Comprehensive Assessment of the Influence of Applying Two Kinds of Chicken-Manure-Processed Organic Fertilizers on Soil Properties, Mineralization of Nitrogen, and Yields of Three Crops
Abstract
1. Introduction
1.1. Characteristics of Organic Fertilizer
1.2. Mineralization of Organic Fertilizer
1.3. Impact of Organic Fertilizer on the Environment
1.4. Objectives
2. Materials and Methods
2.1. Field Experiment and Analysis
2.2. Crop Harvest and Analysis
2.3. Incubation Experiment
2.4. Statistical Analysis
3. Results and Discussion
3.1. Basic Properties of Soil, Organic Fertilizer, and Irrigation Water
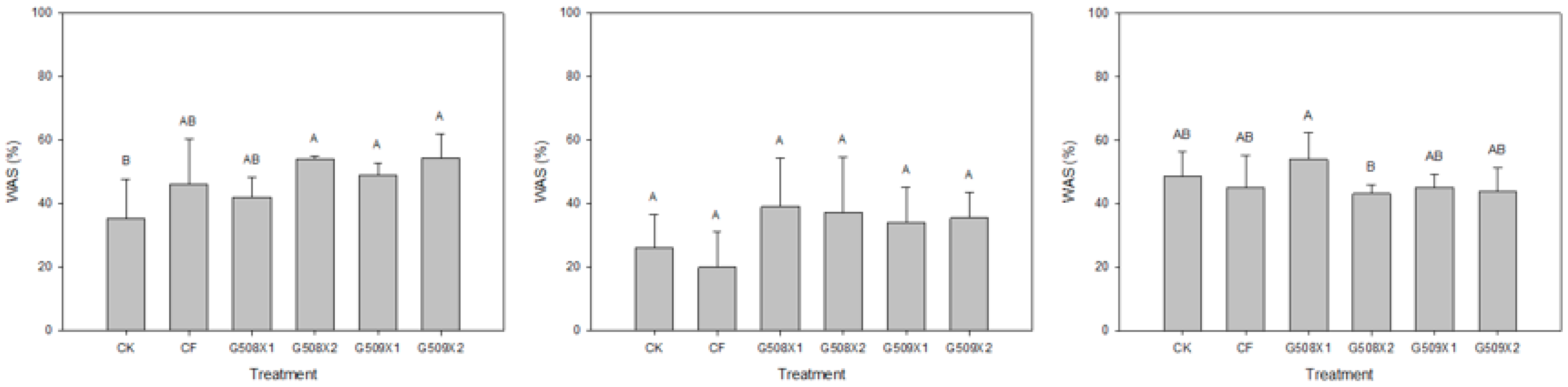
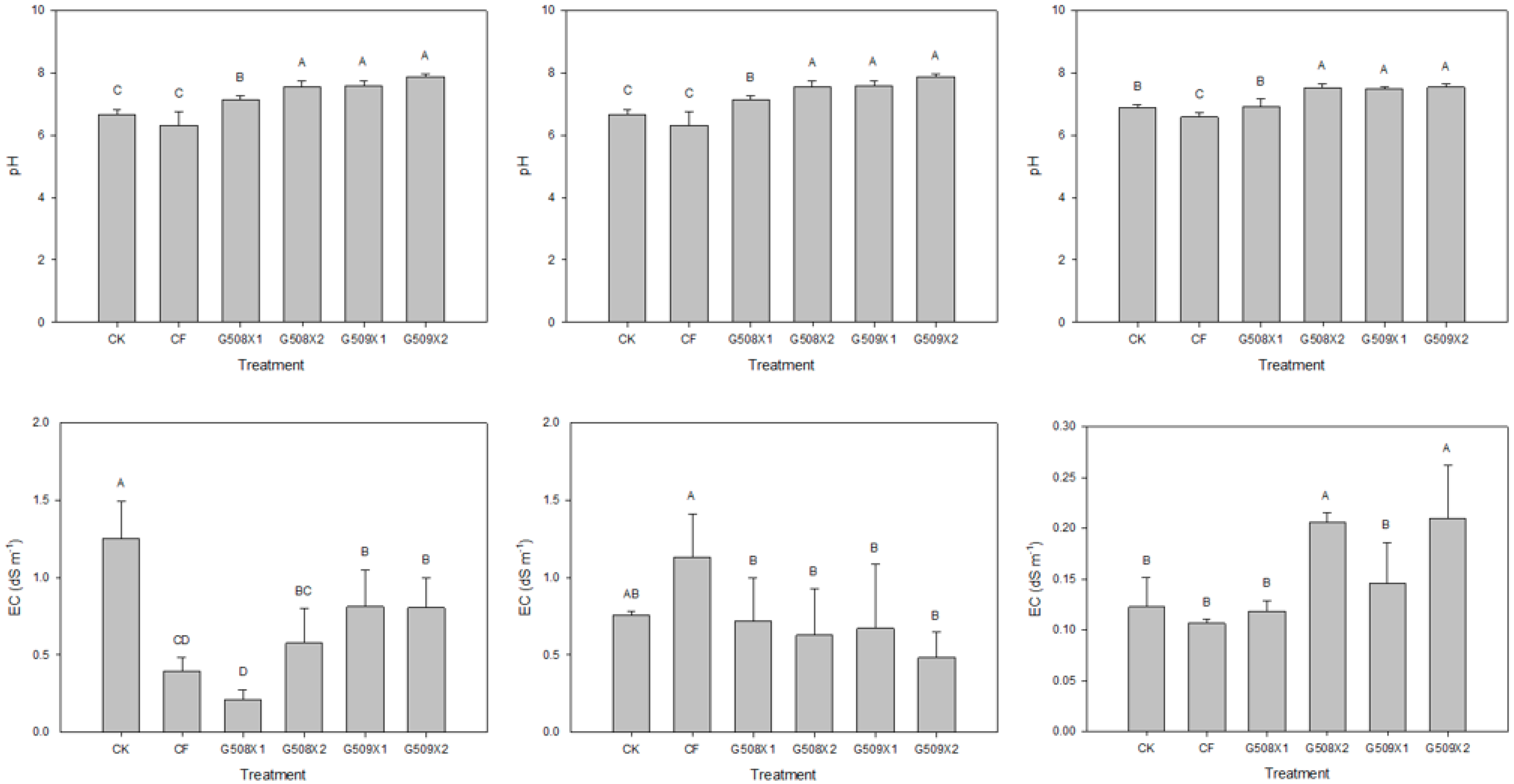
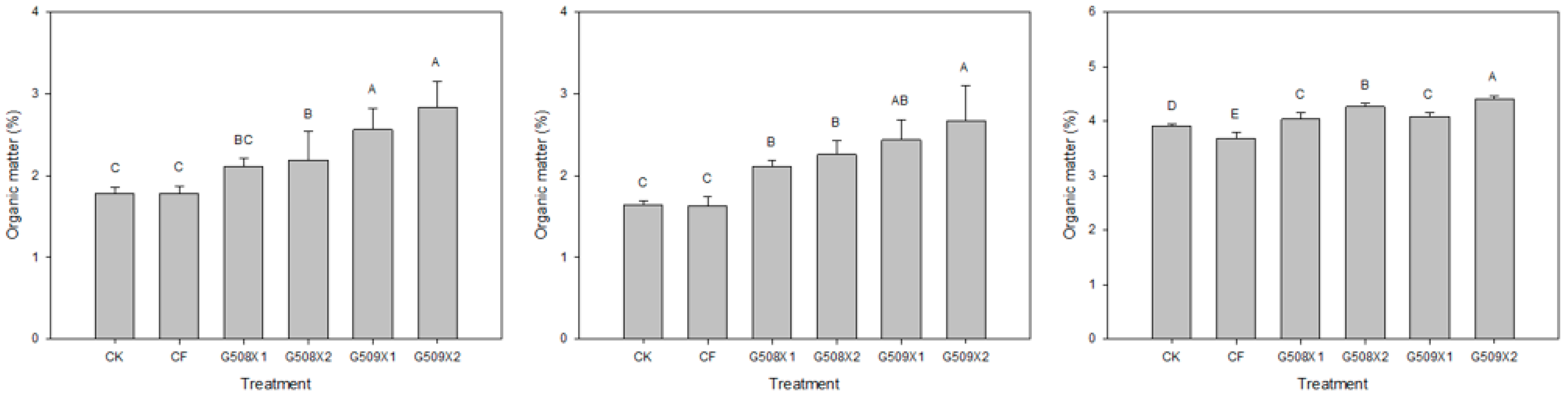
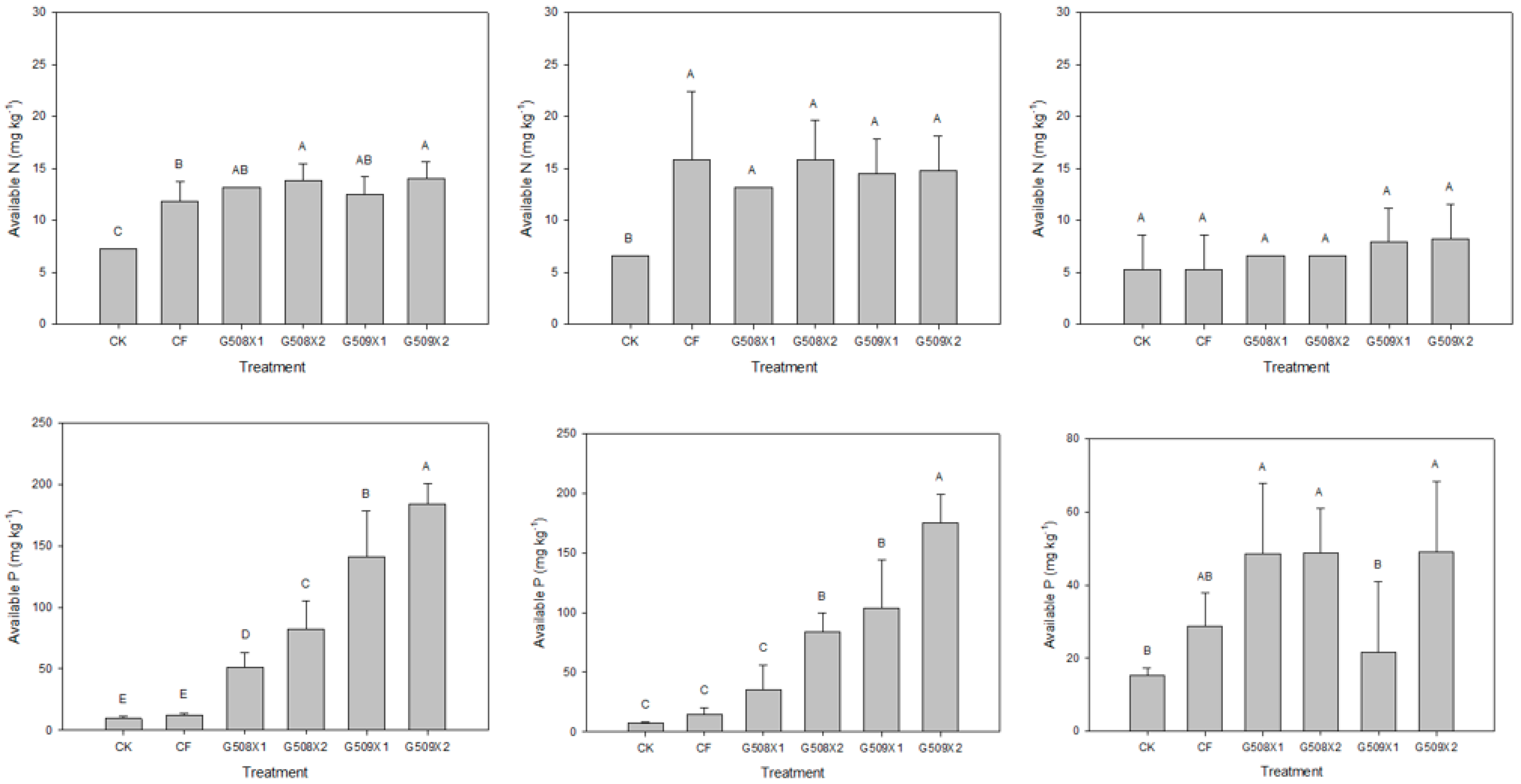
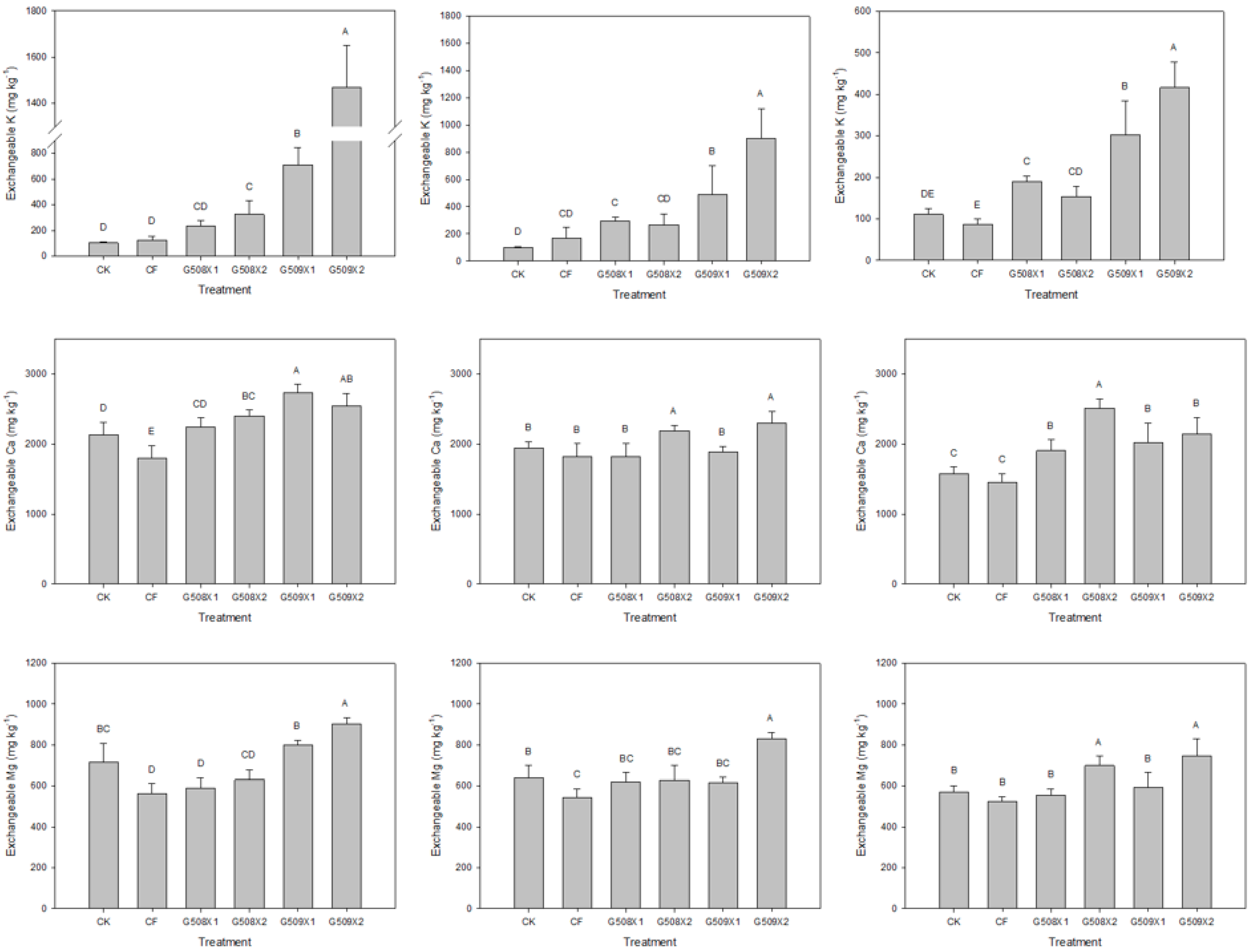
3.2. Influence on Soil Properties
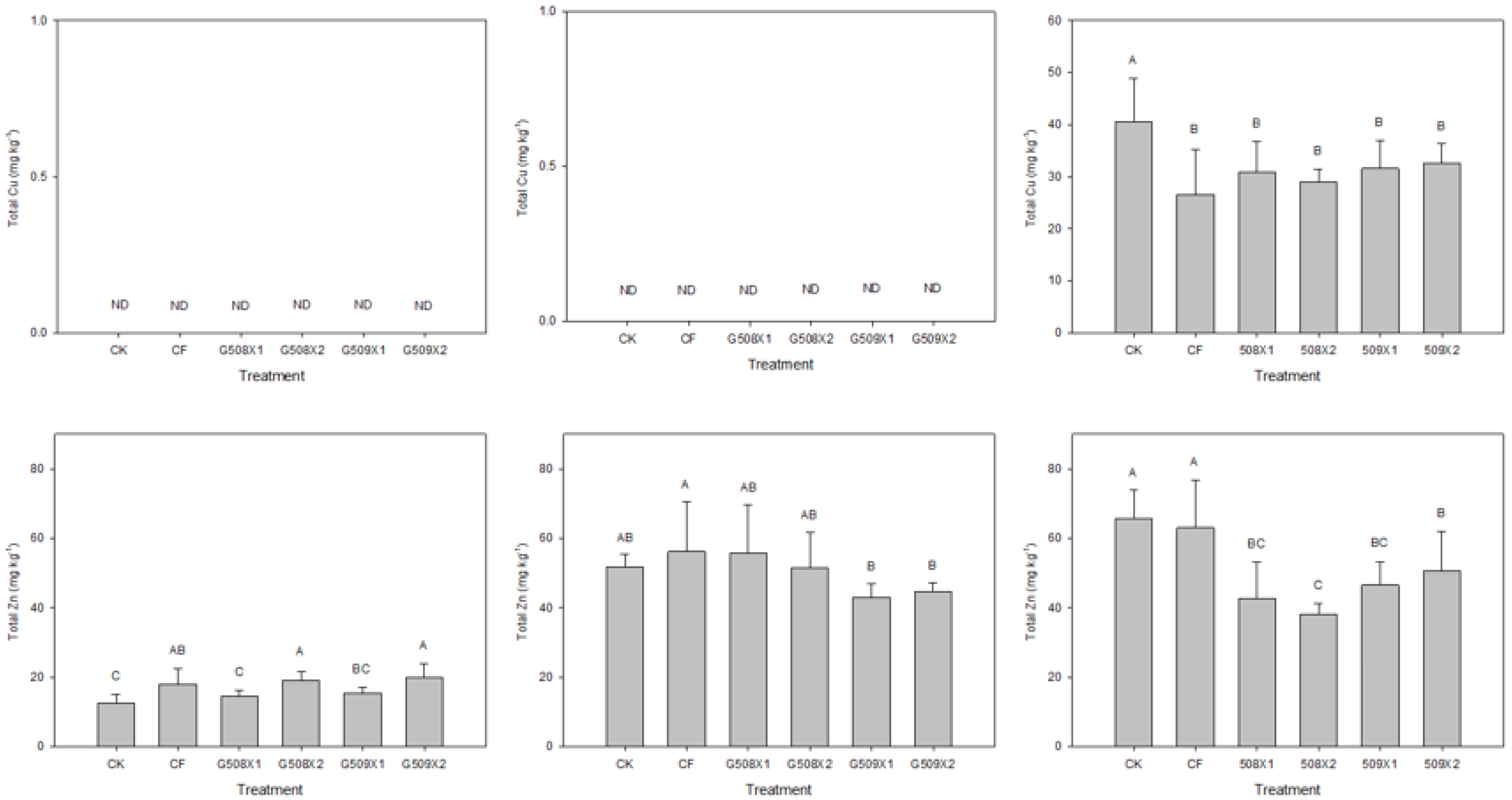
3.3. Results of the Incubation Experiment
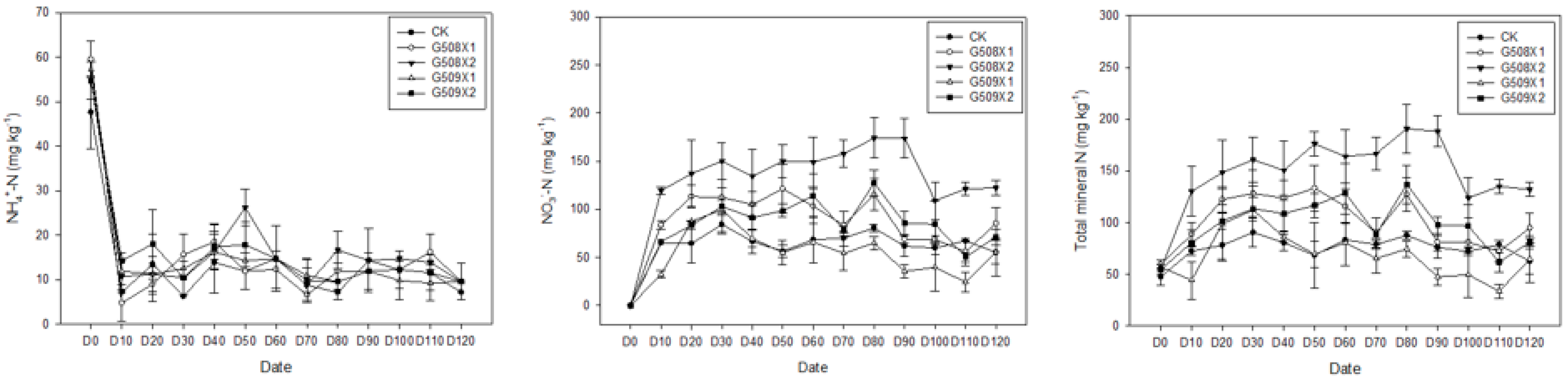
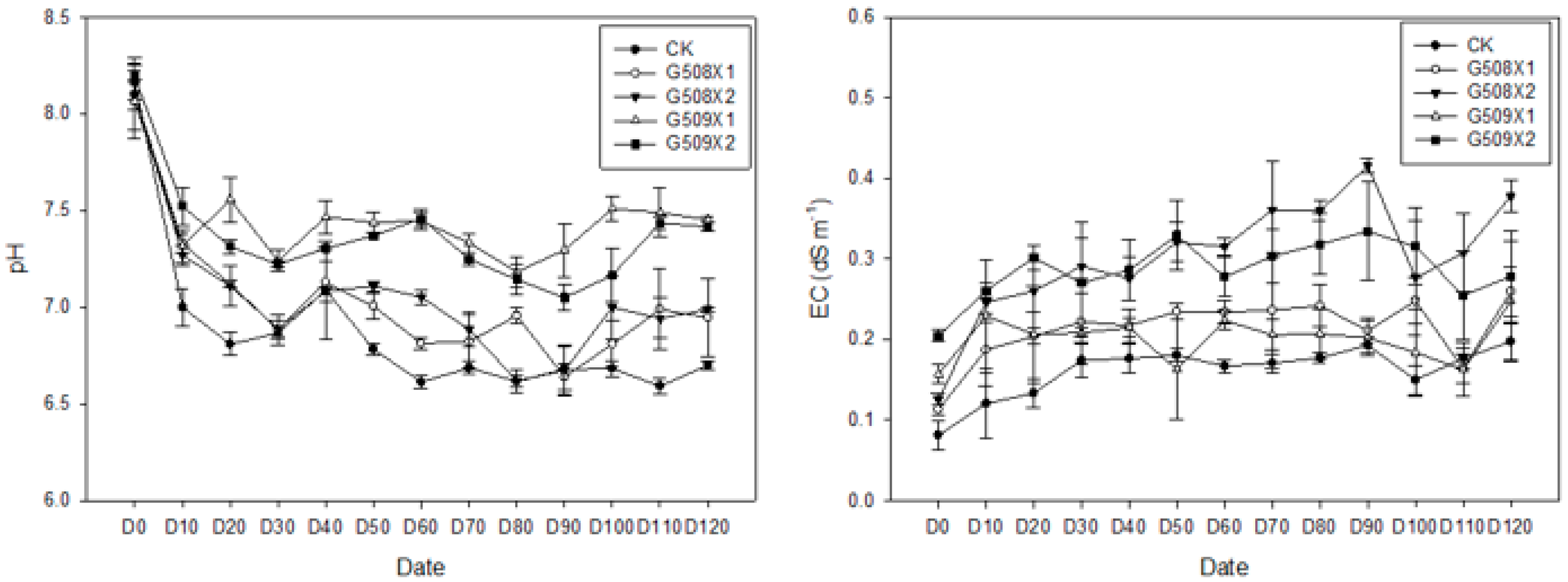
3.3.1. Influence on the Soil’s Available N
3.3.2. Influence on Soil’s pH and EC
3.4. Influence on Crops
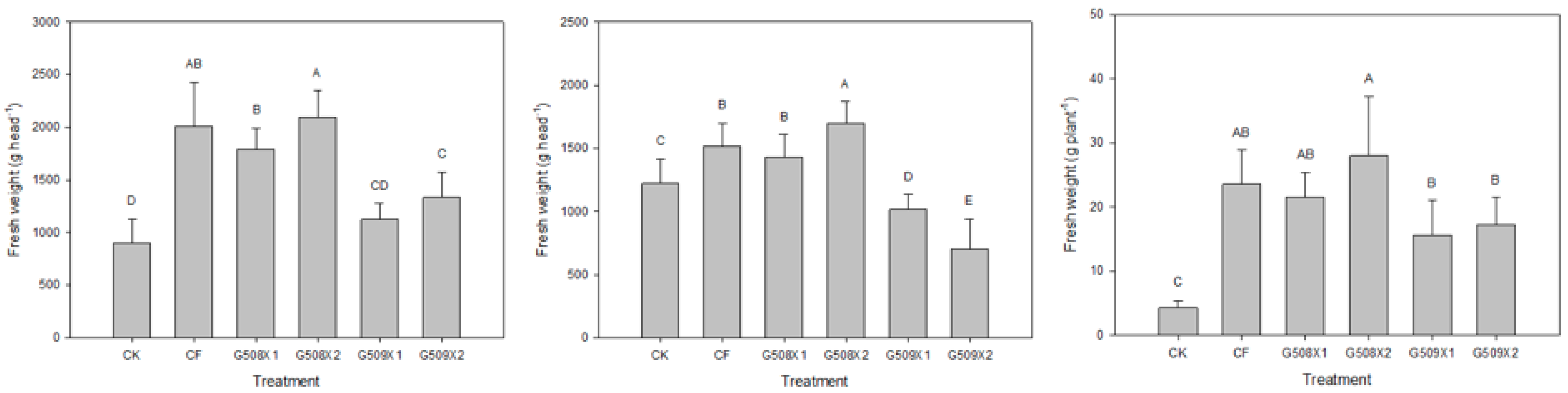
3.4.1. Influence on Crop Yield and Agronomic Traits
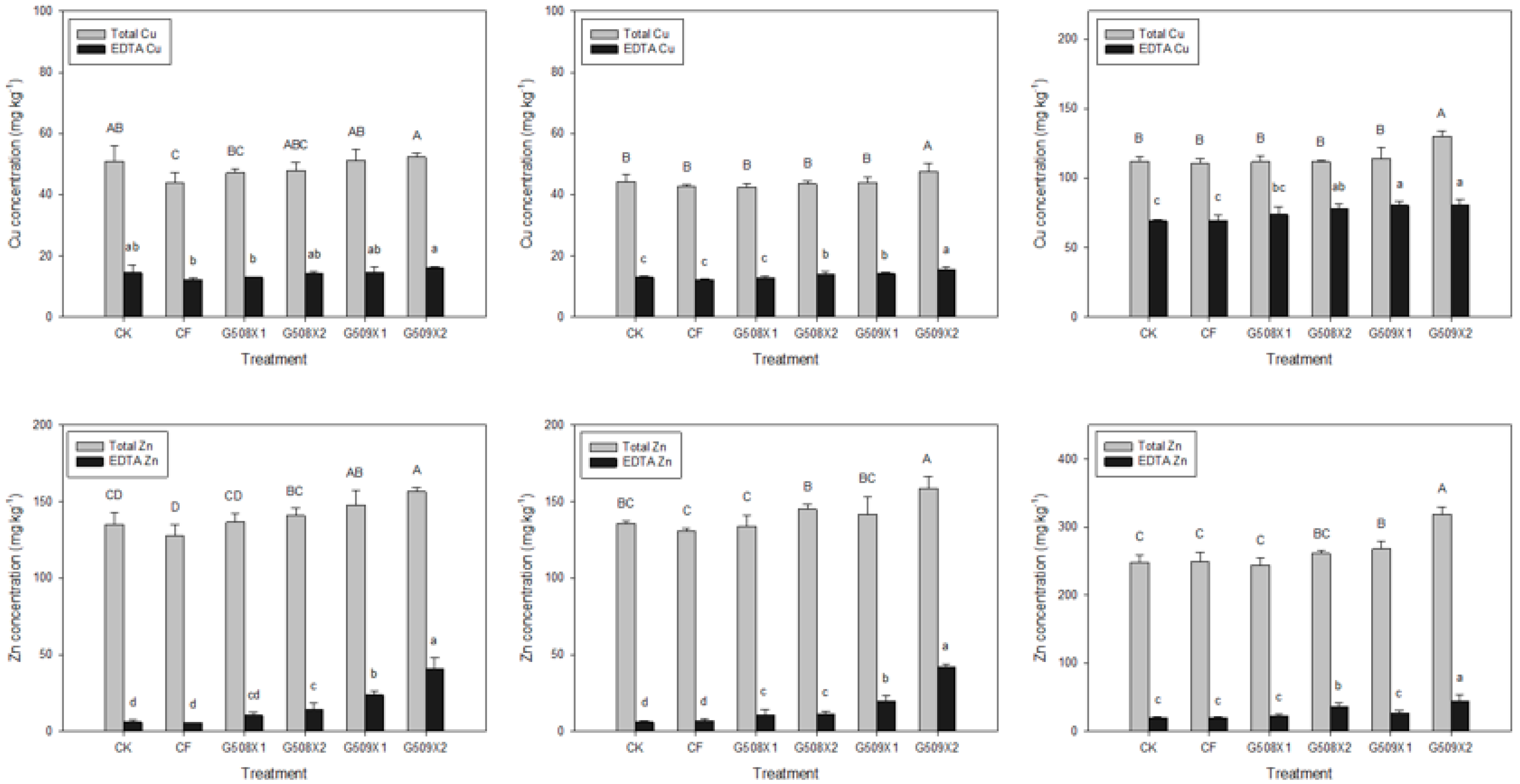
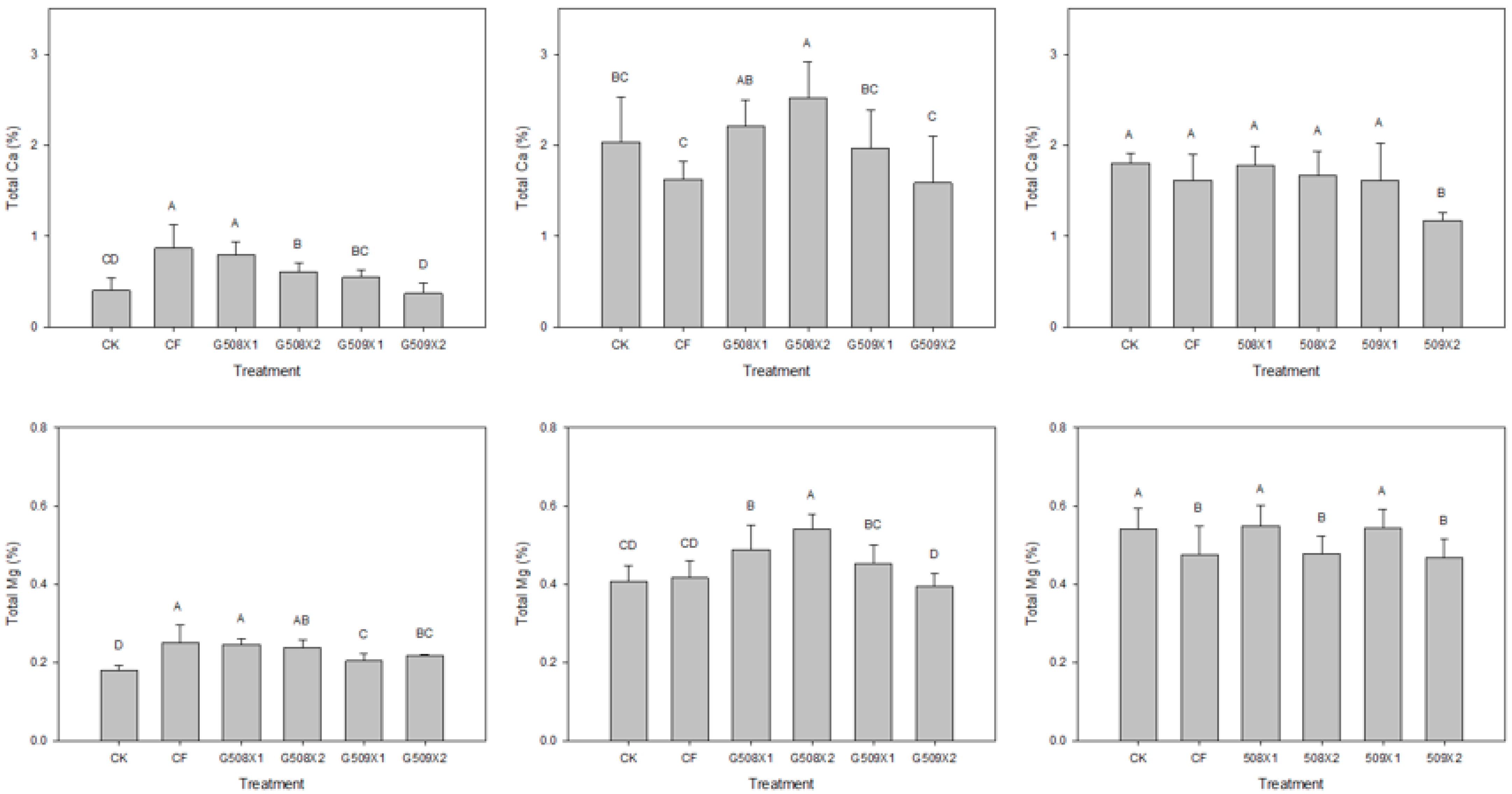
3.4.2. Influence on Element Content
3.5. Nutrient Absorption Proportion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Soil Property | pH | EC | WAS | OM | Avail. N | Avail. P | Exch. K | Exch. Ca | Exch. Mg | Total Cu | Total Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dS m−1 | ---- % ---- | -------------------------------------- mg kg−1 -------------------------------------- | ||||||||||
| Mean | 7.07 | 0.17 | 35.81 | 1.22 | 8.31 | 10.1 | 147.5 | 1608 | 406.7 | 44.19 | 149.2 | |
| Block A | Min. | 6.79 | 0.12 | 27.14 | 0.98 | 4.61 | 7.02 | 189.8 | 1483 | 389.6 | 40.39 | 136.7 |
| Max. | 7.46 | 0.29 | 44.43 | 1.39 | 12.5 | 14.1 | 120.8 | 1811 | 423.3 | 50.85 | 169.7 | |
| Mean | 6.42 | 0.22 | 46.05 | 3.69 | 8.98 | 17.7 | 160.5 | 1938 | 473.5 | 124.7 | 292.3 | |
| Block B | Min. | 6.05 | 0.13 | 42.05 | 3.25 | 6.59 | 7.38 | 126.4 | 1747 | 451.4 | 113.6 | 259.3 |
| Max. | 7.02 | 0.32 | 50.49 | 4.00 | 11.9 | 44.0 | 205.0 | 2160 | 499.8 | 140.9 | 344.3 | |
| Inlet | pH | EC | K | Ca | Mg |
|---|---|---|---|---|---|
| dS m−1 | ------------------------------- mg L−1 ------------------------------- | ||||
| Water 1 | 8.12 ± 0.04 | 0.80 ± 0.01 | 8.6 ± 0.6 | 65.2 ± 0.0 | 24.6 ± 2.4 |
| Water 2 | 8.28 ± 0.04 | 0.56 ± 0.01 | 7.8 ± 0.6 | 71.3 ± 0.4 | 24.6 ± 0.4 |
| Water 3 | 8.13 ± 0.01 | 0.53 ± 0.00 | 6.1 ± 0.2 | 61.2 ± 1.6 | 21.0 ± 0.3 |
| Water 4 | 8.20 ± 0.05 | 0.62 ± 0.01 | 9.6 ± 0.1 | 69.6 ± 1.2 | 25.6 ± 0.1 |
| Water 5 | 8.24 ± 0.07 | 0.81 ± 0.00 | 12.0 ± 0.2 | 77.6 ± 0.4 | 28.4 ± 1.5 |
| Water 6 | 8.13 ± 0.16 | 0.61 ± 0.07 | 6.1 ± 0.0 | 57.1 ± 0.0 | 21.8 ± 6.0 |
| Water 7 | 8.34 ± 0.01 | 0.67 ± 0.00 | 10.7 ± 0.3 | 80.2 ± 0.8 | 26.8 ± 1.0 |
References
- Mamo, M.; Molina, J.A.E.; Rosen, C.J.; Halbach, T.R. Nitrogen and carbon mineralization in soil amended with municipal solid waste compost. Can. J. Soil Sci. 1999, 79, 535–542. [Google Scholar] [CrossRef]
- Hudson, B.D. Soil organic-matter and available water capacity. J. Soil Water Conserv. 1994, 49, 189–194. [Google Scholar]
- Feng, G.; Adeli, A.; Read, J.; McCarty, J.; Jenkins, J. Consequences of pelletized poultry litter applications on soil physical and hydraulic properties in reduced tillage, continuous cotton system. Soil Tillage Res. 2019, 194, 104309. [Google Scholar] [CrossRef]
- Bayu, W.; Rethman, N.F.G.; Hammes, P.S.; Alemu, G. Application of farmyard manure improved the chemical and physical properties of the soil in a semi-arid area in Ethiopia. Biol. Agric. Hortic. 2006, 24, 293–300. [Google Scholar] [CrossRef]
- Adeli, A.; Varco, J.J.; Sistani, K.; Rowe, D. Effects of swine lagoon effluent relative to commercial fertilizer applications on warm-season forage nutritive value. Agron. J. 2005, 97, 408–417. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Zhang, S.; Wang, Y. What could promote farmers to replace chemical fertilizers with organic fertilizers? J. Clean. Prod. 2018, 199, 882–890. [Google Scholar] [CrossRef]
- Tewolde, H.; Buehring, N.; Feng, G.; Way, T. Managing soil nutrient buildup by rotating crops and fertilizers following repeated poultry litter applications. Soil Sci. Soc. Am. J. 2021, 85, 340–352. [Google Scholar] [CrossRef]
- Dikinya, O.; Mufwanzala, N. Chicken manure enhanced soil fertility and productivity: Effects of application rates. J. Soil Sci. Environ. Manag. 2010, 3, 46–54. [Google Scholar]
- Amos, H.; Izundu, C.; Audu, I. Effect of chicken manure on the performance of vegetable maize (Zea mays saccharate) varieties under irrigation. Discourse J. Agric. Food Sci. 2013, 12, 190–195. [Google Scholar]
- Ahmed, A.M.S.; Abu-Zreig, M.; Abdalla, M.A.; Yamanaka, N.; Elhadi, E.A.; Rezig, F.A.M. Integration of composts with NPK improved soil fertility, growth and yield of sorghum in sandy desert soils of Sudan. Int. J. Agric. Biol. 2020, 23, 373–380. [Google Scholar] [CrossRef]
- Huang, X.; Jia, Z.; Guo, J.; Li, T.; Sun, D.; Meng, H.; Yu, G.; He, X.; Ran, W.; Zhang, S.; et al. Ten-year long-term organic fertilization enhances carbon sequestration and calcium-mediated stabilization of aggregate-associated organic carbon in a reclaimed Cambisol. Geoderma 2019, 355, 113880. [Google Scholar] [CrossRef]
- Mumbach, G.L.; Gatiboni, L.C.; de Bona, F.D.; Schmitt, D.E.; Correa, J.C.; Gabriel, C.A.; Dall’Orsoletta, D.J.; Iochims, D.A. Agronomic efficiency of organomineral fertilizer in sequential grain crops in southern Brazil. Agron. J. 2020, 112, 3037–3049. [Google Scholar] [CrossRef]
- Ismale, F.; Ndayiragije, A.; Fangueiro, D. New fertilizer strategies combining manure and urea for improved rice growth in Mozambique. Agronomy 2021, 11, 783. [Google Scholar] [CrossRef]
- Abuarab, M.E.; El-Mogy, M.M.; Hassan, A.M.; Abdeldaym, E.A.; Abdelkader, N.H.; El-Sawy, M.B.I. The effects of root aeration and different soil conditioners on the nutritional values, yield, and water productivity of potato in clay loam soil. Agronomy 2019, 9, 418. [Google Scholar] [CrossRef]
- Cassity-Duffey, K.; Cabrera, M.; Gaskin, J.; Franklin, D.; Kissel, D.; Saha, U. Nitrogen mineralization from organic materials and fertilizers: Predicting N release. Soil Sci. Soc. Am. J. 2020, 84, 522–533. [Google Scholar] [CrossRef]
- Azeez, J.O.; Van Averbeke, W. Nitrogen mineralization potential of three animal manures applied on a sandy clay loam soil. Bioresour. Technol. 2010, 101, 5645–5651. [Google Scholar] [CrossRef] [PubMed]
- Rasouli-Sadaghiani, M.H.; Moradi, N. Effect of poultry, cattle, sheep manures and sewage sludge on N mineralisation. Chem. Ecol. 2014, 30, 666–675. [Google Scholar] [CrossRef]
- Rothé, M.; Darnaudery, M.; Thuries, L. Organic fertilizers, green manures and mixtures of the two revealed their potential as substitutes for inorganic fertilizers used in pineapple cropping. Sci. Hortic. 2019, 257, 108691. [Google Scholar] [CrossRef]
- Gordillo, R.M.; Cabrera, M.L. Mineralizable nitrogen in broiler litter: I. Effect of selected litter chemical characteristics. J. Environ. Qual. 1997, 26, 1672–1679. [Google Scholar] [CrossRef]
- Paul, E.A.; Clark, F.E. Soil Microbiology and Biochemistry, 2nd ed.; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Cui, L.; Li, D.; Wu, Z.; Xue, Y.; Xiao, F.; Zhang, L.; Song, Y.; Li, Y.; Zheng, Y.; Zhang, J.; et al. Effects of nitrification inhibitors on soil nitrification and ammonia volatilization in three soils with different pH. Agronomy 2021, 11, 1674. [Google Scholar] [CrossRef]
- Bergamasco, M.A.M.; Braos, L.B.; Lopes, S.L.; Cruz, M.C.P. Nitrogen mineralization and nitrification in two soil with different pH levels. Commun. Soil Sci. Plant Anal. 2019, 50, 2873–2880. [Google Scholar] [CrossRef]
- Eneji, A.E.; Irshad, M.; Honna, T.; Yamamoto, S.; Endo, T.; Masuda, T. Potassium, calcium, and magnesium mineralization in manure-treated soils. Commun. Soil Sci. Plant Anal. 2003, 34, 1669–1679. [Google Scholar] [CrossRef]
- Huang, C.C.; Chen, Z.S. Carbon and nitrogen mineralization of sewage sludge compost in soils with a different initial pH. Soil Sci. Plant Nutr. 2009, 55, 715–724. [Google Scholar] [CrossRef]
- Cabaleiro, F.A.; Sainz, M.J.; Seoane-Labandeira, S.; Lopez-Mosquera, M.E. Salt effect of dehydrated broiler litter on organic lettuce. Biol. Agric. Hortic. 2018, 34, 107–119. [Google Scholar] [CrossRef]
- Wang, X.H.; Song, Y.P.; Ren, J.; Liu, T.K.; Hou, X.L.; Li, Y. Response of biomass and photosynthesis in non-heading Chinese cabbage to excess copper. J. Anim. Plant Sci. 2013, 23, 1659–1665. [Google Scholar]
- Ali, S.; Rizwan, M.; Ullah, N.; Bharwana, S.A.; Waseem, M.; Farooq, M.A.; Abbasi, G.H.; Farid, M. Physiological and biochemical mechanisms of silicon-induced copper stress tolerance in cotton (Gossypium hirsutum L.). Acta Physiol. Plant. 2016, 38, 262. [Google Scholar] [CrossRef]
- Chiou, W.Y.; Hsu, F.C. Copper toxicity and prediction models of copper content in leafy vegetables. Sustainability 2019, 11, 6215. [Google Scholar] [CrossRef]
- Warne, M.S.J.; Heemsbergen, D.; McLaughlin, M.; Bell, M.; Broos, K.; Whatmuff, M.; Barry, G.; Nash, D.; Pritchard, D.; Penney, N. Models for the field-based toxicity of copper and zinc salts to wheat in 11 Australian soils and comparison to laboratory-based models. Environ. Pollut. 2008, 156, 707–714. [Google Scholar] [CrossRef]
- Tiecher, T.L.; Ceretta, C.A.; Tiecher, T.; Ferreira, P.A.A.; Nicoloso, F.T.; Soriani, H.H.; Rossato, L.V.; Mimmo, T.; Cesco, S.; Lourenzi, C.R.; et al. Effects of zinc addition to a copper-contaminated vineyard soil on sorption of Zn by soil and plant physiological responses. Ecotoxicol. Environ. Saf. 2016, 129, 109–119. [Google Scholar] [CrossRef]
- Du, W.; Yang, J.; Peng, Q.; Liang, X.; Mao, H. Comparison study of zinc nanoparticles and zinc sulphate on wheat growth: From toxicity and zinc biofortification. Chemosphere 2019, 227, 109–116. [Google Scholar] [CrossRef]
- Lafond, S.; Pare, T.; Dinel, H.; Schnitzer, M.; Chambers, J.R.; Jaouich, A. Composting duck excreta enriched wood shavings: C and N transformations and bacterial pathogen reductions. J. Environ. Sci. Health B 2002, 37, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Turner, C. The thermal inactivation of E-coli in straw and pig manure. Bioresour. Technol. 2002, 84, 57–61. [Google Scholar] [CrossRef]
- Wilkinson, K.G.; Tee, E.; Tomkins, R.B.; Hepworth, G.; Premier, R. Effect of heating and aging of poultry litter on the persistence of enteric bacteria. Poult. Sci. 2011, 90, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Pandey, P.; Li, Y.; Venkitasamy, C.; Chen, Z.; Gallardo, R.; Weimer, B.; Jay-Russell, M.; Weimer, B. Assessment of gaseous ozone treatment on Salmonella Typhimurium and Escherichia coli O157:H7 reductions in poultry litter. Waste Manag. 2020, 117, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Farkas, R.; Hogsette, J.A.; Borzsonyi, L. Development of Hydrotaea aenescens and Musca domestica (Diptera: Muscidae) in poultry and pig manures of different moisture content. Environ. Entomol. 1998, 27, 695–699. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johmston, C.T., Sumner, M.E., Eds.; SSSA Inc.: Madison, WI, USA; ASA Inc.: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Rhoades, J.D. Salinity: Electrical conductivity and total dissolved solids. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johmston, C.T., Sumner, M.E., Eds.; SSSA Inc.: Madison, WI, USA; ASA Inc.: Madison, WI, USA, 1996; pp. 417–435. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johmston, C.T., Sumner, M.E., Eds.; SSSA Inc.: Madison, WI, USA; ASA Inc.: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Kemper, W.; Rosenau, R. Aggregate stability and size distribution. In Methods of Soil Analysis: Part 1. Physical and Mineralogica Methods; Klute, A., Campbell, G.S., Jackson, R.D., Mortland, M.M., Nielsen, D.R., Eds.; SSSA Inc.: Madison, WI, USA; ASA Inc.: Madison, WI, USA, 1986; pp. 425–442. [Google Scholar]
- Mulvaney, R.L. Nitrogen—Inorganic forms. In Methods of Soil Analysis. Part 3. Book Ser. 5. Chemical Method; Sparks, D.L., Ed.; SSSA Inc.: Madison, WI, USA; ASA Inc.: Madison, WI, USA, 1996; pp. 1123–1184. [Google Scholar]
- Mehlich, A. Mehlich-3 soil test extractent a modification of Mehlich-2 extractent. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; USDA Circ. 939; USDA: Washington, DC, USA, 1954.
- Kuo, S. Phosphorus. In Methods of Soil Analysis. Part 3. Book Ser. 5. Chemical Method; Sparks, D.L., Ed.; SSSA Inc.: Madison, WI, USA; ASA Inc.: Madison, WI, USA, 1996; pp. 869–919. [Google Scholar]
- EPA/Taiwan. Method Code No: NIEA S321.65B; Environmental Protection Administration of Taiwan ROC: Taipei, Taiwan, 2018.
- Wear, J.I.; Evans, C.E. Relationship of zinc uptake by corn and sorghum to soil zinc measured by three extractant. Soil Sci. Soc. Am. Proc. 1968, 32, 543–546. [Google Scholar] [CrossRef]
- Gardner, W.H. Water content. In Methods of Soil Analysis: Part 1. Physical and Mineralogica Methods; Klute, A., Campbell, G.S., Jackson, R.D., Mortland, M.M., Nielsen, D.R., Eds.; SSSA Inc.: Madison, WI, USA; ASA Inc.: Madison, WI, USA, 1986; pp. 493–544. [Google Scholar]
- Loncarevic, S.; Johannessen, G.S.; Rørvik, L.M. Bacteriological quality of organically grown leaf lettuce in Norway. Lett. Appl. Microbiol. 2005, 41, 186–189. [Google Scholar] [CrossRef]
- Wan, Y.; Huang, Q.; Wang, Q.; Ma, Y.; Su, D.; Qiao, Y.; Jiang, R.; Li, H. Ecological risk of copper and zinc and their different bioavailability change in soil-rice system as affected by biowaste application. Ecotoxicol. Environ. Saf. 2020, 192, 110301. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oade, J.M. Organic matter and water-stable aggregates in soil. J. Soil Sci. Plant Nutr. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Haynes, R.J.; Swift, R.S. Stability of soil aggregates in relation to organic-constituents and soil-water content. J. Soil Sci. 1990, 41, 73–83. [Google Scholar] [CrossRef]
- Arshad, M.A.; Lowery, B.; Grossman, B. Physical tests for monitoring soil quality. In Methods for Assessing Soil Quality; Doran, J.W., Jones, A.J., Eds.; SSSA Spec. Publ.: Madison, WI, USA, 1996; pp. 123–141. [Google Scholar]
- Blazier, M.A.; Patterson, W.B.; Hotard, S.L. Straw harvesting, fertilization, and fertilizer type alter soil microbiological and physical properties in a loblolly pine plantation in the mid-south USA. Biol. Fertil. Soils 2008, 45, 145–153. [Google Scholar] [CrossRef]
- He, Z.; Tazisong, I.A.; Senwo, Z.N.; Zhang, D. Soil properties and macro cations status impacted by long-term applied poultry litter. Commun. Soil Sci. Plant Anal. 2008, 39, 858–872. [Google Scholar] [CrossRef]
- Moritsugu, M.; Kawasaki, T.; Suzuki, T. Comparison of absorption rates between ammonium and nitrate nitrogen in plants. Bull. Res. Inst. Bioresourc. Okayama Univ. 1995, 3, 91–103. [Google Scholar]
- Matsumoto, S.; Ae, N.; Yamagata, M. Nitrogen uptake response of vegetable crops to organic materials. Soil Sci. Plant Nutr. 1999, 45, 269–278. [Google Scholar] [CrossRef][Green Version]
- Yang, C.; Du, W.; Zhang, L.; Dong, Z. Effects of sheep manure combined with chemical fertilizers on maize yield and quality and spatial and temporal distribution of soil inorganic nitrogen. Complexity 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Zhang, F.; Niu, J.; Zhang, W.; Chen, X.; Li, C.; Yuan, L.; Xie, J. Potassium nutrition of crops under varied regimes of nitrogen supply. Plant Soil 2010, 335, 21–34. [Google Scholar] [CrossRef]
- Pii, Y.; Cesco, S.; Mimmo, T. Shoot ionome to predict the synergism and antagonism between nutrients as affected by substrate and physiological status. Plant Physiol. Biochem. 2015, 94, 48–56. [Google Scholar] [CrossRef]
- Laborda, F.; Bolea, E.; Gorriz, M.P.; Martin-Ruiz, M.P.; Ruiz-Begueria, S.; Castillo, J.R. A speciation methodology to study the contributions of humic-like and fulvic-like acids to the mobilization of metals from compost using size exclusion chromatography-ultraviolet absorption-inductively coupled plasma mass spectrometry and deconvolution analysis. Anal. Chim. Acta 2008, 606, 1–8. [Google Scholar]
- Brunetto, G.; Bastos de Melo, G.W.; Terzano, R.; Del Buono, D.; Astolfi, S.; Tomasi, N.; Pii, Y.; Mimmo, T.; Cesco, S. Copper accumulation in vineyard soils: Rhizosphere processes and agronomic practices to limit its toxicity. Chemosphere 2016, 162, 293–307. [Google Scholar] [CrossRef]
- Sabiene, N.; Paulauskas, V.; Chen, S.Y.; Lin, J.G. Variations of metal distribution in sewage sludge composting. Waste Manag. 2008, 28, 1637–1644. [Google Scholar]
- Long, X.X.; Yang, X.E.; Ni, W.Z.; Ye, Z.Q.; He, Z.L.; Calvert, D.V.; Stoffella, J.P. Assessing zinc thresholds for phytotoxicity and potential dietary toxicity in selected vegetable crops. Commun. Soil Sci. Plant Anal. 2003, 34, 1421–1434. [Google Scholar] [CrossRef]
- Antonious, G.F.; Kochhar, T.S.; Coolong, T. Yield, quality, and concentration of seven heavy metals in cabbage and broccoli grown in sewage sludge and chicken manure amended soil. J. Environ. Sci. Health A 2012, 47, 1955–1965. [Google Scholar] [CrossRef] [PubMed]
- Bitzer, C.C.; Sims, J.T. Estimating the availability of nitrogen in poultry manure through laboratoly and field studies. J. Environ. Qual. 1988, 17, 47–54. [Google Scholar] [CrossRef]
- Brinson, S.E.; Cabrera, M.L.; Tyson, S.C. Ammonia volatilization from surface-applied, fresh and composted poultry litter. Plant Soil 1994, 167, 213–218. [Google Scholar] [CrossRef]
- Lin, Y.; Watts, D.B.; Torbert, H.A.; Howe, J.A.; Feng, Y. Integration of poultry litter and mineral nitrogen on growth and yield of winter canola. Agron. J. 2020, 112, 2496–2505. [Google Scholar] [CrossRef]
- Pinto, R.; Brito, L.M.; Coutinho, J. Organic production of horticultural crops with green manure, composted farmyard manure and organic fertiliser. Biol. Agric. Hortic. 2017, 33, 269–284. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Agbede, T.M.; Aboyeji, C.M.; Dunsin, O.; Simeon, V.T. Effects of biochar and poultry manure on soil characteristics and the yield of radish. Sci. Hortic. 2019, 243, 457–463. [Google Scholar] [CrossRef]
- James, D.W.; Hurst, C.J.; Tindall, T.A. Alfalfa cultivar response to phosphorus and potassium deficiency elemental composition of the herbage. J. Plant Nutr. 1995, 18, 2447–2464. [Google Scholar] [CrossRef]
- Magallanes-Quintanar, R.; Valdez-Cepeda, R.D.; Olivares-Saenz, E.; Perez-Veyna, O.; Garcia-Hernandez, J.L.; Lopez-Martinez, J.D. Compositional nutrient diagnosis in maize grown in a calcareous soil. J. Plant Nutr. 2006, 29, 2019–2033. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, X.; Tian, Y.; Chen, Y.; Chen, L. Disturibution of nutrients in camellia oleifera abel. and their correlation with soil nutrients over the period of fruit maturation. Bangladesh J. Bot. 2020, 49, 499–505. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Jiang, Y.; Li, G.; Cui, J.; Wang, Y.; Zhang, H.; Wang, S.; Xu, S.; Wang, R. The accumulation and health risk of heavy metals in vegetables around a zinc smelter in northeastern China. Environ. Sci. Pollut. Res. 2016, 23, 25114–25126. [Google Scholar] [CrossRef] [PubMed]
- Šturm, M.; Kacjan-Marsic, N.; Zupanc, V.; Bracic-Zeleznik, B.; Lojen, S.; Pintar, M. Effect of different fertilisation and irrigation practices on yield, nitrogen uptake and fertiliser use efficiency of white cabbage (Brassica oleracea var. capitata L.). Sci. Hortic. 2010, 125, 103–109. [Google Scholar] [CrossRef]
- Gao, N.; Liu, Y.; Wu, H.; Zhang, P.; Yu, N.; Zhang, Y.; Zou, H.; Fan, Q.; Zhang, Y. Interactive effects of irrigation and nitrogen fertilizer on yield, nitrogen uptake, and recovery of two successive Chinese cabbage crops as assessed using N-15 isotope. Sci. Hortic. 2017, 215, 117–125. [Google Scholar] [CrossRef]

| Fertilizer | Unit | G508 | G509 | Standards Announced by the AFA of the COA of Taiwan | |
|---|---|---|---|---|---|
| G508 | G509 | ||||
| Water content | % | 13 | 10 | <20 | <20 |
| pH | 8 | 9 | 5–9 | 5–9 | |
| OM | % | 54 | 46 | >40 | >40 |
| TN | % | 5.0 | 3.1 | >2.5 | 1–4 |
| P2O5 | % | 4 | 6 | 1–6 | 1–6 |
| K2O | % | 3.2 | 3.6 | 0.5–5 | 0.5–5 |
| CaO | % | 9.5 | 12.3 | N/A | N/A |
| MgO | % | 12.6 | 16.4 | N/A | N/A |
| As | mg kg−1 | 1.3 | 1.0 | <25 | <25 |
| Cd | mg kg−1 | ND | ND | <2.0 | <2.0 |
| Cr | mg kg−1 | ND | 2.5 | <150 | <150 |
| Cu | mg kg−1 | 23 | 67 | <100 | <100 |
| Hg | mg kg−1 | <1.0 | <1.0 | <1.0 | <1.0 |
| Ni | mg kg−1 | ND | ND | <25 | <25 |
| Pb | mg kg−1 | ND | ND | <150 | <150 |
| Zn | mg kg−1 | 294 | 470 | <500 | <500 |
| Coliform bacteria | MPN g−1 | <1 | N/A | <1 × 103 | N/A |
| Cabbage | Chinese Cabbage | Water Spinach | ||||||
|---|---|---|---|---|---|---|---|---|
| Dry Weight | Shoot Height | Head Diameter | Dry Weight | Shoot Height | Head Diameter | Dry Weight | Shoot Height | |
| Treatment | g head−1 | ----------- cm ----------- | g head−1 | ------------- cm ------------- | g plant−1 | cm | ||
| CK | 69.6 ± 0.1D | 14.9 ± 1.1D | 12.0 ± 1.0C | 53.4 ± 6.1AB | 25.2 ± 1.6ABC | 17.3 ± 1.6AB | 0.4 ± 0.1C | 34.9 ± 4.4C |
| CF | 124.2 ± 19.0AB | 24.0 ± 1.8A | 14.1 ± 1.0A | 51.3 ± 10.5AB | 23.7 ± 1.5CD | 18.4 ± 1.1A | 1.9 ± 0.4B | 79.7 ± 14.0A |
| G508X1 | 110.5 ± 10.0BC | 18.0 ± 1.5C | 13.8 ± 0.3A | 45.8 ± 7.0BC | 25.8 ± 1.7AB | 16.5 ± 1.3B | 2.0 ± 0.3AB | 62.7 ± 10.9B |
| G508X2 | 133.8 ± 15.4A | 24.1 ± 2.2A | 14.3 ± 0.9A | 59.2 ± 4.5A | 26.6 ± 2.1A | 18.5 ± 0.9A | 2.5 ± 0.4A | 77.4 ± 8.7A |
| G509X1 | 92.5 ± 16.8C | 18.7 ± 1.3BC | 13.0 ± 0.6B | 39.5 ± 4.4C | 24.6 ± 1.9BC | 14.5 ± 1.0C | 1.5 ± 0.4B | 57.6 ± 7.8B |
| G509X2 | 99.9 ± 14.3C | 19.8 ± 1.0B | 12.9 ± 0.7B | 45.9 ± 1.1BC | 22.8 ± 2.8D | 13.4 ± 2.3C | 1.6 ± 0.4B | 63.5 ± 10.8B |
| Treatment | Cabbage (%) | ||||||
| N | P | K | Ca | Mg | Cu | Zn | |
| G508X1 | 5.11 | 12.81 | 31.43 | <5 | <5 | ND | <5 |
| G508X2 | <5 | 10.71 | 24.77 | <5 | <5 | ND | <5 |
| G509X1 | <5 | <5 | 8.10 | <5 | <5 | ND | <5 |
| G509X2 | <5 | <5 | 6.30 | <5 | <5 | ND | <5 |
| Treatment | Chinese cabbage (%) | ||||||
| N | P | K | Ca | Mg | Cu | Zn | |
| G508X1 | <5 | <5 | 8.00 | <5 | <5 | ND | <5 |
| G508X2 | <5 | <5 | 22.02 | <5 | <5 | ND | <5 |
| G509X1 | <5 | <5 | <5 | <5 | <5 | ND | <5 |
| G509X2 | <5 | <5 | <5 | <5 | <5 | ND | <5 |
| Treatment | Water spinach (%) | ||||||
| N | P | K | Ca | Mg | Cu | Zn | |
| G508X1 | 36.13 | 177.2 | 489.1 | 26.25 | 6.13 | <5 | <5 |
| G508X2 | 23.37 | 115.9 | 351.3 | 16.00 | <5 | <5 | <5 |
| G509X1 | 23.15 | 57.54 | 214.3 | 7.13 | <5 | <5 | <5 |
| G509X2 | 11.38 | 29.72 | 151.0 | <5 | <5 | <5 | <5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-M.; Lai, H.-Y. Comprehensive Assessment of the Influence of Applying Two Kinds of Chicken-Manure-Processed Organic Fertilizers on Soil Properties, Mineralization of Nitrogen, and Yields of Three Crops. Agronomy 2022, 12, 2355. https://doi.org/10.3390/agronomy12102355
Hsu C-M, Lai H-Y. Comprehensive Assessment of the Influence of Applying Two Kinds of Chicken-Manure-Processed Organic Fertilizers on Soil Properties, Mineralization of Nitrogen, and Yields of Three Crops. Agronomy. 2022; 12(10):2355. https://doi.org/10.3390/agronomy12102355
Chicago/Turabian StyleHsu, Chun-Mai, and Hung-Yu Lai. 2022. "Comprehensive Assessment of the Influence of Applying Two Kinds of Chicken-Manure-Processed Organic Fertilizers on Soil Properties, Mineralization of Nitrogen, and Yields of Three Crops" Agronomy 12, no. 10: 2355. https://doi.org/10.3390/agronomy12102355
APA StyleHsu, C.-M., & Lai, H.-Y. (2022). Comprehensive Assessment of the Influence of Applying Two Kinds of Chicken-Manure-Processed Organic Fertilizers on Soil Properties, Mineralization of Nitrogen, and Yields of Three Crops. Agronomy, 12(10), 2355. https://doi.org/10.3390/agronomy12102355








