic axes | genetic transformation test | 6.4 to 12.7 5.3 to 10.4 |
| ICCV1 and ICCV6 [150] | A. tumefaciens strain LBA4404 | nptII | 35S::nptII 35S::uidA | kanamycin | GUS | uidA | embryonic axes | genetic transformation test | 1.16 to 1.96 |
| ICC4918 [151] | A. tumefaciens strain LBA4404 | nptII | NOS::nptII 35S::uidA | kanamycin | GUS | uidA | immature cotyledon | genetic transformation test | non-informed |
| Italian cultivars [152] | A. tumefaciens strain LBA4404 | nptII | NOS::nptII 35S::uidA | kanamycin | GUS | uidA | embryonic axes | genetic transformation test | 4 |
| non-informed [152] | A. tumefaciens strain LBA4404 | nptII | NOS::nptII 35S::uidA | kanamycin | GUS | nptII and uidA | embryo axes | genetic transformation test | non-informed |
| Pusa256 [8] | A. tumefaciens strain R1601 | nptII | ?::nptII | kanamycin | unused | nptII | leaf and stem explants | genetic transformation test | non-informed |
4. Next Steps—Breeding Approaches
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NASA Earth Observatory. A July of Extremes. 2022. Available online: https://earthobservatory.nasa.gov/images/150152/a-july-of-extremes (accessed on 1 August 2022).
- Awasthi, R.; Kaushal, N.; Vadez, V.; Turner, N.C.; Berger, J.; Siddique, K.H.; Nayyar, H. Individual and combined effects of transient drought and heat stress on carbon assimilation and seed filling in chickpea. Funct. Plant Biol. 2014, 41, 1148–1167. [Google Scholar]
- Ray, D.K.; Gerber, J.S.; MacDonald, G.K.; West, P.C. Climate variation explains a third of global crop yield variability. Nat. Comm. 2015, 6, 5989. [Google Scholar] [CrossRef]
- Snowdon, R.J.; Wittkop, B.; Chen, T.W.; Stahl, A. Crop adaptation to climate change as a consequence of long-term breeding. Theor. Appl. Genet. 2021, 134, 1613–1623. [Google Scholar] [CrossRef]
- Cooper, M.; Voss-Fels, K.P.; Messina, C.D.; Tang, T.; Hammer, G.L. Tackling G × E × M interactions to close on-farm yield-gaps: Creating novel pathways for crop improvement by predicting contributions of genetics and management to crop productivity. Theor. Appl. Genet. 2021, 134, 1625–1644. [Google Scholar] [CrossRef]
- Fernie, A.R.; Bachem, C.W.B.; Helariutta, Y.; Neuhaus, H.E.; Prat, S.; Ruan, Y.L.; Stitt, M.; Sweetlove, L.J.; Tegeder, M.; Wahl, V.; et al. Synchronization of developmental, molecular and metabolic aspects of source-sink interactions. Nat. Plants 2020, 6, 55–66. [Google Scholar] [CrossRef]
- Joshi, P.K.; Rao, P.P. Global pulses scenario: Status and outlook. Ann. N. Y. Acad. Sci. 2017, 1392, 6–17. [Google Scholar] [CrossRef]
- Mohapatra, T.; Sharma, R.P. Agrobacterium mediated genetic transformation of chickpea, Cicer arietinum L. Indian J. Exp. Biol. 1991, 29, 758–761. [Google Scholar]
- Kumar, S.; Thakur, P.; Kaushal, N.; Malik, J.A.; Gaur, P.; Nayyar, H. Effect of varying high temperatures during reproductive growth on reproductive function, oxidative stress and seed yield in chickpea genotypes differing in heat sensitivity. Arch. Agron. Soil Sci. 2013, 59, 823–843. [Google Scholar] [CrossRef]
- Kumari, P.; Rastogi, A.; Yadav, S. Effects of Heat stress and molecular mitigation approaches in orphan legume, Chickpea. Mol. Biol. Rep. 2020, 47, 4659–4670. [Google Scholar] [CrossRef]
- Rani, A.; Devi, P.; Jha, U.C.; Sharma, K.D.; Siddique, K.H.; Nayyar, H. Developing climate-resilient chickpea involving physiological and molecular approaches with a focus on temperature and drought stresses. Front. Plant Sci. 2020, 10, 1759. [Google Scholar] [CrossRef]
- Fang, X.; Turner, N.C.; Yan, G.; Li, F.; Siddique, K.H. Flower numbers, pod production, pollen viability, and pistil function are reduced and flower and pod abortion increased in chickpea (Cicer arietinum L.) under terminal drought. J. Exp. Bot. 2010, 61, 335–345. [Google Scholar] [CrossRef]
- Devasirvatham, V.; Gaur, P.; Raju, T.; Trethowan, R.; Tan, D. Field response of chickpea (Cicer arietinum L.) to high temperature. Field Crops Res. 2015, 172, 59–71. [Google Scholar] [CrossRef] [Green Version]
- Devasirvatham, V.; Tan, D.K.Y. Impact of high temperature and drought stresses on chickpea production. Agronomy 2018, 8, 145. [Google Scholar] [CrossRef]
- Tardieu, F.; Tuberosa, R. Dissection and modelling of abiotic stress tolerance in plants. Curr. Opin. Plant Biol. 2010, 13, 206–212. [Google Scholar] [CrossRef]
- Leport, L.; Turner, N.C.; French, R.J.; Barr, M.D.; Duda, R.; Daves, S.L.; Tennant, D.; Siddique, K.H.M. Physiological responses of chickpea genotypes to terminal drought in a Mediterranean-type environment. Eur. J. Agron. 1999, 11, 279–291. [Google Scholar] [CrossRef]
- Korbu, L.; Fikre, A.; Tesfaye, K.; Funga, A.; Bekele, D.; Ojiewo, C.O. Response of chickpea to varying moisture stress conditions in Ethiopia. Agrosyst. Geosci. Environ. 2022, 5, e20234. [Google Scholar] [CrossRef]
- Anamul Karim, A.N.M.; Kumer Sarker, U.; Khairul Hasan, A.; Islam, N.; Romij Uddin, M. Physiological and biochemical responses of chickpea (Cicer arietinum L.) genotypes to different moisture stresses. Turk. J. Field Crops 2022, 27, 1–9. [Google Scholar]
- Nayyar, H.; Kaur, S.; Singh, S.; Upadhyaya, H.D. Differential sensitivity of Desi (small-seeded) and Kabuli (large-seeded) chickpea genotypes to water stress during seed filling: Effects on accumulation of seed reserves and yield. J. Sci. Food Agric. 2006, 86, 2076–2082. [Google Scholar] [CrossRef]
- Takagi, H.; Tamiru, M.; Abe, A.; Yoshida, K.; Uemura, A.; Yaegashi, H.; Obara, T.; Oikawa, K.; Utsushi, H.; Kanzaki, E.; et al. MutMap accelerates breeding of a salt-tolerant rice cultivar. Nat. Biotechnol. 2015, 33, 445–449. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; Liu, H.; Zhao, J.; Li, T.; Hou, J.; Zhang, X.; Hao, C. Global status of 47 major wheat loci controlling yield, quality, adaptation and stress resistance selected over the last century. BMC Plant Biol. 2019, 19, 5. [Google Scholar] [CrossRef]
- Christopher, J.T.; Christopher, M.J.; Borrell, A.K.; Fletcher, S.; Chenu, K. Stay-green traits to improve wheat adaptation in well- watered and water-limited environments. J. Exp. Bot. 2016, 67, 5159–5172. [Google Scholar] [CrossRef] [PubMed]
- Maphosa, L.; Richards, M.F.; Norton, S.L.; Nguyen, G.N. Breeding for abiotic stress adaptation in chickpea (Cicer arietinum L.): A comprehensive review. Crops Breed. Genet. Genom. 2020, 4, e200015. [Google Scholar]
- Varshney, R.K.; Roorkiwal, M.; Sun, S.; Bajaj, P.; Chitikineni, A.; Thudi, M.; Singh, N.P.; Du, X.; Upadhyaya, H.D.; Khan, A.W.; et al. A chickpea genetic variation map based on the sequencing of 3366 genomes. Nature 2021, 599, 622–627. [Google Scholar] [CrossRef]
- Roorkiwal, M.; Bharadwaj, C.; Barmukh, R.; Dixit, G.P.; Thudi, M.; Gaur, P.M.; Chaturvedi, S.K.; Fikre, A.; Hamwieh, A.; Kumar, S.; et al. Integrating genomics for chickpea improvement: Achievements and opportunities. Theor. Appl. Genet. 2020, 133, 1703–1720. [Google Scholar] [CrossRef] [Green Version]
- Jha, U.C.; Nayyar, H.; Palakurthi, R.; Jha, R.; Valluri, V.; Bajaj, P.; Chitikineni, A.; Singh, N.P.; Varshney, R.K.; Thudi, M. Major QTLs and potential candidate genes for heat stress tolerance identified in chickpea (Cicer arietinum L.). Front. Plant Sci. 2021, 12, 1241. [Google Scholar] [CrossRef] [PubMed]
- Deokar, A.A.; Taran, B. Genome-wide analysis of the aquaporin gene family in chickpea (Cicer arietinum L.). Front. Plant Sci. 2016, 7, 1802. [Google Scholar] [CrossRef]
- Deokar, A.A.; Kondawar, V.; Kohli, D.; Aslam, M.; Jain, P.K.; Karuppayil, S.M.; Varshney, R.K.; Srinivasan, R. The CarERF genes in chickpea (Cicer arietinum L.) and the identification of CarERF116 as abiotic stress responsive transcription factor. Funct. Integr. Genom. 2015, 15, 27–46. [Google Scholar] [CrossRef]
- Gu, H.; Jia, Y.; Wang, X.; Chen, Q.; Shi, S.; Ma, L.; Zhang, J.; Zhang, H.; Ma, H. Identification and characterization of a LEA family gene CarLEA4 from chickpea (Cicer arietinum L.). Mol. Biol. Rep. 2012, 39, 3565–3572. [Google Scholar] [CrossRef]
- Thudi, M.; Upadhyaya, H.D.; Rathore, A.; Gaur, P.M.; Krishnamurthy, L.; Roorkiwal, M.; Nayak, S.N.; Chaturvedi, S.K.; Basu, P.S.; Gangarao, N.V.P.R.; et al. Genetic dissection of drought and heat tolerance in chickpea through genome-wide and candidate gene-based association mapping approaches. PLoS ONE 2014, 9, e96758. [Google Scholar]
- Roorkiwal, M.; Nayak, S.N.; Thudi, M.; Upadhyaya, H.D.; Brunel, D.; Mournet, P.; This, D.; Sharma, P.C.; Varshney, R.K. Allele diversity for abiotic stress responsive candidate genes in chickpea reference set using gene based SNP markers. Front. Plant Sci. 2014, 5, 248. [Google Scholar] [CrossRef]
- Ullah, M.; Qian, N.P.M.; Yannarelli, G.; Akbar, A. Heat shock protein 20 promotes sirtuin 1-dependent cell proliferation in induced pluripotent stem cells. World J. Stem Cells 2021, 13, 659. [Google Scholar] [CrossRef]
- Ullah, A.; Farooq, M.; Rehman, A.; Hussain, M.; Siddique, K.H. Zinc nutrition in chickpea (Cicer arietinum): A review. Crops Pasture Sci. 2020, 71, 199–218. [Google Scholar] [CrossRef]
- Ullah, S.; Khan, J.; Hayat, K.; Abdelfattah Elateeq, A.; Salam, U.; Yu, B.; Ma, Y.; Wang, H.; Tang, Z.H. Comparative study of growth, cadmium accumulation and tolerance of three chickpea (Cicer arietinum L.) cultivars. Plants 2020, 9, 310. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V. ROS-activated ion channels in plants: Biophysical characteristics, physiological functions and molecular nature. Int. J. Mol. Sci. 2018, 19, 1263. [Google Scholar] [CrossRef]
- Gupta, N.; Ram, H.; Kumar, B. Mechanism of Zinc absorption in plants: Uptake, transport, translocation and accumulation. Rev. Environ. Sci. Biotechnol. 2016, 15, 89–109. [Google Scholar] [CrossRef]
- Karim, M.; Zhang, Y.Q.; Zhao, R.R.; Chen, X.P.; Zhang, F.S.; Zou, C.Q. Alleviation of drought stress in winter wheat by late foliar application of zinc, boron, and manganese. J. Plant Nutr. Soil Sci. 2012, 175, 142–151. [Google Scholar] [CrossRef]
- Hera, M.H.R.; Hossain, M.; Paul, A.K. E_ect of foliar zinc spray on growth and yield of heat tolerant wheat under water stress. Int. J. Biol. Environ. Eng. 2018, 1, 10–16. [Google Scholar]
- Umair Hassan, M.; Aamer, M.; Umer Chattha, M.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y.; et al. The critical role of zinc in plants facing the drought stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Tului, V.; Janmohammadi, M.; Abbasi, A.; Vahdati-Khajeh, S.; Nouraein, M. Influence of iron, zinc and bimetallic Zn-Fe nanoparticles on growth and biochemical characteristics in chickpea (Cicer arietinum) cultivars. Poljopr. Sumar. 2021, 67, 179–193. [Google Scholar]
- Venugopalan, V.; Nath, R.; Sengupta, K.; Pal, A.; Banerjee, S.; Banerjee, P.; Chandran, M.; Roy, S.; Sharma, L.; Hossain, A.; et al. Foliar Spray of Micronutrients Alleviates Heat and Moisture Stress in Lentil (Lens culinaris Medik) Grown Under Rainfed Field Conditions. Front. Plant Sci. 2022, 13, 847743. [Google Scholar] [CrossRef]
- Hadi, M.R.H.S.; Bazargani, P.; Darzi, M.T. Effects of irrigation treatment and zinc foliar application on yield and yield components of chickpea (Cicer arietinum L.). IJFAS 2013, 2, 720–724. [Google Scholar]
- El-Beltagi, H.S.; Mohamed, H.I.; Sofy, M.R. Role of ascorbic acid, glutathione and proline applied as singly or in sequence combination in improving chickpea plant through physiological change and antioxidant defense under different levels of irrigation intervals. Molecules 2020, 25, 1702. [Google Scholar] [CrossRef] [PubMed]
- Mladenov, V.; Fotopoulos, V.; Kaiserli, E.; Karalija, E.; Maury, S.; Baranek, M.; Segal, N.; Testillano, P.S.; Vassileva, V.; Pinto, G.; et al. Deciphering the epigenetic alphabet involved in transgenerational stress memory in crops. Int. J. Mol. Sci. 2021, 22, 7118. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Kanwal, H.; Kausar, A.; Ilyas, A.; Akhter, N.; Ilyas, M.; Nisa, Z.; Khalid, H. Seed priming with zinc modulate growth, pigments and yield of chickpea (Cicer arietinum L.) under water deficit conditions. Appl. Ecol. Environ. Res. 2019, 17, 147–160. [Google Scholar] [CrossRef]
- Farooq, M.; Almamari, S.A.D.; Rehman, A.; Al-Busaidi, W.M.; Wahid, A.; Al-Ghamdi, S.S. Morphological, physiological and biochemical aspects of zinc seed priming-induced drought tolerance in faba bean. Sci. Hortic. 2021, 281, 109894. [Google Scholar] [CrossRef]
- Shariatmadari, M.H.; Parsa, M.; Nezami, A.; Kafi, M. The effects of hormonal priming on emergence, growth and yield of chickpea under drought stress in glasshouse and field. Biosci. Res. 2017, 14, 34–41. [Google Scholar]
- Ahmad, P.; Raja, V.; Ashraf, M.; Wijaya, L.; Bajguz, A.; Alyemeni, M.N. Jasmonic acid (JA) and gibberellic acid (GA3) mitigated Cd-toxicity in chickpea plants through restricted cd uptake and oxidative stress management. Sci. Rep. 2021, 11, 19768. [Google Scholar] [CrossRef]
- Mazid, M. Seed priming application of gibberellic acid on growth, biochemical, yield attributes and protein status of chickpea (Cicer arietinum L. cv. DCP 92-3). IJGEB 2014, 5, 17–22. [Google Scholar]
- Aziz, T.; Pekşen, E. Seed priming with gibberellic acid rescues chickpea (Cicer arietinum L.) from chilling stress. Acta Physiol. Plant 2020, 42, 139. [Google Scholar] [CrossRef]
- Kaur, S.; Gupta, A.K.; Kaur, N. Effect of osmo-and hydropriming of chickpea seeds on seedling growth and carbohydrate metabolism under water deficit stress. Plant Growth Regul. 2002, 37, 17–22. [Google Scholar] [CrossRef]
- Zare, I.; Mohammadi, G.; Sohrabi, Y.; Kahrizi, D.; Khah, E.M.; Yari, K. Effect of different hydropriming times on the quantitative and qualitative characteristics of chickpea (Cicer arietinum L.). Afr. J. Biotechnol. 2011, 10, 14844–14850. [Google Scholar] [CrossRef]
- Elkoca, E.; Haliloglu, K.; Esitken, A.; Ercisli, S. Hydro-and osmopriming improve chickpea germination. Acta Agric. Scand B Soil Plant Sci. 2007, 57, 193–200. [Google Scholar] [CrossRef]
- Novák, V. Physiological drought—How to quantify it? In Bioclimatology and Natural Hazards; Springer: Dordrecht, The Netherlands, 2009; pp. 89–95. [Google Scholar]
- Asadi, M.; Heidari, M.A.; Kazemi, M.; Filinejad, A.R. Salicylic acid induced changes in some physiological parameters in chickpea (Cicer arietinum L.) under salt stress. J. Agric. Technol. 2013, 9, 311–316. [Google Scholar]
- Ceritoğlu, M.; Erman, M. Mitigation of salinity stress on chickpea germination by salicylic acid priming. Uluslararası Tarım ve Yaban Hayatı Bilimleri Dergisi 2020, 6, 582–591. [Google Scholar] [CrossRef]
- Mehboob, N.; Minhas, W.A.; Naeem, M.; Yasir, T.A.; Naveed, M.; Farooq, S.; Hussain, M. Seed priming with boron and Bacillus sp. MN54 inoculation improves productivity and grain boron concentration of chickpea. Crops Pasture Sci. 2022, 73, 494–502. [Google Scholar] [CrossRef]
- Hussain, M.; Mehboob, N.; Naveed, M.; Shehzadi, K.; Yasir, T.A. Optimizing boron seed coating level and boron-tolerant bacteria for improving yield and biofortification of chickpea. J. Soil Sci. Plant Nutr. 2020, 20, 2471–2478. [Google Scholar] [CrossRef]
- Rafique, M.; Naveed, M.; Mustafa, A.; Akhtar, S.; Munawar, M.; Kaukab, S.; Ali, H.M.; Siddiqui, M.H.; Salem, M.Z. The combined effects of gibberellic acid and rhizobium on growth, yield and nutritional status in chickpea (Cicer arietinum L.). Agronomy 2021, 11, 105. [Google Scholar] [CrossRef]
- Kalra, N.; Suneja, P.; Mendiratta, N.; Gupta, N. Simulating the impact of climate change and its variability on growth and yield of crops. Clim. Change Environ. Sustain. 2013, 1, 11–19. [Google Scholar] [CrossRef]
- Brockwell, J.; Bottomley, P.J. Recent advances in inoculant technology and prospects for the future. Soil Biol. Biochem. 1995, 27, 683–697. [Google Scholar] [CrossRef]
- Prasuna, M.L. Biological studies on the effect of agrochemicals on nodulation of some cultivated legumes. J. Ind. Pollut. Control 2014, 30, 317–319. [Google Scholar]
- Kumar, N.; Srivastava, P.; Vishwakarma, K.; Kumar, R.; Kuppala, H.; Maheshwari, S.K.; Vats, S. The Rhizobium–Plant Symbiosis: State of the Art. In Plant Microbe Symbiosis; Varma, A., Tripathi, S., Prasad, R., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Sindhu, S.; Dahiya, A.; Gera, R.; Sindhu, S.S. Mitigation of abiotic stress in legume-nodulating rhizobia for sustainable crop production. Agric. Res. 2020, 9, 444–459. [Google Scholar] [CrossRef]
- Bhargava, Y.; Murthy, J.S.R.; Kumar, T.V.R.; Rao, M.N. Phenotypic, stress tolerance and plant growth promoting characteristics of rhizobial isolates from selected wild legumes of semiarid region, Tirupati, India. Adv. Microbiol. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Gebremedhin, W.; Assefa, F.; Thuita, M.; Masso, C. Nutritionally versatile, abiotic stress resistant and symbiotically effective chickpea (Cicer arietinum L.) root nodulating rhizobial isolates from eastern, southeastern and southern ethiopia. eJBio 2018, 14, 87–99. [Google Scholar]
- Khalid, R.; Zhang, X.X.; Hayat, R.; Ahmed, M. Molecular Characteristics of Rhizobia Isolated from Arachis hypogaea Grown under Stress Environment. Sustainability 2020, 12, 6259. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, J.P. Does plant-microbe interaction confer stress tolerance to plants: A review. Microbiol. Res. 2018, 207, 41–52. [Google Scholar] [CrossRef]
- Sati, D.; Pande, V.; Pandey, S.C.; Samant, M. Recent advances in PGPR and molecular mechanisms involved in drought stress resistance. J. Soil Sci. Plant Nutr. 2022, 1–19. [Google Scholar] [CrossRef]
- Chang, W.S.; Van de Mortel, M.; Nielsen, L.; de Guzman, G.N.; Li, X.; Halverson, L.J. Alginate production by Pseudomonas putida creates a hydrated microenvironment and contributes to biofilm architecture and stress tolerance under water limiting conditions. J. Bacteriol. 2007, 189, 8290–8299. [Google Scholar] [CrossRef]
- Chaudhary, T.; Gera, R.; Shukla, P. Deciphering the potential of Rhizobium pusense MB-17a, a plant growth-promoting root endophyte, and functional annotation of the genes involved in the metabolic pathway. Front. Bioeng. Biotechnol. 2021, 8, 617034. [Google Scholar] [CrossRef]
- Defez, R.; Andreozzi, A.; Bianco, C. The overproduction of indole 3 acetic acid IAA in endophytes upregulates nitrogen fixation in both bacterial cultures and inoculated rice plants. Microb. Ecol. 2017, 74, 441–452. [Google Scholar] [CrossRef]
- Vargas, L.; de Carvalho, T.L.G.; Ferreira, P.C.G.; Baldani, V.L.D.; Baldani, J.I.; Hemerly, A.S. Early responses of rice Oryza sativa L seedlings to inoculation with beneficial diazotrophic bacteria are dependent on plant and bacterial genotypes. Plant Soil 2012, 356, 127–137. [Google Scholar] [CrossRef]
- Ali, S.; Hayat, K.; Iqbal, A.; Xie, L. Implications of abscisic acid in the drought stress tolerance of plants. Agronomy 2020, 10, 1323. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, M.S.; Krishnamachari, V.; Payton, P.; Sun, Y.; Grimson, M.; Farag, M.A.; Ryu, C.M.; Allen, R.; Melo, I.S.; et al. Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 2007, 226, 839–851. [Google Scholar] [CrossRef]
- Santos, R.; Hérouart, D.; Puppo, A.; Touati, D. Critical protective role of bacterial superoxide dismutase in Rhizobium–legume symbiosis. Mol. Microbiol. 2000, 38, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Kumari, V.; Germida, J.; Vujanovic, V. Legume endosymbionts: Drought stress tolerance in second-generation chickpea (Cicer arietinum) seeds. J. Agron. Crops Sci. 2018, 204, 529–540. [Google Scholar] [CrossRef]
- Srivastava, S.; Chaudhry, V.; Mishra, A.; Chauhan, P.S.; Rehman, A.; Yadav, A.; Tuteja, N.; Nautiyal, C.S. Gene expression profiling through microarray analysis in Arabidopsis thaliana colonized by Pseudomonas putida MTCC5279, a plant growth promoting rhizobacterium. Plant Signal. Behav. 2012, 7, 235–245. [Google Scholar] [CrossRef]
- Davies, W.J.; Zhang, J.; Yang, J.; Dodd, I.C. Novel crop science to improve yield and resource use efficiency in water limited agriculture. J. Agric. Sci. 2010, 149, 123–131. [Google Scholar] [CrossRef]
- Mantri, N.L.; Ford, R.; Coram, T.E.; Pang, E.C. Transcriptional profiling of chickpea genes differentially regulated in response to high-salinity, cold and drought. BMC Genom. 2007, 8, 303. [Google Scholar] [CrossRef]
- Joseph, B.; Patra, R.R.; Lawrence, R. Characterization of plant growth promoting rhizobacteria associated with chickpea (Cicer arietinum L.). Int. J. Plant Prod. 2007, 1, 141–152. [Google Scholar]
- Ahmad, M.; Naseer, I.; Hussain, A.; Zahid Mumtaz, M.; Mustafa, A.; Hilger, T.; Ahmad Zahir, Z.; Xu, M. Appraising endophyte–plant symbiosis for improved growth, nodulation, nitrogen fixation and abiotic stress tolerance: An experimental investigation with chickpea (Cicer arietinum L.). Agronomy 2019, 9, 621. [Google Scholar] [CrossRef]
- Tiwari, S.; Lata, C.; Chauhan, P.S.; Nautiyal, C.S. Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol. Biochem. 2016, 99, 108–117. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Rahman, M.A.; Guo, J.; Kang, Z.; Babar, M. Comparative physiological and metabolic analysis reveals a complex mechanism involved in drought tolerance in chickpea (Cicer arietinum L.) induced by PGPR and PGRs. Sci. Rep. 2019, 9, 2097. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, Z.; Faizan, S.; Gulzar, B.; Hakeem, K.R. Inoculation of Rhizobium alleviates salinity stress through modulation of growth characteristics, physiological and biochemical attributes, stomatal activities and antioxidant defence in Cicer arietinum L. J. Plant Growth Regul. 2021, 40, 2148–2163. [Google Scholar] [CrossRef]
- Gaur, P.M.; Krishnamurthy, L.; Kashiwagi, J. Improving drought avoidance root traits in chickpea (Cicer arietinum L.)-current status of research at ICRISAT. Plant Prod. Sci. 2008, 11, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Nautiyal, C.S.; Srivastava, S.; Chauhan, P.S.; Seem, K.; Mishra, A.; Sopory, S.K. Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol. Biochem. 2013, 66, 1–9. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.J.; Shurigin, V.V.; Hashem, A.; Abd_Allah, E.F. Endophytic bacteria improve plant growth, symbiotic performance of chickpea (Cicer arietinum L.) and induce suppression of root rot caused by Fusarium solani under salt stress. Front. Microbiol. 2017, 8, 1887. [Google Scholar] [CrossRef]
- Benjelloun, I.; Thami Alami, I.; Douira, A.; Udupa, S.M. Phenotypic and genotypic diversity among symbiotic and non-symbiotic bacteria present in chickpea nodules in Morocco. Front. Microbiol. 2019, 10, 1885. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; Orozco-Mosqueda, M.D.C.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Glick, B.R.; Todorovic, B.; Czarny, J.; Cheng, Z.; Duan, J.; McConkey, B. Promotion of Plant Growth by Bacterial ACC Deaminase. Crit. Rev. Plant Sci. 2007, 26, 227–242. [Google Scholar] [CrossRef]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur. J. Plant Pathol. 2007, 119, 329–339. [Google Scholar] [CrossRef]
- Chernin, L.; Chet, I. Microbial Enzymes in the Biocontrol of Plant Pathogens and Pests. In Water Policy and Planning in a Variable and Changing Climate; Informa UK Limited: Colchester, UK, 2002; Volume 84, pp. 179–234. [Google Scholar]
- Prasanna, R.; Ramakrishnan, B.; Simranjit, K.; Ranjan, K.; Kanchan, A.; Hossain, F.; Nain, L. Cyanobacterial and rhizobial inoculation modulates the plant physiological attributes and nodule microbial communities of chickpea. Arch. Microbiol. 2017, 199, 1311–1323. [Google Scholar] [CrossRef]
- Neumann, E.; George, E. Nutrient uptake: The arbuscular mycorrhiza fungal symbiosis as a plant nutrient acquisition strategy. In Arbuscular Mycorrhizas: Physiology and Function; Springer: Dordrecht, The Netherlands, 2010; pp. 137–167. [Google Scholar]
- González-Guerrero, M.; Escudero, V.; Saéz, Á.; Tejada-Jiménez, M. Transition metal transport in plants and associated endosymbionts: Arbuscular mycorrhizal fungi and rhizobia. Front. Plant Sci. 2016, 7, 1088. [Google Scholar] [CrossRef] [PubMed]
- Golubkina, N.; Krivenkov, L.; Sekara, A.; Vasileva, V.; Tallarita, A.; Caruso, G. Prospects of arbuscular mycorrhizal fungi utilization in production of Allium plants. Plants 2020, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Moarrefzadeh, N.; Khateri, H.; Sharifi, R. The effect of some defense inducing volatile compounds against chickpea Ascochyta blight. Plant. Prot. Sci. J. Agric. 2021, 44, 43–58. [Google Scholar]
- Hashem, A.; Kumar, A.; Al-Dbass, A.M.; Alqarawi, A.A.; Al-Arjani, A.B.F.; Singh, G.; Farooq, M.; Abd_Allah, E.F. Arbuscular mycorrhizal fungi and biochar improves drought tolerance in chickpea. Saudi J. Biol. Sci. 2019, 26, 614–624. [Google Scholar] [CrossRef]
- Sohrabi, M.; Mohammadi, H.; Mohammadi, A.H. Influence of AM fungi, Glomus mosseae and Glomus intraradices on chickpea growth and root-rot disease caused by Fusarium solani f. sp. pisi under greenhouse conditions. J. Agric. Sci. Technol. A 2015, 17, 1919–1929. [Google Scholar]
- Marulanda, A.; Barea, J.M.; Azcón, R. Stimulation of plant growth and drought tolerance by native microorganisms (AM fungi and bacteria) from dry environments: Mechanisms related to bacterial effectiveness. J. Plant Growth Regul. 2009, 28, 115–124. [Google Scholar] [CrossRef]
- Badhan, S.; Ball, A.S.; Mantri, N. First report of CRISPR/Cas9 mediated DNA-free editing of 4CL and RVE7 genes in chickpea protoplasts. Int. J. Mol. Sci. 2021, 22, 396. [Google Scholar] [CrossRef]
- Das, A.; Parihar, A.K.; Barpete, S.; Kumar, S.; Gupta, S. Current perspectives on reducing the β-ODAP content and improving potential agronomic traits in grass pea (Lathyrus sativus L.). Front. Plant Sci. 2021, 12, 703275. [Google Scholar] [CrossRef]
- Choudhury, A.; Rajam, M.V. Genetic transformation of legumes: An update. Plant Cell Rep. 2021, 40, 1813–1830. [Google Scholar] [CrossRef]
- Das Bhowmik, S.S.; Cheng, A.Y.; Long, H.; Tan, G.Z.H.; Hoang, T.M.L.; Karbaschi, M.R.; Williams, B.; Higgins, T.J.V.; Mundree, S.G. Robust genetic transformation system to obtain non-chimeric transgenic chickpea. Front. Plant Sci. 2019, 10, 524. [Google Scholar] [CrossRef]
- Sen, S.; Chakraborty, J.; Ghosh, P.; Basu, D.; Das, S. Chickpea WRKY70 regulates the expression of a homeodomain-leucine zipper (HD-Zip) I transcription factor CaHDZ12, which confers abiotic stress tolerance in transgenic tobacco and chickpea. Plant. Cell Physiol. 2017, 58, 1934–1952. [Google Scholar] [CrossRef] [PubMed]
- Ghanti Kiran Kumar, S.; Sujata, K.G.; Kumar, V.; Nataraja Karba, N.; Srinath Rao, M.; Kavi Kishor, P.B. Heterologous expression of P5CS gene in chickpea enhances salt tolerance without affecting yield. Biol. Plant 2011, 55, 634–640. [Google Scholar] [CrossRef]
- Bhatnagar-Mathur, P.; Vadez, V.; Jyostna Devi, M.; Lavanya, M.; Vani, G.; Sharma, K.K. Genetic engineering of chickpea (Cicer arietinum L.) with the P5CSF129A gene for osmoregulation with implications on drought tolerance. Mol. Breed. 2009, 23, 591–606. [Google Scholar] [CrossRef]
- Sawardekar, S.V.; Katageri, I.S.; Salimath, P.M.; Kumar, P.A.; Kelkar, V.G. Standardization of in-vitro genetic transformation technique in chickpea (Cicer arietinum L.) for pod-borer resistance. Adv. Agric. Res. Technol. J. 2017, 1, 1198–1205. [Google Scholar]
- Das, A.; Datta, S.; Thakur, S.; Shukla, A.; Ansari, J.; Sujayanand, G.K.; Chaturvedi, S.K.; Kumar, P.A.; Singh, N.P. Expression of a chimeric gene encoding insecticidal crystal protein Cry1Aabc of Bacillus thuringiensis in chickpea (Cicer arietinum L.) confers resistance to gram pod borer (Helicoverpa armigera Hubner.). Front. Plant Sci. 2017, 8, 1423. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, M.; Singh, A.K.; Sanyal, I.; Altosaar, I.; Amla, D.V. Pyramiding of modified cry1Ab and cry1Ac genes of Bacillus thuringiensis in transgenic chickpea (Cicer arietinum L.) for improved resistance to pod borer insect Helicoverpa armigera. Euphytica 2011, 182, 87–102. [Google Scholar] [CrossRef]
- Chakraborty, J.; Sen, S.; Ghosh, P.; Sengupta, A.; Basu, D.; Das, S. Homologous promoter derived constitutive and chloroplast targeted expression of synthetic cry1Ac in transgenic chickpea confers resistance against Helicoverpa armigera. Plant Cell Tissue Organ. Cult. 2016, 125, 521–535. [Google Scholar] [CrossRef]
- Khatodia, S.; Kharb, P.; Batra, P.; Kumar, P.A.; Chowdhury, V.K. Molecular characterization of Bt chickpea (Cicer arietinum L.) plants carrying cry1Aa3 gene. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 632–642. [Google Scholar]
- Acharjee, S.; Sarmah, B.K.; Kumar, P.A.; Olsen, K.; Mahon, R.; Moar, W.J.; Moore, A.; Higgins, T.J.V. Transgenic chickpeas (Cicer arietinum L.) expressing a sequence-modified cry2Aa gene. Plant Sci. 2010, 178, 333–339. [Google Scholar] [CrossRef]
- Indurker, S.; Misra, H.S.; Eapen, S. Agrobacterium-mediated transformation in chickpea (Cicer arietinum L.) with an insecticidal protein gene: Optimisation of different factors. Physiol. Mol. Biol. Plants 2010, 16, 273–284. [Google Scholar] [CrossRef]
- Chakraborti, D.; Sarkar, A.; Mondal, H.A.; Das, S. Tissue specific expression of potent insecticidal, Allium sativum leaf agglutinin (ASAL) in important pulse crop, chickpea (Cicer arietinum L.) to resist the phloem feeding Aphis craccivora. Transgenic Res. 2009, 18, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Razavi, A.; Malhotra, I.; Ghosh, A.; Pusztai-Carey, M.; Marks, J.; King, C. Antibodies as epidemiological markers of genetically modified crop exposure: Detection of Cry1Ab-specific IgG. Food Agric. Immunol. 2017, 28, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, B.K.; Moore, A.; Tate, W.; Molvig, L.; Morton, R.L.; Rees, D.P.; Chiaiese, P.; Chrispeels, M.J.; Tabe, L.M.; Higgins, T.J.V. Transgenic chickpea seeds expressing high levels of a bean α-amylase inhibitor. Mol. Breed. 2004, 14, 73–82. [Google Scholar] [CrossRef]
- Ignacimuthu, S.; Prakash, S. Agrobacterium-mediated transformation of chickpea with α-amylase inhibitor gene for insect resistance. J. Biosci. 2006, 31, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Basso, M.F.; Duarte, K.E.; Santiago, T.R.; de Souza, W.R.; de Oliveira Garcia, B.; da Cunha, B.D.B.; Kobayashi, A.K.; Molinari, H.B.C. Efficient genome editing and gene knockout in Setaria viridis with CRISPR/Cas9 directed gene editing by the non-homologous end-joining pathway. Plant Biotechnol. 2021, 38, 227–238. [Google Scholar] [CrossRef]
- Anwar, A.; Kim, J.K. Transgenic breeding approaches for improving abiotic stress tolerance: Recent progress and future perspectives. Int. J. Mol. Sci. 2020, 21, 2695. [Google Scholar] [CrossRef]
- Sadhu, S.; Jogam, P.; Gande, K.; Banoth, R.; Penna, S.; Peddaboina, V. Optimization of different factors for an Agrobacterium-mediated genetic transformation system using embryo axis explants of chickpea (Cicer arietinum L.). J. Plant Biotechnol. 2022, 49, 61–73. [Google Scholar] [CrossRef]
- Ganguly, S.; Ghosh, G.; Ghosh, S.; Purohit, A.; Chaudhuri, R.K.; Das, S.; Chakraborti, D. Plumular meristem transformation system for chickpea: An efficient method to overcome recalcitrant tissue culture responses. Plant Cell Tissue Organ. Cult. 2020, 142, 493–504. [Google Scholar] [CrossRef]
- Mandal, D.; Sinharoy, S. A toolbox for nodule development studies in chickpea: A hairy-root transformation protocol and an efficient laboratory strain of Mesorhizobium sp. Mol. Plant. Microbe Interact. 2019, 32, 367–378. [Google Scholar] [CrossRef]
- Aggarwal, P.R.; Nag, P.; Choudhary, P.; Chakraborty, N.; Chakraborty, S. Genotype-independent Agrobacterium rhizogenes-mediated root transformation of chickpea: A rapid and efficient method for reverse genetics studies. Plant Methods 2018, 14, 55. [Google Scholar] [CrossRef]
- Srivastava, J.; Datta, S.; Mishra, S.P. Development of an efficient Agrobacterium mediated transformation system for chickpea (Cicer arietinum). Biologia 2017, 72, 153–160. [Google Scholar] [CrossRef]
- Kumar, B.K.; Mahalakshmi, L.S.; Kumar, S.M.; Devi, B.P. Optimized Agrobacterium-mediated genetic transformation in chickpea (Cicer arietinum L.) cultivar swetha (ICCV-2). Trends Biosci. 2014, 7, 2237–2244. [Google Scholar]
- Tripathi, L.; Singh, A.K.; Singh, S.; Singh, R.; Chaudhary, S.; Sanyal, I.; Amla, D.V. Optimization of regeneration and Agrobacterium-mediated transformation of immature cotyledons of chickpea (Cicer arietinum L.). Plant Cell Tissue Organ. Cult. 2013, 113, 513–527. [Google Scholar] [CrossRef]
- Sharmin, R.A.; Akter, J.; Sarker, R.H.; Hoque, M.I. Agrobacterium-mediated genetic transformation of local cultivars of chickpea (Cicer arietinum L.). Plant Tissue Cult. Biotechnol. 2012, 22, 41–50. [Google Scholar] [CrossRef]
- Kharb, P.; Batra, P.; Chowdhury, V. A Novel Process of Genetic Transformation in Chickpea Using Agrobacterium. 2012. Available online: https://www.allindianpatents.com/patents/252590-a-novel-process-of-genitic-transformatiohn-in-chickpea-using-agrobacterium (accessed on 1 August 2022).
- Yadav, I.S.; Singh, N.P. In vitro regeneration and genetic transformation of diverse genotypes of chickpea (Cicer arietinum L.). Indian J. Genet. Plant Breed. 2011, 71, 320–328. [Google Scholar]
- Moshtaghi, N.; Bagheri, A.; Sharifi, A. A comparison of two selectable marker gene systems used in the transformation of chickpea (Cicer arietinum L.). Transgenic Plant J. 2011, 5, 67–71. [Google Scholar]
- Bhattacharjee, B.; Mohan, M.; Nair, S. Transformation of chickpea: Effect of genotype, explant, Agrobacterium-strain and composition of culture medium. Biol. Plant 2010, 54, 21–32. [Google Scholar] [CrossRef]
- Patil, G.; Deokar, A.; Jain, P.K.; Thengane, R.J.; Srinivasan, R. Development of a phosphomannose isomerase-based Agrobacterium-mediated transformation system for chickpea (Cicer arietinum L.). Plant Cell Rep. 2009, 28, 1669–1676. [Google Scholar] [CrossRef]
- Akbulut, M.; Yücel, M.; Öktem, H.A. Analysis and optimization of DNA delivery into chickpea (Cicer arietinum L.) seedlings by Agrobacterium tumefaciens. Afr. J. Biotechnol. 2008, 7, 1011–1017. [Google Scholar]
- Pathak, M.R.; Hamzah, R.Y. An effective method of sonication-assisted Agrobacterium-mediated transformation of chickpeas. Plant Cell Tissue Organ. Cult. 2008, 93, 65–71. [Google Scholar] [CrossRef]
- Sanyal, I.; Amla, D.V. Genetic transformation of chickpea (Cicer arietinum L.) using cotyledonary node explants. In Handbook of New Technologies for Genetic Improvement of Legumes, 1st ed.; Kirt, P., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 147–158. [Google Scholar]
- Malhotra, S.; Shikha; Batra, P.; Chowdhury, V.K. Agrobacterium-mediated genetic transformation in chickpea (Cicer arietinum): Optimization of protocol and development of insect resistant chickpea. Natl. J. Plant Improv. 2007, 9, 82–87. [Google Scholar]
- Sharma, K.K.; Bhatnagar-Mathur, P.; Jayanand, B. Chickpea (Cicer arietinum L.). In Agrobacterium Protocols; Wang, K., Ed.; Humana Press: Totowa, NJ, USA, 2006; pp. 313–324. [Google Scholar]
- Sanyal, I.; Singh, A.K.; Kaushik, M.; Amla, D.V. Agrobacterium-mediated transformation of chickpea (Cicer arietinum L.) with Bacillus thuringiensis cry1Ac gene for resistance against pod borer insect Helicoverpa armigera. Plant Sci. 2005, 168, 1135–1146. [Google Scholar] [CrossRef]
- Polowick, P.L.; Baliski, D.S.; Mahon, J.D. Agrobacterium tumefaciens-mediated transformation of chickpea (Cicer arietinum L.): Gene integration, expression and inheritance. Plant Cell Rep. 2004, 23, 485–491. [Google Scholar] [CrossRef]
- Tewari-Singh, N.; Sen, J.; Kiesecker, H.; Reddy, V.S.; Jacobsen, H.J.; Guha-Mukherjee, S. Use of a herbicide or lysine plus threonine for non-antibiotic selection of transgenic chickpea. Plant Cell Rep. 2004, 22, 576–583. [Google Scholar] [CrossRef]
- Senthil, G.; Williamson, B.; Dinkins, R.D.; Ramsay, G. An efficient transformation system for chickpea (Cicer arietinum L.). Plant Cell Rep. 2004, 23, 297–303. [Google Scholar] [CrossRef]
- Khawar, K.M.; Ozcan, S. Hairy root transformation in Turkish chickpea (Cicer arietinum L) cultivars. Biotechnol. Biotechnol. Equip. 2004, 18, 51–54. [Google Scholar] [CrossRef]
- Sanyal, I.; Singh, A.; Singh, A.; Amla, D. Agrobacterium tumefaciens-mediated transformation of chickpea (Cicer arietinum L.) using mature embryonic axes and cotyledonary nodes. Indian J. Biotechnol. 2003, 2, 524–532. [Google Scholar]
- Krishnamurthy, K.V.; Suhasini, K.; Sagare, A.P.; Meixner, M.; de Kathen, A.; Pickardt, T.; Schieder, O. Agrobacterium mediated transformation of chickpea (Cicer arietinum L.) embryo axes. Plant Cell Rep. 2000, 19, 235–240. [Google Scholar] [CrossRef]
- Husnain, T.; Fatima, T.; Rafi-ul-Islam; Riazuddin, S. Plant regeneration and expression of beta-glucuronidase gene in hypocotyl tissues of chickpea (Cicer arietinum L.). Pak. J. Biol. Sci. 2000, 3, 842–845. [Google Scholar] [CrossRef]
- Kar, S.; Basu, D.; Das, S.; Ramkrishnan, N.A.; Mukherjee, P.; Nayak, P.; Sen, S.K. Expression of cryIA(c) gene of Bacillus thuringiensis in transgenic chickpea plants inhibits development of pod-borer (Heliothis armigera) larvae. Transgenic Res. 1997, 6, 177–185. [Google Scholar] [CrossRef]
- Altinkut, A.; Gözükirmiz, N.; Bajroviç, K.; Gözükirmizi, N. High Percentage of Regeneration and Transformation in Chickpea, ActaHorticulturae, 447th ed.; International Society for Horticultural Science (ISHS): Leuven, Belgium, 1997; pp. 319–320. [Google Scholar]
- Kar, S.; Johnson, T.M.; Nayak, P.; Sen, S.K. Efficient transgenic plant regeneration through Agrobacterium-mediated transformation of chickpea (Cicer arietinum L.). Plant Cell Rep. 1996, 16, 32–37. [Google Scholar] [CrossRef]
- Ramana, R.V.; Venu, C.; Jayasree, T.; Sadanadam, A. Direct somatic embryogenesis and transformation in Cicer arietinum L.). Indian J. Exp. Biol. 1996, 34, 716–718. [Google Scholar]
- Fontana, G.S.; Santini, L.; Caretto, S.; Frugis, G.; Mariotti, D. Genetic transformation in the grain legume Cicer arietinum L. (chickpea). Plant Cell Rep. 1993, 12, 194–198. [Google Scholar] [CrossRef]
- Anbessa, Y.; Warkentin, T.; Bueckert, R.; Vandenberg, A. Short internode, double podding and early flowering effects on maturity and other agronomic characters in chickpea. Field Crops Res. 2007, 102, 43–50. [Google Scholar] [CrossRef]
- Millan, T.; Clarke, H.J.; Siddique, K.H.; Buhariwalla, H.K.; Gaur, P.M.; Kumar, J.; Gil, J.; Kahl, G.; Winter, P. Chickpea molecular breeding: New tools and concepts. Euphytica 2006, 147, 81–103. [Google Scholar] [CrossRef] [Green Version]
| Gene | Correlation to Stress |
|---|---|
| Aquaporins gene family (CaAQPs) [27] | Biotic and abiotic stress |
| CarERF116 [28] | Abiotic stress response |
| CarLEA4 [29] | Plant developmental processes |
| Abscisic acid stress and ripening gene (ASR) [30] | Reproductive processes |
| Drought responsive element binding protein (DREB) [31] | Heat and drought stress response |
| Dehydration responsive element binding (DREB1) [31] | Induced by dehydration and high-salt stresses |
| CAP2 gene (DREB2A) [31] | Regulates expression of water stress-inducible genes |
| SNF-1relatedproteinkinase (AKIN) [31] | Response to nutritional and environmental stresses in plants |
| Amino aldehyde dehydrogenase (AMADH) [31] | Osmotic stress, dehydration, and salt stress tolerance |
| CAP2 promoter [31] | Induce a set of abiotic stress-related genes |
| Dehydrin (DHN) [31] | Induced by environmental stress, dehydration, or low temperature |
| ERECTA (fragment 7F-5R) [31] | Mediates plants’ responses to disease and stress |
| ERECTA (fragment 8F-8R) [31] | Mediates plants’ responses to disease and stress |
| Myb transcription factor [31] | Response to biotic and abiotic stresses |
| Sucrose synthase (SuSy) [31] | Sugar metabolism pathway |
| Sucrose phosphate synthase (SPS) [31] | Induced by drought and mannitol |
| Heat shock proteins [32] | Heat stress resistance |
| Pollen-specific leucine-rich repeat extensin-like protein 1 [32] | Heat stress resistance |
| Transcription factor CAULIFLOWER A-like [32] | Heat stress resistance |
| Heat shock protein-binding protein [32] | Heat stress resistance |
| Heat shock amino-terminal domain protein [32] | Heat stress resistance |
| PHOTOPERIOD-INDEPENDENT EARLY FLOWERING 1isoform X1 [32] | Heat stress resistance |
| Heat shock protein/heat shock factor protein HSF24-like [32] | Heat stress resistance |
| Calmodulin-binding heat-shock protein [32] | Heat stress resistance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karalija, E.; Vergata, C.; Basso, M.F.; Negussu, M.; Zaccai, M.; Grossi-de-Sa, M.F.; Martinelli, F. Chickpeas’ Tolerance of Drought and Heat: Current Knowledge and Next Steps. Agronomy 2022, 12, 2248. https://doi.org/10.3390/agronomy12102248
Karalija E, Vergata C, Basso MF, Negussu M, Zaccai M, Grossi-de-Sa MF, Martinelli F. Chickpeas’ Tolerance of Drought and Heat: Current Knowledge and Next Steps. Agronomy. 2022; 12(10):2248. https://doi.org/10.3390/agronomy12102248
Chicago/Turabian StyleKaralija, Erna, Chiara Vergata, Marcos Fernando Basso, Miriam Negussu, Michele Zaccai, Maria Fatima Grossi-de-Sa, and Federico Martinelli. 2022. "Chickpeas’ Tolerance of Drought and Heat: Current Knowledge and Next Steps" Agronomy 12, no. 10: 2248. https://doi.org/10.3390/agronomy12102248
APA StyleKaralija, E., Vergata, C., Basso, M. F., Negussu, M., Zaccai, M., Grossi-de-Sa, M. F., & Martinelli, F. (2022). Chickpeas’ Tolerance of Drought and Heat: Current Knowledge and Next Steps. Agronomy, 12(10), 2248. https://doi.org/10.3390/agronomy12102248









