Variation Characteristics of Glucosinolate Contents in Leaf Mustard (Brassica juncea)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Morphological and Phenotypic Characterization
2.3. Extraction of Glucosinolates
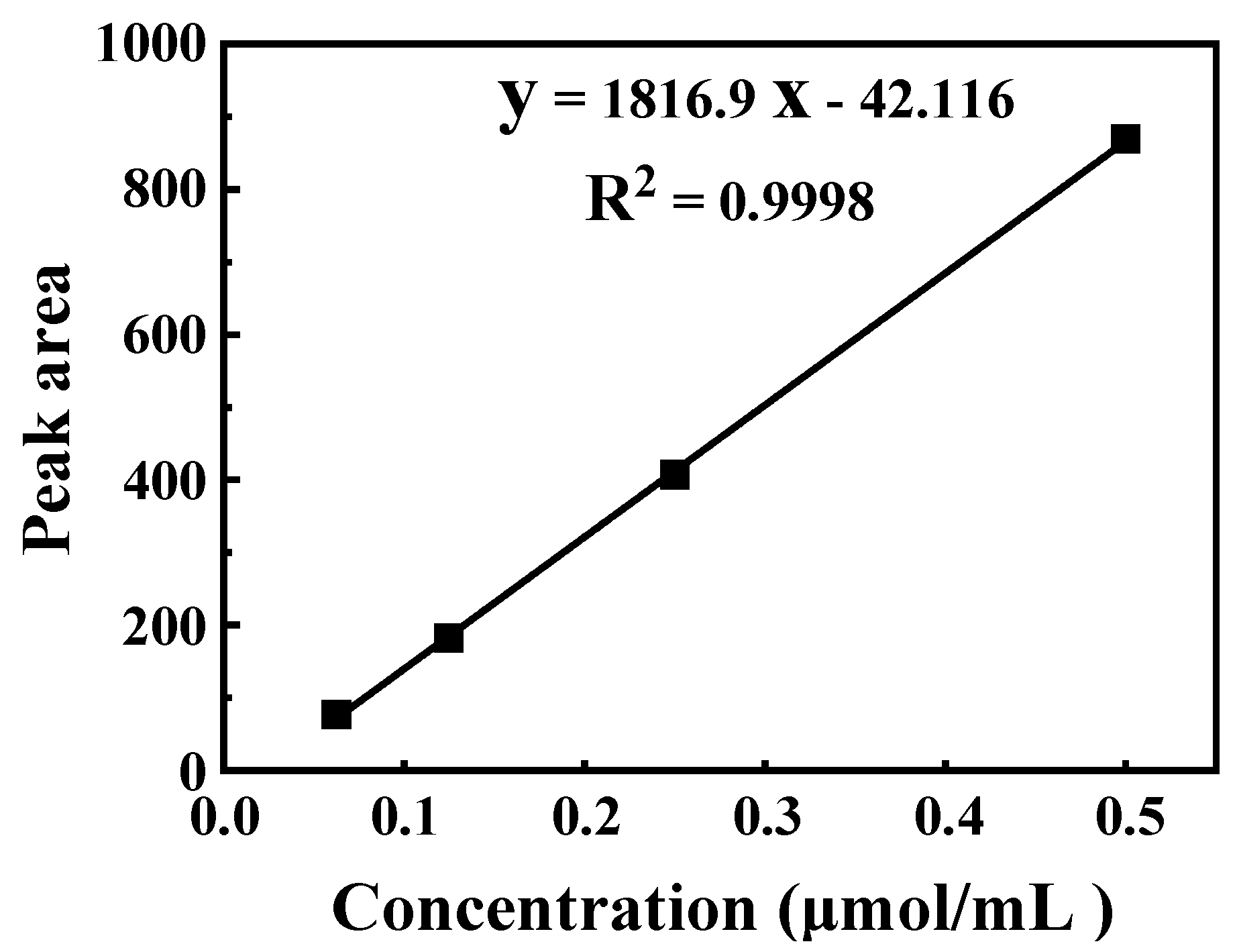
2.4. Quantification of Glucosinolate by HPLC
2.5. Statistical Analysis
3. Results
3.1. Morphological and Phenotypic Characterization of 60 Mustard Resources
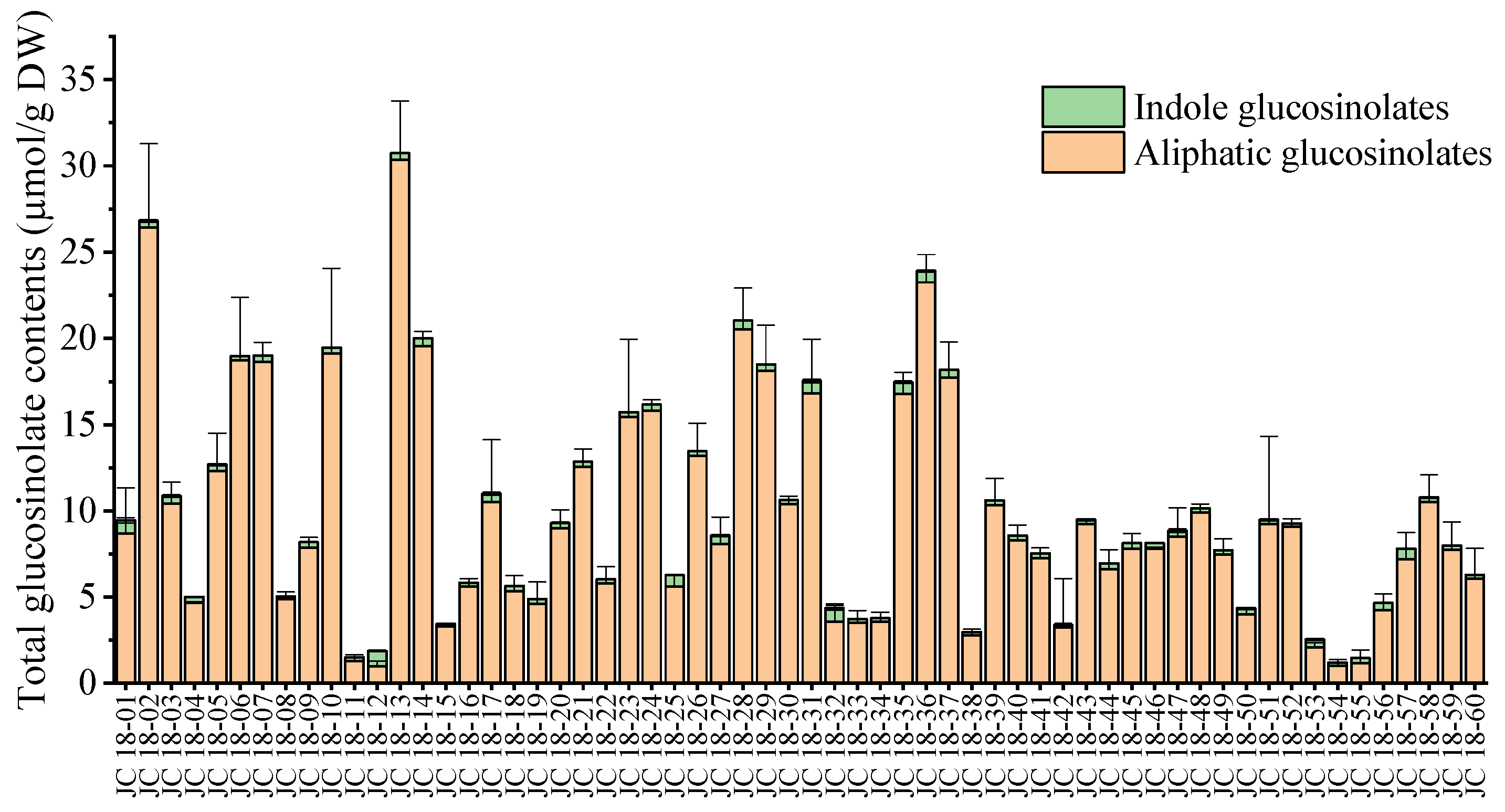
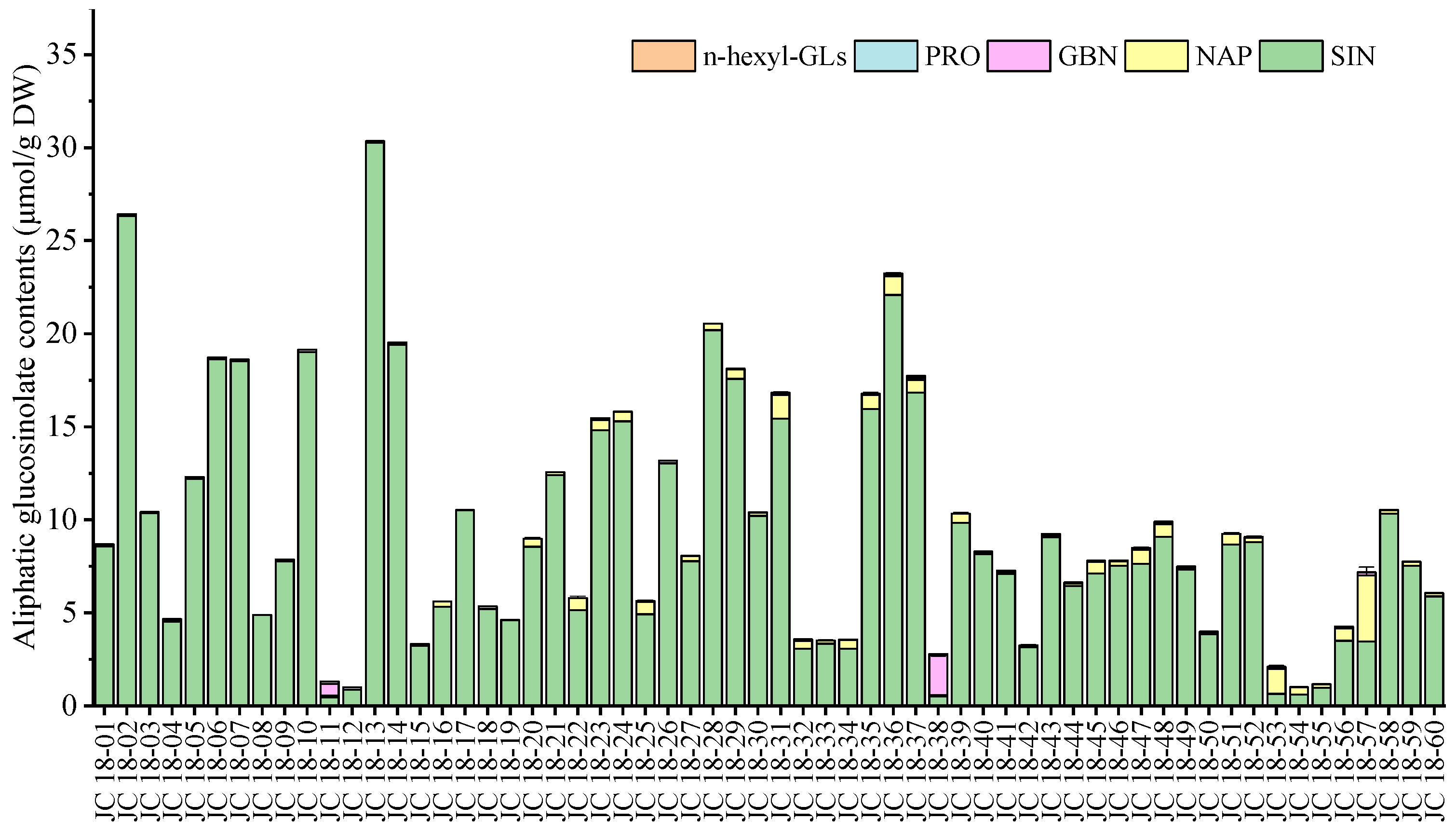
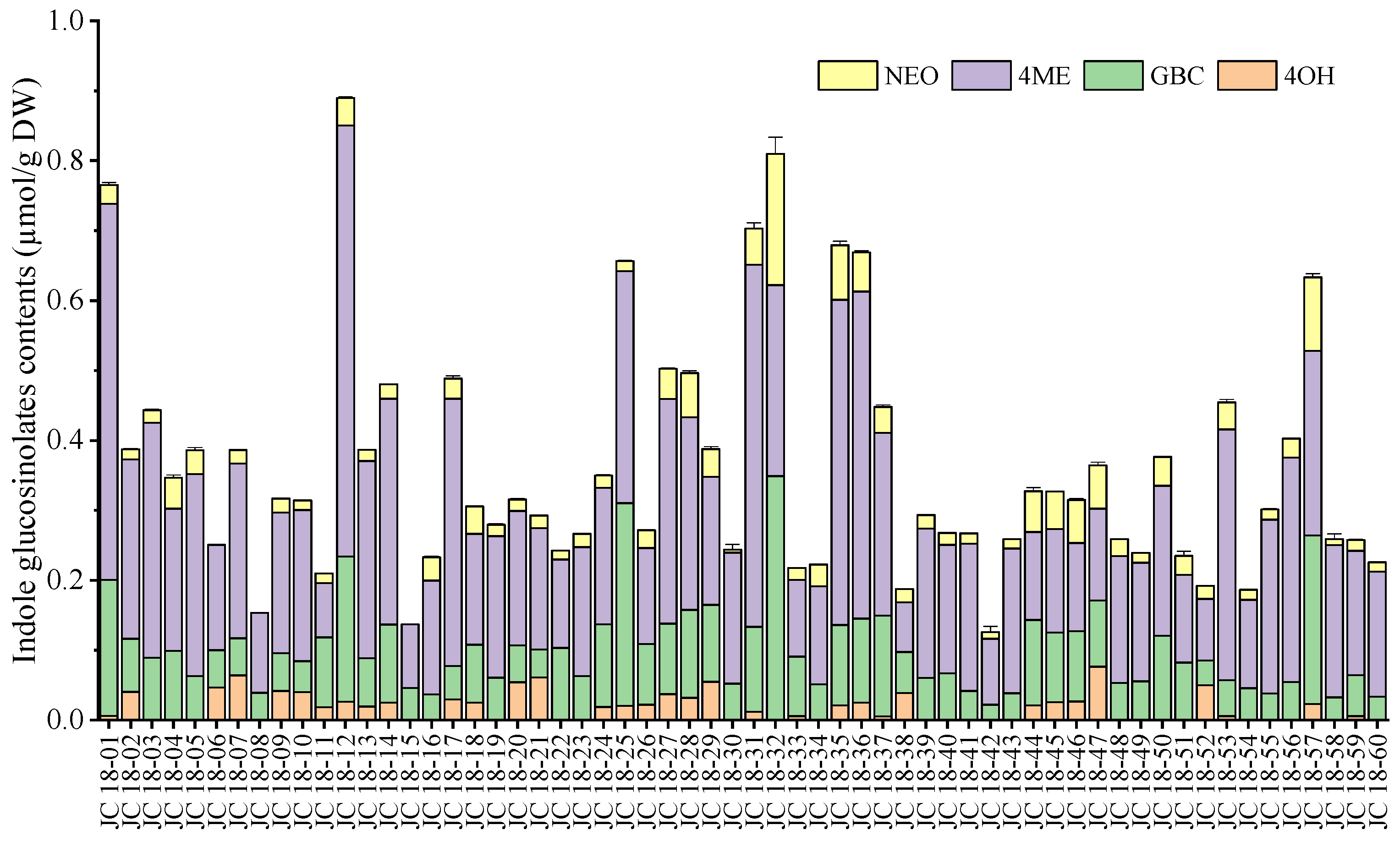
3.2. Evaluation of Glucosinolate Compounds in Leaves Based on 60 Mustard Resources
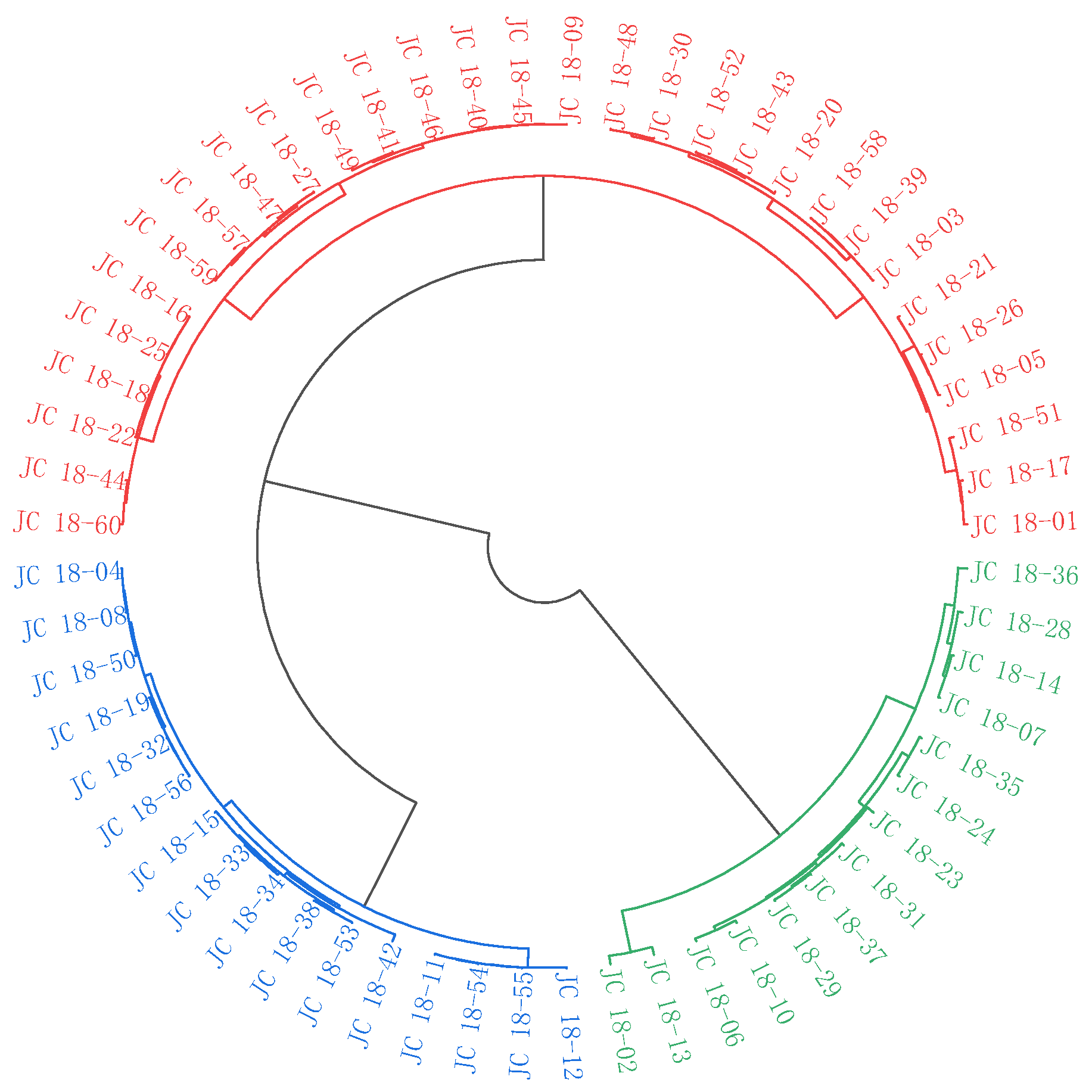
3.3. Cluster Analysis
3.3.1. Cluster Analysis of 60 Mustard Resources Based on Phenotypic Characterization
3.3.2. Cluster Analysis of 60 Mustard Resources Based on Glucosinolate Contents
3.4. Correlation Analysis of Agronomic Characters and Glucosinolate Contents in 60 Mustard Resources
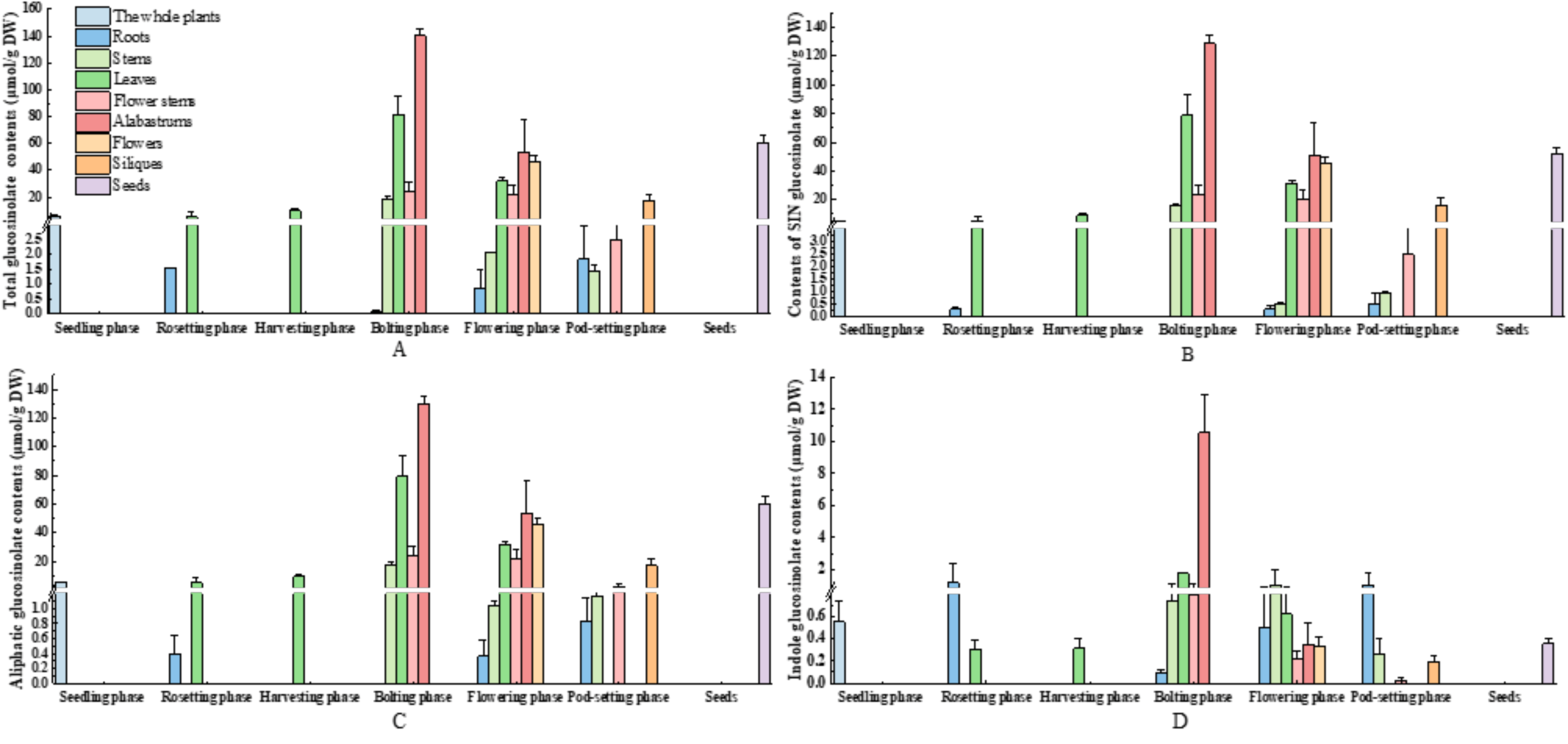
3.5. Diversity Distribution of Glucosinolate in Various Parts and Developmental Stages of Mustard Resource JC 18-56
3.5.1. Evaluation of Glucosinolates in Various Parts of Mustard Resource JC 18-56
3.5.2. Evaluation of Glucosinolates in Different Stages of Mustard Resource JC 18-56
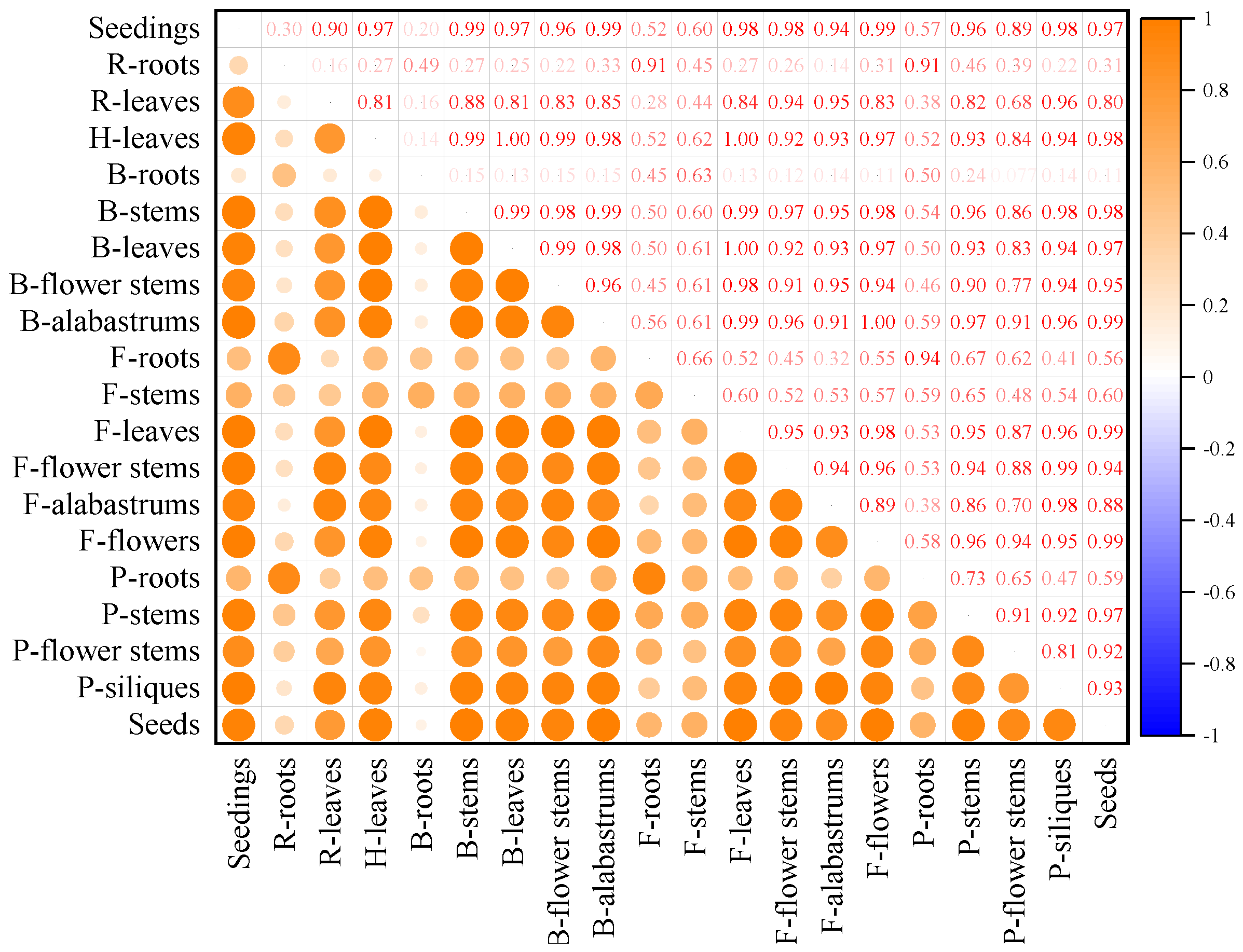
3.6. Correlation between Different Tissues in the Whole Growth Period of Mustard Resource JC 18-56
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, X.M.; Chen, B.Y.; Lu, G.Y.; Wang, H.Z.; Xu, K.; Gao, G.Z.; Song, Y.C. Genetic diversity in oil and vegetable mustard (Brassica juncea) landraces revealed by SRAP markers. Genet. Resour. Crop Evol. 2009, 56, 1011–1022. [Google Scholar] [CrossRef]
- Sun, X.Q.; Qu, Y.Q.; Li, M.M.; Song, X.L.; Hang, Y.Y. Genetic diversity, genetic structure and migration routes of wild Brassica juncea in China assessed by SSR markers. Genet. Resour. Crop Evol. 2018, 65, 1581–1590. [Google Scholar] [CrossRef]
- Yang, Y.G.; Liu, N.C.; Chen, X.Q.; Chen, C.L.; Zhou, G.F.; Liao, K.L. A study on classification of mustard. Acta Hortic. Sin. 1989, 1, 114–121. [Google Scholar]
- Hopkins, R.J.; Dam, N.V.; Loon, J.V. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. EntoMogy 2009, 54, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Bisht, N.C. Biotic elicitors and mechanical damage modulate glucosinolate accumulation by coordinated interplay of glucosinolate biosynthesis regulators in polyploid Brassica juncea. Phytochemistry 2015, 117, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kliebenstein, D.J.; Kroymann, J.; Mitchell-Olds, T. The glucosinolate-myrosinase system in an ecological and evolutionary context. Curr. Opin. Plant Biol. 2005, 8, 264–271. [Google Scholar] [CrossRef]
- Yan, X.F.; Chen, S.X. Regulation of plant glucosinolate metabolism. Planta 2007, 226, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Chhajed, S.; Mostafa, I.; He, Y.; Abou–Hashem, M.; El–Domiaty, M.; Chen, S.X. Glucosinolate biosynthesis and the Glucosinolate–Myrosinase system in plant defense. Agronomy 2020, 10, 1786. [Google Scholar] [CrossRef]
- Augustine, R.; Mukhopadhyay, A.; Bisht, N.C. Targeted silencing of BjMYB28 transcription factor gene directs development of low glucosinolate lines in oilseed Brassica juncea. Plant Biotechnol. J. 2013, 11, 855–866. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Nambiar, D.M.; Kumari, J.; Augustine, R.; Kumar, P.; Bajpai, P.K.; Bisht, N.C. GTR1 and GTR2 transporters differentially regulate tissue–specific glucosinolate contents and defence responses in the oilseed crop Brassica juncea. Plant Cell Environ. 2021, 44, 2729–2743. [Google Scholar] [CrossRef] [PubMed]
- McGregor, D.I.; Mullin, W.J.; Fenwick, G.R. Analytical methodology for determining glucosinolate composition and content. J. Assoc. Anal. Chem. 1983, 66, 825–849. [Google Scholar] [CrossRef]
- Antonious, G.F.; Bomford, M.; Vincelli, P. Screening Brassica species for glucosinolate content. J. Environ. Sci. Health Part B 2009, 44, 311–316. [Google Scholar] [CrossRef]
- Cools, K.; Terry, L.A. The effect of processing on the glucosinolate profile in mustard seed. Food Chem. 2018, 252, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Edger, P.P.; Hall, J.C.; Harkess, A.; Tang, M.; Coombs, J.; Mohammadin, S.; Schranz, M.E.; Xiong, Z.; Leebens–Mack, J.; Meyers, B.C.; et al. Brassicales phylogeny inferred from 72 plastid genes: A reanalysis of the phylogenetic localization of two paleopolyploid events and origin of novel chemical defenses. Am. J. Bot. 2018, 105, 463–469. [Google Scholar] [CrossRef]
- Padilla, G.; Cartea, M.E.; Velasco, P.; Haro, A.D.; Orda’s, A. Variation of glucosinolates in vegetable crops of Brassica rapa. Phytochemistry 2007, 68, 536–545. [Google Scholar] [CrossRef]
- Yang, B.; Quiros, C.F. Survey of glucosinolate variation in leaves of Brassica rapa crops. Genet. Resour. Crop. Evol. 2010, 57, 1079–1089. [Google Scholar] [CrossRef]
- Sodhi, Y.S.; Mukhopadhyay, A.; Arumugam, N.; Verma, J.K.; Gupta, V.; Pental, D.; Pradhan, A.K. Genetic analysis of total glucosinolate in crosses involving a high glucosinolate Indian variety and a low glucosinolate line of Brassica juncea. Plant Breed. 2010, 121, 508–511. [Google Scholar] [CrossRef]
- Mao, S.X.; Wang, J.W.; Wu, Q.; Liang, M.T.; Yuan, Y.M.; Wu, T.; Liu, M.Y.; Wu, Q.Y.; Huang, K. Effect of selenium-sulfur interaction on the anabolism of sulforaphane in broccoli. Phytochemistry 2020, 179, 112499. [Google Scholar] [CrossRef]
- Bednarek, P.; Piślewska-Bednarek, M.; Ver Loren van Themaat, E.; Maddula, R.K.; Svatoš, A.; Schulze-Lefert, P. Conservation and clade–specific diversification of pathogen-inducible tryptophan and indole glucosinolate metabolism in Arabidopsis thaliana relatives. New Phytol. 2011, 192, 713–726. [Google Scholar] [CrossRef]
- Simon, M.; Tamara, G. Regulation of glucosinolate biosynthesis. J. Exp. Bot. 2020, 72, 70–91. [Google Scholar]
- Sun, B.; Liu, N.; Zhao, Y.T.; Yan, H.Z.; Wang, Q.M. Variation of glucosinolates in three edible parts of Chinese kale (Brassica alboglabra Bailey) varieties. Food Chem. 2011, 124, 941–947. [Google Scholar] [CrossRef]
- Chen, X.J. Investigation of Glucosinolates and Their Effect Factors in Chinese Brassica Vegetables; Zhejiang University: Hangzhou, China, 2006. [Google Scholar]
- Li, Z.S.; Zheng, S.N.; Liu, Y.M.; Fang, Z.Y.; Yang, L.M.; Zhuang, M.; Zhang, Y.Y.; Lv, H.H.; Wang, Y.; Xu, D.H. Characterization of glucosinolates in 80 broccoli genotypes and different organs using UHPLC-Triple-TOF-MS method. Food Chem. 2021, 334, 127519. [Google Scholar] [CrossRef] [PubMed]
- Li, P.W. Glucosinolate and Their Relationship between Leaves and Seeds in Brassica napus; Huazhong Agricultural University: Wuhan, China, 2007. [Google Scholar]
- Bhandari, S.R.; Jo, J.S.; Lee, J.G. Comparison of glucosinolate profiles in different tissues of nine Brassica Crops. Molecules 2015, 20, 15827–15841. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Study on Insect Resistance with Glucosinolate Accumulation and Changes in Glucosinolate Concentrations after Feeding by Pests in Brassicaceae and Characterization of Glucosinolate Biosynthetic Genes in Brassica nigra and Brassica juncea; Zhejiang A&F University: Zhejiang, China, 2019. [Google Scholar]
- Sun, B.; Tian, Y.X.; Chen, Q.; Zhang, Y.; Luo, Y.; Wang, Y.; Li, M.Y.; Gong, R.G.; Wang, X.R.; Zhang, F.; et al. Variations in the glucosinolates of the individual edible parts of three stem mustards (Brassica juncea). R. Soc. Open Sci. 2019, 6, 182054. [Google Scholar] [CrossRef]
- Brown, P.D.; Tokuhisa, J.G.; Reichelt, M.; Gershenzon, J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 2003, 62, 471–481. [Google Scholar] [CrossRef]
- Jeon, B.W.; Oh, M.H.; Kim, E.O.; Kim, H.S.; Chae, W.B. Different vegetative growth stages of Kimchi cabbage (Brassica rapa L.) exhibit specific glucosinolate composition and content. Hortic. Environ. Biotechnol. 2018, 59, 355–362. [Google Scholar] [CrossRef]
- Kim, Y.B.; Li, X.H.; Kim, S.J.; Kim, H.H.; Lee, J.; Kim, H.R.; Park, S.U. MYB transcription factors regulate glucosinolate biosynthesis in different organs of Chinese cabbage (Brassica rapa ssp.pekinensis). Molecules 2013, 18, 8682–8695. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhu, Z.J.; Gerendas, J.; Zimmermann, N. Glucosinolates in Chinese Brassica campestris vegetables: Chinese cabbage, purple cai-tai, choysum, pakchoi, and turnip. HortScience 2008, 43, 571–574. [Google Scholar] [CrossRef]
- Dam, N.M.V.; Tytgat, T.O.G.; Kirkegaard, J.A. Root and shoot glucosinolates: A comparison of their diversity, function and interactions in natural and managed ecosystems. Phytochem. Rev. 2009, 8, 171–186. [Google Scholar]
- Petersen, B.L.; Chen, S.X.; Hansen, C.H.; Olsen, C.E.; Halkier, B.A. Composition and content of glucosinolates in developing Arabidopsis thaliana. Planta 2002, 214, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Huseby, S.; Koprivova, A.; Lee, B.R.; Saha, S.; Mithen, R.; Wold, A.B.; Bengtsson, G.B.; Kopriva, S. Diurnal and light regulation of sulphur assimilation and glucosinolate biosynthesis in Arabidopsis. J. Exp. Bot. 2013, 64, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, V.; Weber, K.; Moore, S.S.; Burow, M. Coordination of glucosinolate biosynthesis and turnover under different nutrient conditions. Front. Plant Sci. 2019, 10, 1560. [Google Scholar] [CrossRef] [PubMed]
- Sønderby, I.E.; Geu-Flores, F.; Halkier, B.A. Biosynthesis of glucosinolates-gene discovery and beyond. Trends Plant Sci. 2010, 15, 283–290. [Google Scholar] [CrossRef]
- Tian, Z.T. Genetic Study of Glucosinolate in Rapeseed by Associative Transcriptomics; Chinese Academy of Agricultural Sciences: Beijing, China, 2018. [Google Scholar]


| Germplasms | Origin | Germplasms | Origin | Germplasms | Origin | Germplasms | Origin |
|---|---|---|---|---|---|---|---|
| JC 18-01 | Beijing | JC 18-16 | Hunan | JC 18-31 | Fujian | JC 18-46 | Fujian |
| JC 18-02 | Beijing | JC 18-17 | Hunan | JC 18-32 | Fujian | JC 18-47 | Fujian |
| JC 18-03 | Beijing | JC 18-18 | Hunan | JC 18-33 | Fujian | JC 18-48 | Fujian |
| JC 18-04 | Beijing | JC 18-19 | Hunan | JC 18-34 | Fujian | JC 18-49 | Fujian |
| JC 18-05 | Beijing | JC 18-20 | Hunan | JC 18-35 | Fujian | JC 18-50 | Fujian |
| JC 18-06 | Beijing | JC 18-21 | Hunan | JC 18-36 | Fujian | JC 18-51 | Fujian |
| JC 18-07 | Beijing | JC 18-22 | Hunan | JC 18-37 | Fujian | JC 18-52 | Fujian |
| JC 18-08 | Beijing | JC 18-23 | Hunan | JC 18-38 | Fujian | JC 18-53 | Fujian |
| JC 18-09 | Hunan | JC 18-24 | Hunan | JC 18-39 | Fujian | JC 18-54 | Fujian |
| JC 18-10 | Hunan | JC 18-25 | Hunan | JC 18-40 | Fujian | JC 18-55 | Fujian |
| JC 18-11 | Hunan | JC 18-26 | Hunan | JC 18-41 | Fujian | JC 18-56 | Hunan |
| JC 18-12 | Hunan | JC 18-27 | Hunan | JC 18-42 | Fujian | JC 18-57 | Guangxi |
| JC 18-13 | Hunan | JC 18-28 | Hunan | JC 18-43 | Fujian | JC 18-58 | Guangxi |
| JC 18-14 | Hunan | JC 18-29 | Hunan | JC 18-44 | Fujian | JC 18-59 | Guangxi |
| JC 18-15 | Hunan | JC 18-30 | Hunan | JC 18-45 | Fujian | JC 18-60 | Guangxi |
| Stages | Samples |
|---|---|
| Seedling phase | The whole seedlings |
| Rosetting phase | Roots, leaves |
| Harvesting phase | Roots, leaves |
| Bolting phase | Roots, stems, leaves, flower stems, alabastrums |
| Flowering phase | Roots, stems, leaves, flower stems, alabastrums, flowers |
| Pod-setting phase | Roots, stems, flower stems, siliques |
| Seed maturity phase | Seeds |
| Quantitative Traits | Values Assigned (Based on the National Standard) |
|---|---|
| Cotyledon color | flavovirens = 1; light green = 2; green = 3; bottle green = 4 |
| Hypocotyl color | white green = 1; flavovirens = 2; light green = 3; green = 4; light purple = 5 |
| Blade profile | mottle-leaf = 1; plate leaf = 2 |
| Phylliform | obovate = 5; broadly obovate = 6; long obovate = 7; elliptical = 8; broadly elliptic = 9; long ova = l0; fan-shaped = 11; lanceolate = 12; oblanceolate = 13 |
| Leaf apical shape | pointed = 1; blunt = 2; circle = 3; wide circle = 4 |
| Leaf margin tooth | entire = 1; undulate = 2; serrulate = 3; serrate = 4; doubly serrate = 5 |
| Leaf cleft | no crack = 0; shallow crack = 1; drastic crack = 2; through shake = 3 |
| Leaf surface | smoothness = 1; slightly wrinkled = 2; wrinkled = 3; many wrinkles = 4 |
| Leaf bristlesr | no = 0; yes = 1 |
| Leaf wax powder | no = 0; yes = 1 |
| Leaf luster | no = 0; yes = 1 |
| Leaf color | flavovirens = 1; light green = 2; green = 3; bottle green = 4; purple = 5; purple green = 6 |
| Petiole color | white = 0; white green = 1; light green = 2; green = 3 |
| Petiole cross section shape | wide flat = 1; wide thick = 2; medium round = 3; narrow circle = 4 |
| Leaf nodules | no = 0; yes = 1 |
| Moss drawing | very early = 1; early = 2; mediun = 3; late = 4; very late = 5 |
| Flower stem color | flavovirens = 1; pale green = 2; green = 3 |
| Plant shape | upright = 1; half upright = 2; plantlet spreading = 3; collapsed = 4 |
| Characters | Maximum | Minimum | Mean | R | S.D. | A.D. | CV% |
|---|---|---|---|---|---|---|---|
| Cotyledon length (mm) | 19.98 | 10.90 | 15.20 | 9.08 | 2.12 | 1.71 | 11.24 |
| Cotyledon width (mm) | 24.72 | 12.52 | 17.49 | 12.20 | 2.61 | 2.10 | 12.02 |
| Groove depth (mm) | 4.94 | 1.82 | 3.22 | 3.12 | 0.69 | 0.59 | 18.28 |
| Plant height (mm) | 176.00 | 38.00 | 90.37 | 138.00 | 43.38 | 39.26 | 43.45 |
| Plant width (mm) | 125.00 | 38.00 | 71.57 | 87.00 | 16.05 | 12.28 | 17.16 |
| Rosette number | 19.00 | 0.00 | 7.87 | 19.00 | 4.10 | 3.13 | 39.80 |
| Leaf length (mm) | 95.00 | 30.00 | 50.89 | 65.00 | 10.70 | 8.20 | 16.10 |
| Leaf width (mm) | 36.00 | 12.00 | 21.93 | 24.00 | 5.31 | 4.27 | 19.46 |
| Petiole width (mm) | 7.00 | 0.60 | 2.30 | 6.40 | 1.22 | 0.93 | 40.41 |
| Main moss height (mm) | 176.00 | 30.00 | 125.07 | 146.00 | 37.42 | 29.04 | 23.22 |
| Main moss thick (mm) | 11.50 | 0.50 | 3.19 | 11.00 | 1.57 | 0.97 | 30.56 |
| Moss per plant | 23.00 | 0.00 | 8.33 | 23.00 | 4.17 | 3.10 | 37.20 |
| Characters | Max./(%) | Min./(%) | Mean | R | S.D. | A.D. | CV% |
|---|---|---|---|---|---|---|---|
| Cotyledon color | 4/(11.67%) | 1/(11.67%) | 2.65 | 3 | 0.83 | 0.69 | 25.97 |
| Hypocotyl color | 5/(43.33%) | 1/(55.00%) | 3.25 | 4 | 1.97 | 1.95 | 60.00 |
| Blade profile | 2/(15.00%) | 1/(85.00%) | 1.85 | 1 | 0.36 | 0.26 | 13.78 |
| Phylliform | 13/(6.67%) | 5/(11.67%) | 8.45 | 8 | 1.86 | 1.33 | 15.76 |
| Leaf apical shape | 4/(18.33%) | 1/(5.00%) | 2.48 | 3 | 0.85 | 0.74 | 29.69 |
| Leaf margin tooth | 5/(13.33%) | 1/(13.33%) | 2.82 | 4 | 1.31 | 1.19 | 42.33 |
| Leaf cleft | 3/(35.00%) | 0/(31.67%) | 1.40 | 3 | 1.25 | 1.15 | 82.38 |
| Leaf surface | 4/(28.33%) | 1/(3.33%) | 1.87 | 3 | 0.69 | 0.49 | 26.31 |
| Leaf luster | 1/(68.33) | 0/(31.67%) | 0.32 | 1 | 0.47 | 0.43 | 136.67 |
| Plant shape | 4/(28.33%) | 1/(16.67%) | 2.25 | 3 | 1.04 | 0.88 | 39.26 |
| Leaf color | 6/(8.33%) | 1/(6.67%) | 3.03 | 5 | 1.05 | 0.56 | 18.35 |
| Leaf wax powder | 1/(11.67%) | 0/(88.33%) | 0.88 | 1 | 0.32 | 0.21 | 23.33 |
| Leaf bristles | 1/(90.00%) | 0/(10.00%) | 0.10 | 1 | 0.30 | 0.18 | 180.00 |
| Petiole color | 3/(8.33%) | 0/(35.00%) | 1.95 | 3 | 0.96 | 0.77 | 39.40 |
| Petiole cross section shape | 4/(26.67%) | 1/(30.00%) | 2.45 | 3 | 1.18 | 1.06 | 43.20 |
| Leaf nodules | 1/(96.67%) | 0/(3.33%) | 0.03 | 1 | 0.18 | 0.06 | 193.33 |
| Moss drawing | 5/(10.00%) | 1/(20.00%) | 3.15 | 4 | 1.24 | 1.00 | 31.59 |
| Flower stem color | 3/(5.00%) | 1/(61.67%) | 2.57 | 2 | 0.59 | 0.53 | 20.82 |
| Cotyledon Length | Cotyledon Width | Groove Depth | Plant Height | Plant Width | Rosette Number | |
|---|---|---|---|---|---|---|
| Glucosinolate contents | 0.036 | −0.045 | 0.019 | −0.038 | 0.144 | −0.097 |
| Leaf length | Leaf width | Petiole width | Main moss height | Main moss thick | Moss per plant | |
| Glucosinolate contents | 0.200 | 0.018 | 0.189 | −0.229 | 0.254 | 0.127 |
| Cotyledon Color | Hypocotyl Color | Blade Profile | Phylliform | Leaf Apical Shape | Leaf Margin Tooth | |
|---|---|---|---|---|---|---|
| Glucosinolate contents | −0.123 | −0.047 | −0.001 | 0.154 | 0.079 | −0.134 |
| Leaf cleft | Leaf surface | Leaf luster | Plant shape | Leaf color | Leaf wax powder | |
| Glucosinolate contents | 0.026 | 0.003 | −0.063 | 0.192 | −0.328 * | 0.088 |
| Leaf bristles | Petiole color | Petiole cross section shape | Leaf nodules | Moss drawing | Flower stem color | |
| Glucosinolate contents | 0.121 | −0.017 | −0.058 | 0.135 | −0.029 | −0.162 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Wang, J.; Mao, S.; Wu, Q.; Tian, Y.; Wang, F.; Wang, P.; Huang, K.; Wu, Q. Variation Characteristics of Glucosinolate Contents in Leaf Mustard (Brassica juncea). Agronomy 2022, 12, 2287. https://doi.org/10.3390/agronomy12102287
Huang H, Wang J, Mao S, Wu Q, Tian Y, Wang F, Wang P, Huang K, Wu Q. Variation Characteristics of Glucosinolate Contents in Leaf Mustard (Brassica juncea). Agronomy. 2022; 12(10):2287. https://doi.org/10.3390/agronomy12102287
Chicago/Turabian StyleHuang, Huiping, Junwei Wang, Shuxiang Mao, Qi Wu, Yuxiao Tian, Feng Wang, Pei Wang, Ke Huang, and Qiuyun Wu. 2022. "Variation Characteristics of Glucosinolate Contents in Leaf Mustard (Brassica juncea)" Agronomy 12, no. 10: 2287. https://doi.org/10.3390/agronomy12102287
APA StyleHuang, H., Wang, J., Mao, S., Wu, Q., Tian, Y., Wang, F., Wang, P., Huang, K., & Wu, Q. (2022). Variation Characteristics of Glucosinolate Contents in Leaf Mustard (Brassica juncea). Agronomy, 12(10), 2287. https://doi.org/10.3390/agronomy12102287





