Deflowering as a Tool to Accelerate Growth of Young Trees in Both Intensive and Super-High-Density Olive Orchards
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment 1—Effects of Different NAA Concentrations on the Vegetative and Reproductive Activities of the Cultivar Moraiolo
2.2. Experiment 2—Effects of Different NAA Concentrations on the Reproductive Activity of the Cultivar Sikitita
2.3. Experiment 3—Effects of NAA Treatments on the Cultivar Moraiolo for Two Consecutive Years
2.4. Statistical Analysis
3. Results
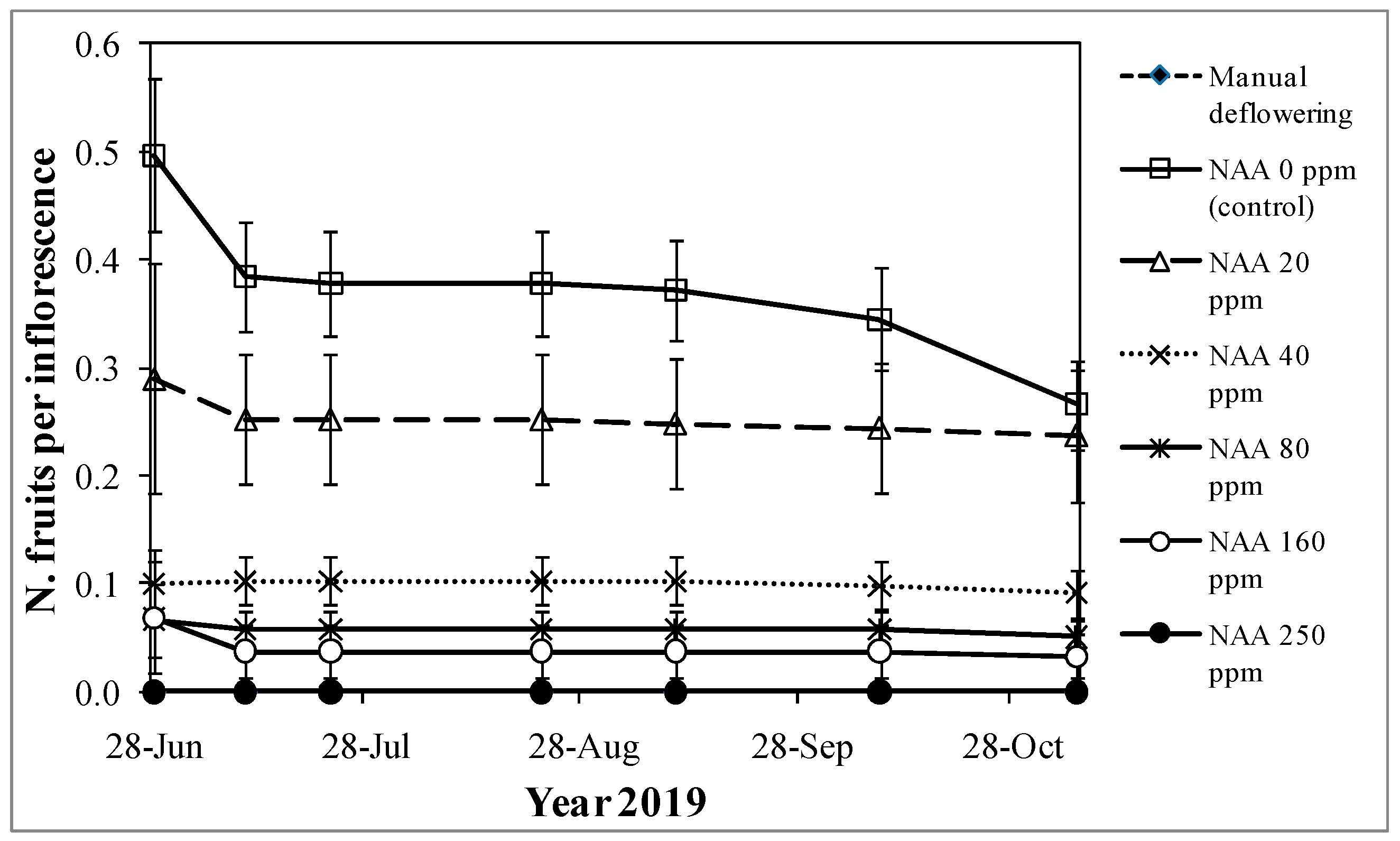
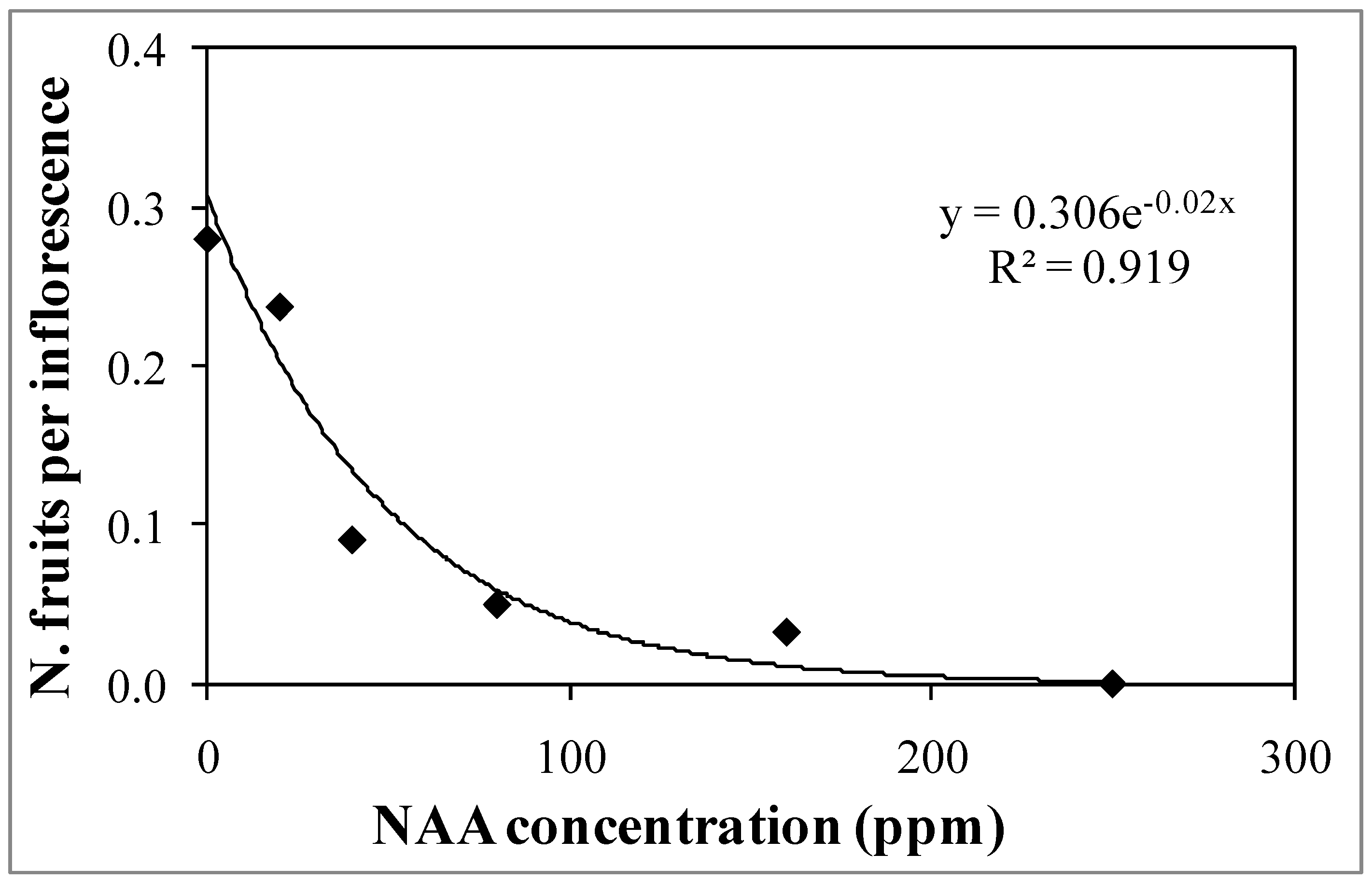
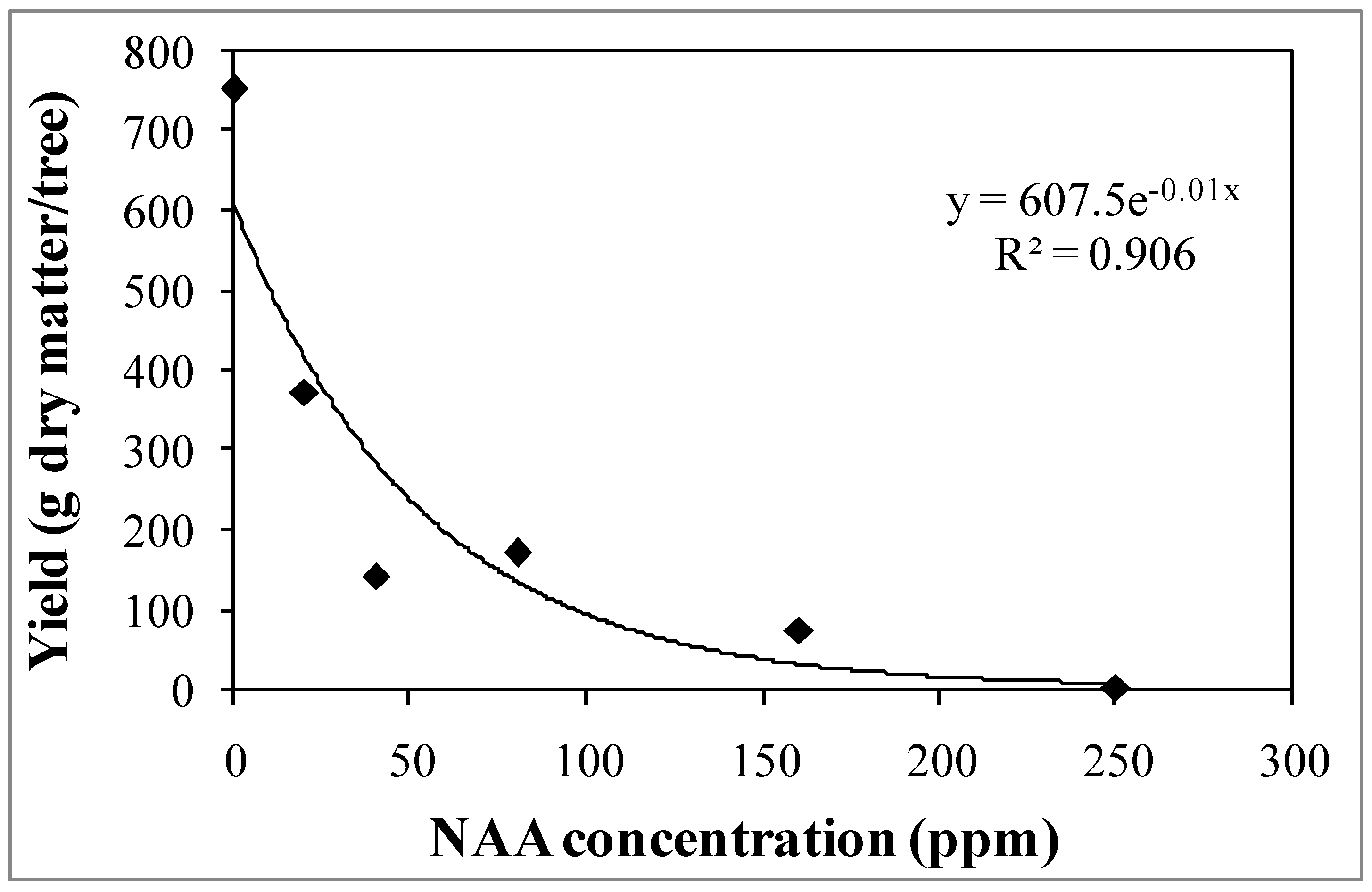
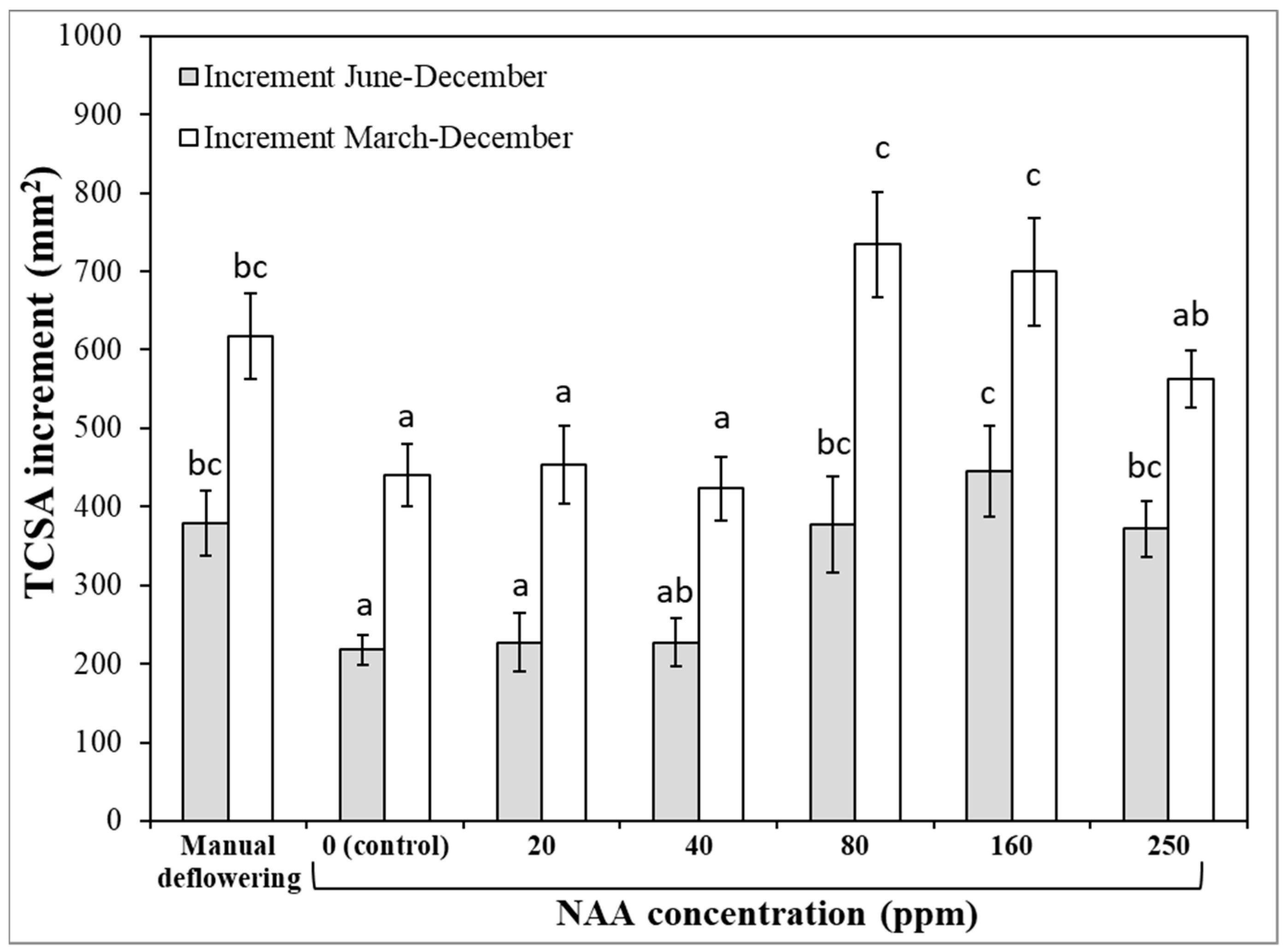
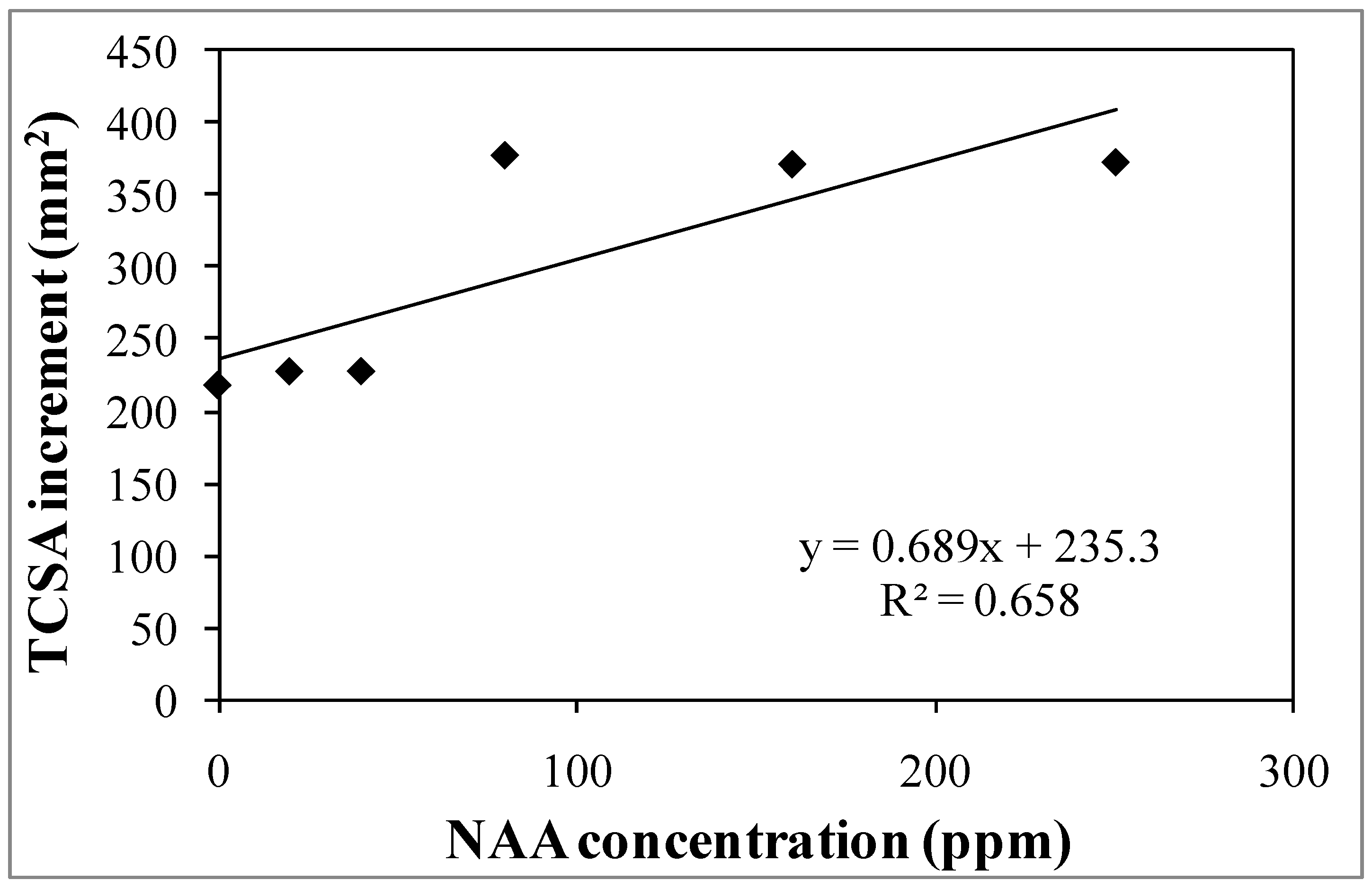
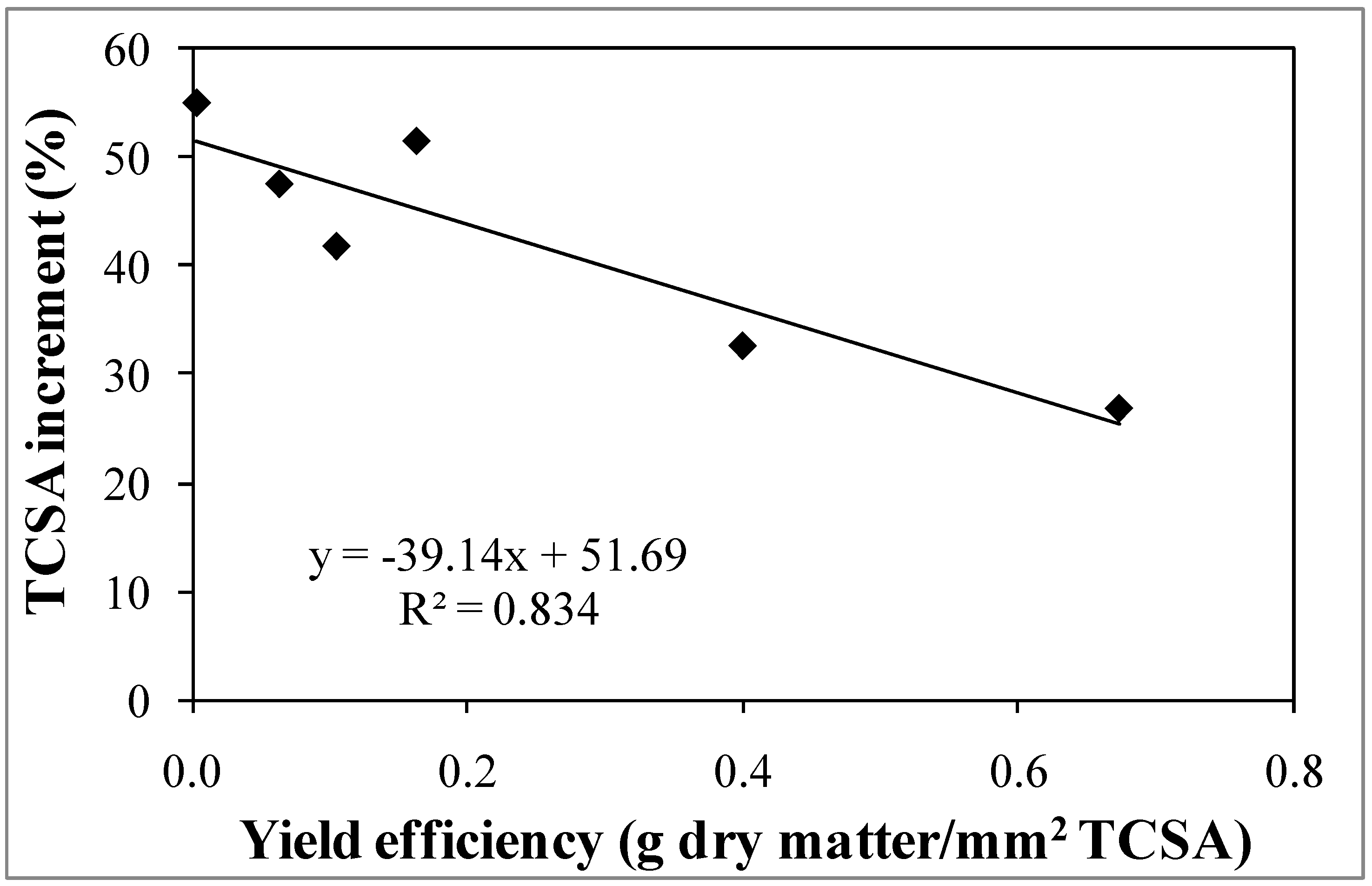
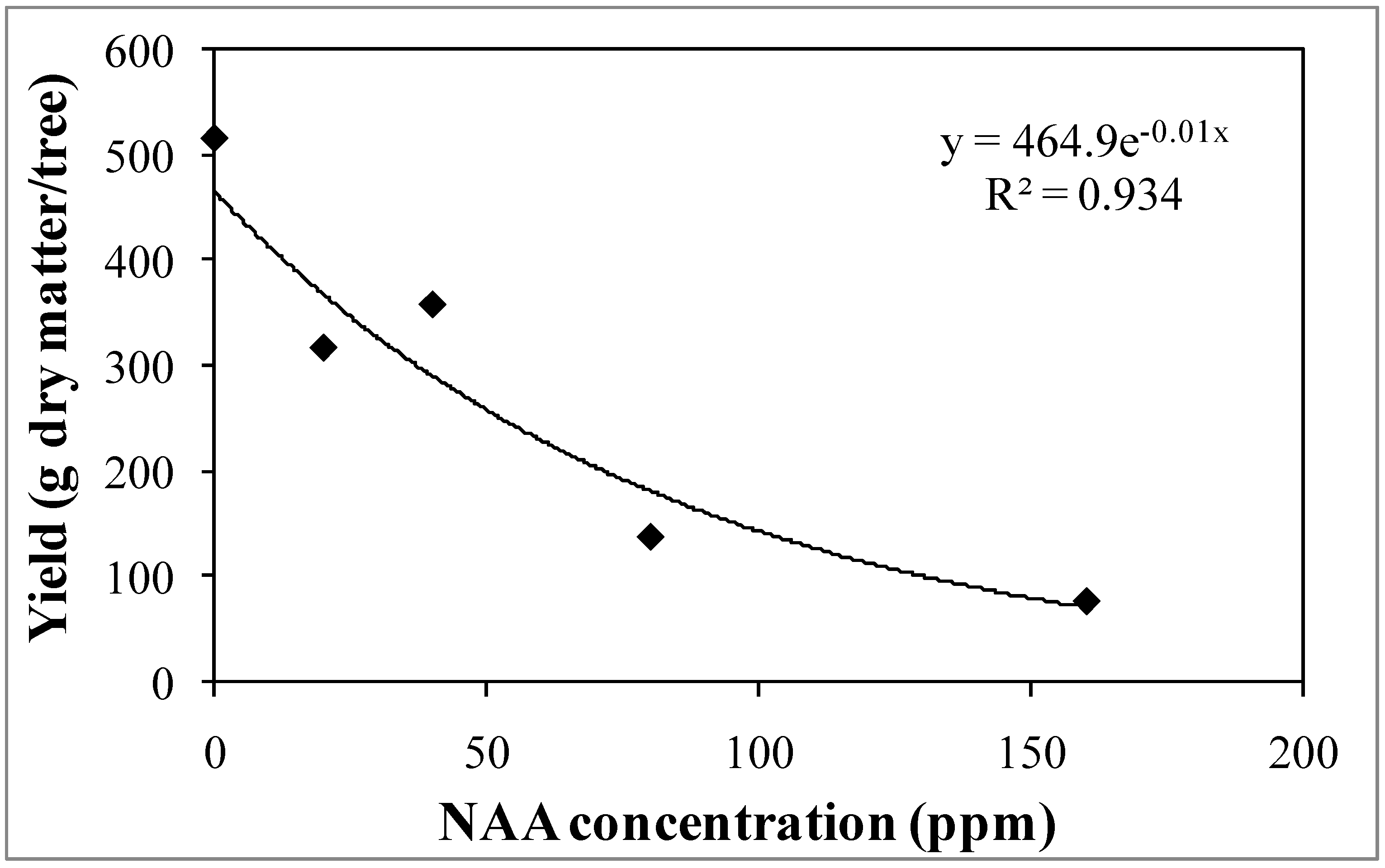
3.1. Experiment 1—Effects of Different NAA Concentrations on the Vegetative and Reproductive Activities of the Cultivar Moraiolo
3.2. Experiment 2—Effects of Different NAA Concentrations on the Reproductive Activity of the Cultivar Sikitita
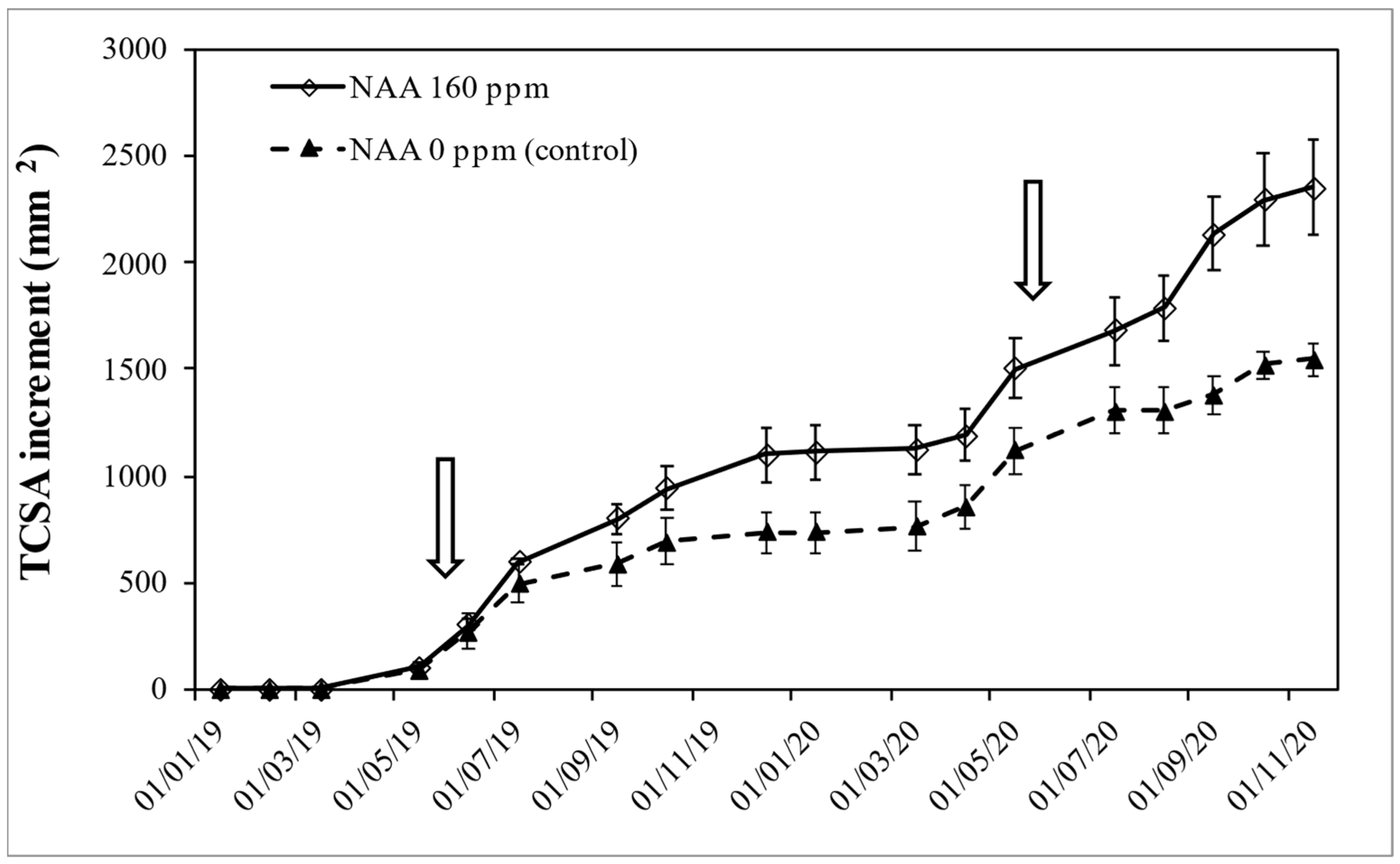
3.3. Experiment 3—Effects of NAA Treatments on Cultivar Moraiolo for Two Consecutive Years
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Famiani, F.; Gucci, R. Moderni Modelli Olivicoli; Collana divulgativa dell’Accademia Vol VII; Accademia Nazionale dell’Olivo e dell’Olio: Spoleto, Italy, 2011; p. 32. [Google Scholar]
- Lo Bianco, R.; Proietti, P.; Regni, L.; Caruso, T. Planting systems for modern olive growing: Strengths and weaknesses. Agriculture 2021, 11, 494. [Google Scholar] [CrossRef]
- Rallo, L.; Barranco, D.; Castro-Garcia, S.; Connor, D.J.; Gomez del Campo, M.; Rallo, P. High-density olive plantations. Hortic. Rev. 2013, 41, 303–384. [Google Scholar]
- Tognetti, R.; d’Andria, R.; Lavini, A.; Morelli, G. The effect of deficit irrigation on crop yield and vegetative development of Olea europaea L. (cvs. Frantoio and Leccino). Eur. J. Agron. 2006, 25, 356–364. [Google Scholar] [CrossRef]
- Pérez-López, D.; Ribas, F.; Moriana, A.; Olmedilla, N.; de Juan, A. The effect of irrigation schedules on the water relations and growth of a young olive (Olea europaea L.) orchard. Agric. Water Manag. 2007, 89, 297–304. [Google Scholar] [CrossRef]
- Gucci, R.; Lodolini, E.M.; Rapoport, H.F. Productivity of olive trees with different water status and crop load. J. Hortic. Sci. Biotechnol. 2007, 82, 648–656. [Google Scholar] [CrossRef]
- Martín-Vertedor, A.I.; Pérez Rodríguez, J.M.; Losada, H.P.; Castiel, E.F. Interactive responses to water deficits and crop load in olive (Olea europaea L., cv. Morisca) I.–Growth and water relations. Agric. Water Manag. 2011, 98, 941–949. [Google Scholar] [CrossRef]
- Rodrigues, M.; Lopes, J.; Pavão, F.; Cabanas, J.; Arrobas, M. Effect of soil management on olive yield and nutritional status of trees in rainfed orchards. Commun. Soil Sci. Plant Anal. 2011, 42, 993–1007. [Google Scholar] [CrossRef]
- Leskovar, D.; Othman, Y.A. Nitrogen management for improving root and shoot components of young ‘Arbequina’ olives. HortScience 2019, 54, 175–180. [Google Scholar] [CrossRef]
- Endeshaw, S.T.; Lodolini, E.M.; Neri, D. Effects of olive shoot residues on shoot and root growth of potted olive plantlets. Sci. Hortic. 2015, 182, 31–40. [Google Scholar] [CrossRef]
- Soriano-Martín, M.L.; Azcón, R.; Barea, J.M.; Porras-Soriano, A.; Marcilla-Goldaracena, I.; Porras-Piedra, A. Reduction of the juvenile period of new olive plantations through the early application of mycorrhizal fungi. Soil Sci. 2006, 171, 52–58. [Google Scholar] [CrossRef]
- Porras-Soriano, A.; Soriano-Martín, M.L.; Porras-Piedra, A.; Azcón, R. Arbuscular mycorrhizal fungi increased growth, nutrient uptake and tolerance to salinity in olive trees under nursery conditions. J. Plant Physiol. 2009, 166, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Almadi, L.; Paoletti, A.; Cinosi, N.; Daher, E.; Rosati, A.; Di Vaio, C.; Famiani, F. A biostimulant based on protein hydrolysates promotes the growth of young olive trees. Agriculture 2020, 10, 618. [Google Scholar] [CrossRef]
- Mazeh, M.; Almadi, L.; Paoleatti, A.; Cinosi, N.; Daher, E.; Tucci, M.; Lodolini, E.M.; Rosati, A.; Famiani, F. Use of organic fertilizer also having a biostimulant action to promote the growth of young olive trees. Agriculture 2021, 11, 593. [Google Scholar] [CrossRef]
- Smith, H.M.; Samach, A. Constraints to obtaining consistent annual yields in perennial tree crops. I: Heavy fruit load dominates over vegetative growth. Plant Sci. 2013, 207, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, M.T.; Shackel, K.A. Alternate bearing in pistachio as a masting phenomenon: Construction cost of reproduction versus vegetative growth and storage. J. Am. Soc. Hortic. Sci. 1998, 123, 1069–1075. [Google Scholar] [CrossRef]
- Costes, E.; Fournier, D.; Salles, J.C. Changes in primary and secondary growth as influenced by crop load in ‘Fantasme’ apricot trees. J. Hortic. Sci. Biotechnol. 2000, 75, 510–519. [Google Scholar] [CrossRef]
- Berman, M.E.; DeJong, T.M. Seasonal patterns of vegetative growth and competition with reproductive sink in peach. J. Hortic. Sci. Biotechnol. 2003, 78, 303–309. [Google Scholar] [CrossRef]
- Obeso, J.R. The cost of reproduction in plants. New Phytol. 2002, 155, 321–348. [Google Scholar] [CrossRef] [PubMed]
- Connor, D.J.; Fereres, E. The physiology of adaptation and yield expression in olive. Hortic. Rev. 2005, 31, 155–229. [Google Scholar]
- Lavee, S. Biennial bearing in olive (Olea europaea). Ann. Ser. Hist. Nat. 2007, 17, 101–112. [Google Scholar]
- Dag, A.; Bustan, A.; Avni, A.; Tzipori, I.; Lavee, S.; Riov, J. Timing of fruit removal affects concurrent vegetative growth and subsequent return bloom and yield in olive (Olea europaea L.). Sci. Hortic. 2010, 123, 469–472. [Google Scholar] [CrossRef]
- Castillo-Llanque, F.; Rapoport, H.F. Relationship between reproductive behavior and new shoot development in 5-year-old branches of olive trees (Olea europaea L.). Trees 2011, 25, 823–832. [Google Scholar] [CrossRef]
- Fernández, F.J.; Ladux, J.L.; Searles, P.S. Dynamics of shoot and fruit growth following fruit thinning in olive trees: Same season and subsequent season responses. Sci. Hortic. 2015, 192, 320–330. [Google Scholar] [CrossRef]
- Chandler, W.H.; Heinicke, A.J. The effect of fruiting on the growth of Oldenburg apple trees. Proc. Amer. Soc. Hort. Sci. 1926, 23, 36–46. [Google Scholar]
- Verheij, E.W.M. Competition in Apple as Influenced by Alar Sprays, Fruiting, Pruning and Tree Spacing. Ph.D. Thesis, Agricultural University, Wageningen, The Netherlands, 1972; 54p. [Google Scholar]
- Forshey, C.G.; Elfving, D.C. The relationship between vegetative growth and fruiting in apple trees. Hortic. Rev. 1989, 11, 229–287. [Google Scholar]
- Embree, C.G.; Myra, M.T.; Nichols, D.S.; Wright, A.H. Effect of blossom density and crop load on growth, fruit quality, and return bloom in ‘Honeycrisp’ apple. HortScience 2007, 42, 1622–1625. [Google Scholar] [CrossRef]
- Rosati, A.; Paoletti, A.; Al Hariri, R.; Morelli, A.; Famiani, F. Partitioning of dry matter into fruit explains cultivar differences in vigor in young olive (Olea europaea L.) trees. HortScience 2018, 53, 491–495. [Google Scholar] [CrossRef]
- Rosati, A.; Paoletti, A.; Pannelli, G.; Famiani, F. Growth is inversely correlated with yield efficiency across cultivars in young olive (Olea europaea L.) trees. HortScience 2017, 52, 1525–1529. [Google Scholar] [CrossRef]
- Rosati, A.; Paoletti, A.; Al Hariri, R.; Morelli, A.; Famiani, F. Resource investments in reproductive growth proportionately limit investments in whole-tree vegetative growth in young olive trees with varying crop loads. Tree Physiol. 2018, 38, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Botton, A.; Vizzotto, G. Fruit thinning: Advances and trends. Hortic. Rev. 2018, 46, 185–226. [Google Scholar]
- Krueger, W.H.; Maranto, J.; Sibbett, G.S. Olive fruit thinning. In Olive Production Manual; Sibbett, G.S., Ferguson, L., Eds.; University of California, Agriculture and Natural Resources, Publication 3353: Oakland, CA, USA, 2005; pp. 101–104. [Google Scholar]
- Dag, A.; Bustan, A.; Avni, A.; Lavee, S.; Riov, J. Fruit thinning using NAA shows potential for reducing biennial bearing of ‘Barnea’ and ‘Picual’ oil olive trees. Crop. Pasture. Sci. 2009, 60, 1124–1130. [Google Scholar] [CrossRef]
- Krueger, W.H.; Heat, Z.; Mulqueeney, B. Effect of spray solution concentration, active ingredient, additives and sequential treatments of Naphtalene Acetic Acid for chemical thinning of Manzanillo table olives (Olea europaea). Acta Hortic. 2002, 586, 267–271. [Google Scholar] [CrossRef]
- Barone, E.; La Mantia, M.; Marchese, A.; Marra, F.P. Improvement in yield and fruit size and quality of the main Italian table olive cultivar ‘Nocellara del Belice’. Sci. Agric. 2014, 71, 52–57. [Google Scholar] [CrossRef]
- Crous, J.J.; Steyn, W.J. Chemical thinning of ‘Barouni’ and ‘Manzanilla’ olives with naphthalene acetic acid under South African conditions. S. Afr. J. Plant Soil 2015, 32, 47–54. [Google Scholar] [CrossRef]
- Hegazi, E.S.; Hegzi, A.A.; ElKoly, D.M. Effect of some chemical thinning agents on fruit set of Manzanillo and Eggezi Shgami olive cultivars. J. Hortic. Sci. Ornam. 2015, 7, 117–123. [Google Scholar]
- Paoletti, A.; Rosati, A.; Famiani, F. Effects of cultivar, fruit presence and tree age on whole-plant dry matter partitioning in young olive trees. Heliyon 2021, 7, e06949. [Google Scholar] [CrossRef]
- DeJong, T.M. Development and environmental control of dry-matter partitioning in peach. HortScience 1999, 34, 1037–1040. [Google Scholar] [CrossRef] [Green Version]
- Wunsche, J.N.; Ferguson, I.B. Crop load interactions in apple. Hortic. Rev. 2005, 31, 231–290. [Google Scholar]
- Monselise, S.P.; Goldschmidt, E.E. Alternate bearing in fruit trees. Hortic. Rev. 1982, 4, 128–173. [Google Scholar]
- Rallo, L.; Suarez, M.P. Seasonal distribution of dry matter within the olive fruit-bearing limb. Adv. Hort. Sci. 1989, 3, 55–59. [Google Scholar]
- Lodolini, E.M.; Neri, D. How growth and reproduction cycles affect alternate bearing in olive. Acta Hortic. 2012, 949, 191–198. [Google Scholar] [CrossRef]
- Di Vaio, C.; Nocerino, S.; Paduano, A.; Sacchi, R. Characterization and evaluation of olive germplasm in southern Italy. J. Sci. Food Agric. 2013, 93, 2458–2462. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Famiani, F.; Cinosi, N.; Paoletti, A.; Farinelli, D.; Rosati, A.; Lodolini, E.M. Deflowering as a Tool to Accelerate Growth of Young Trees in Both Intensive and Super-High-Density Olive Orchards. Agronomy 2022, 12, 2319. https://doi.org/10.3390/agronomy12102319
Famiani F, Cinosi N, Paoletti A, Farinelli D, Rosati A, Lodolini EM. Deflowering as a Tool to Accelerate Growth of Young Trees in Both Intensive and Super-High-Density Olive Orchards. Agronomy. 2022; 12(10):2319. https://doi.org/10.3390/agronomy12102319
Chicago/Turabian StyleFamiani, Franco, Nicola Cinosi, Andrea Paoletti, Daniela Farinelli, Adolfo Rosati, and Enrico Maria Lodolini. 2022. "Deflowering as a Tool to Accelerate Growth of Young Trees in Both Intensive and Super-High-Density Olive Orchards" Agronomy 12, no. 10: 2319. https://doi.org/10.3390/agronomy12102319
APA StyleFamiani, F., Cinosi, N., Paoletti, A., Farinelli, D., Rosati, A., & Lodolini, E. M. (2022). Deflowering as a Tool to Accelerate Growth of Young Trees in Both Intensive and Super-High-Density Olive Orchards. Agronomy, 12(10), 2319. https://doi.org/10.3390/agronomy12102319








