Long-Term Field Biochar Application for Rice Production: Effects on Soil Nutrient Supply, Carbon Sequestration, Crop Yield and Grain Minerals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochar
2.2. Experimental Site
2.3. Experimental Setup
2.4. Plant and Soil Sampling
2.5. Plant and Soil Analyses
2.6. Statistical Analysis
3. Results
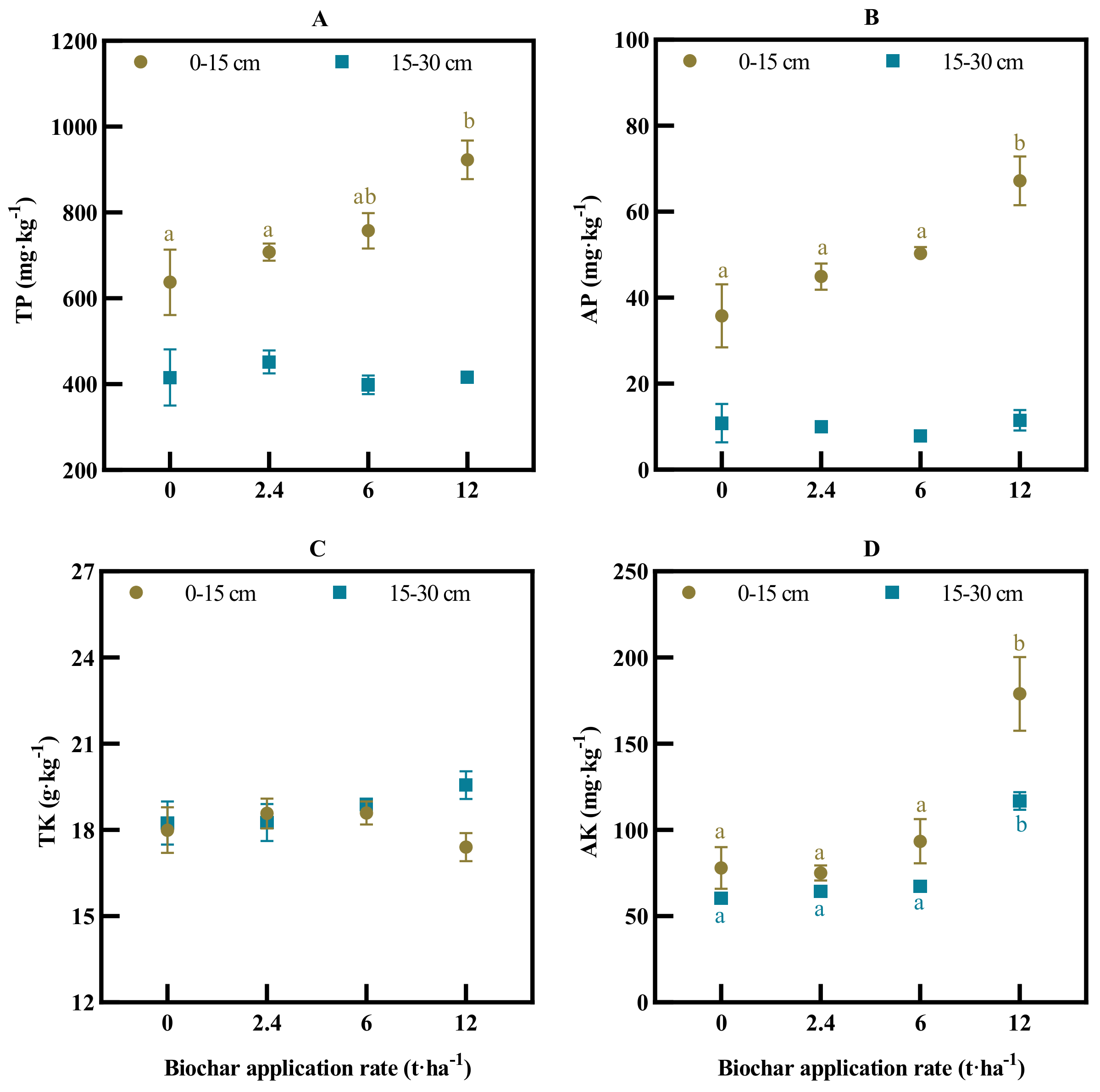
3.1. Soil Physicochemical Properties
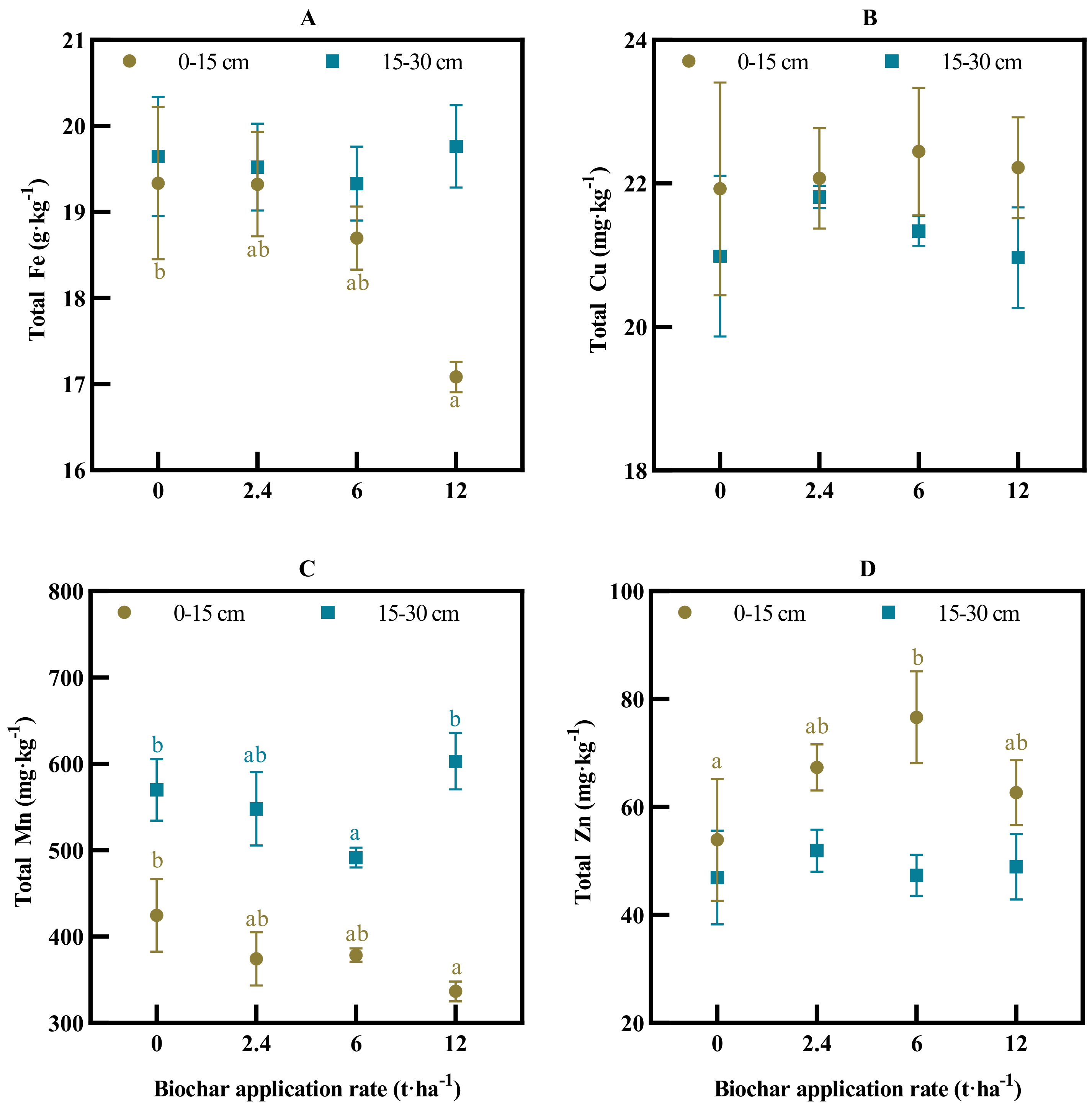
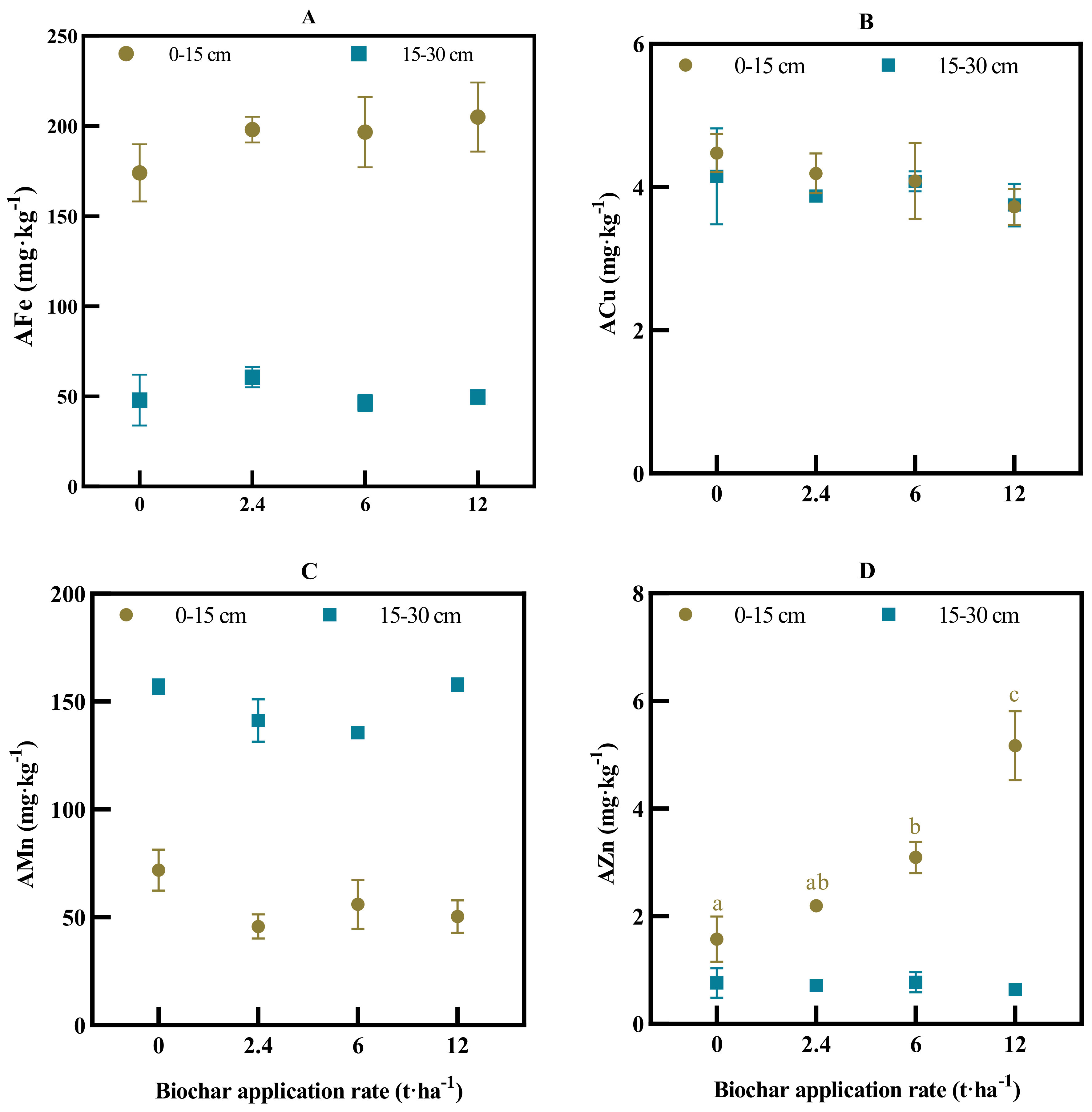
3.2. Soil Total Fe, Mn, Cu, Zn and Their Availability
3.3. Rice Yield and Mineral Nutrients
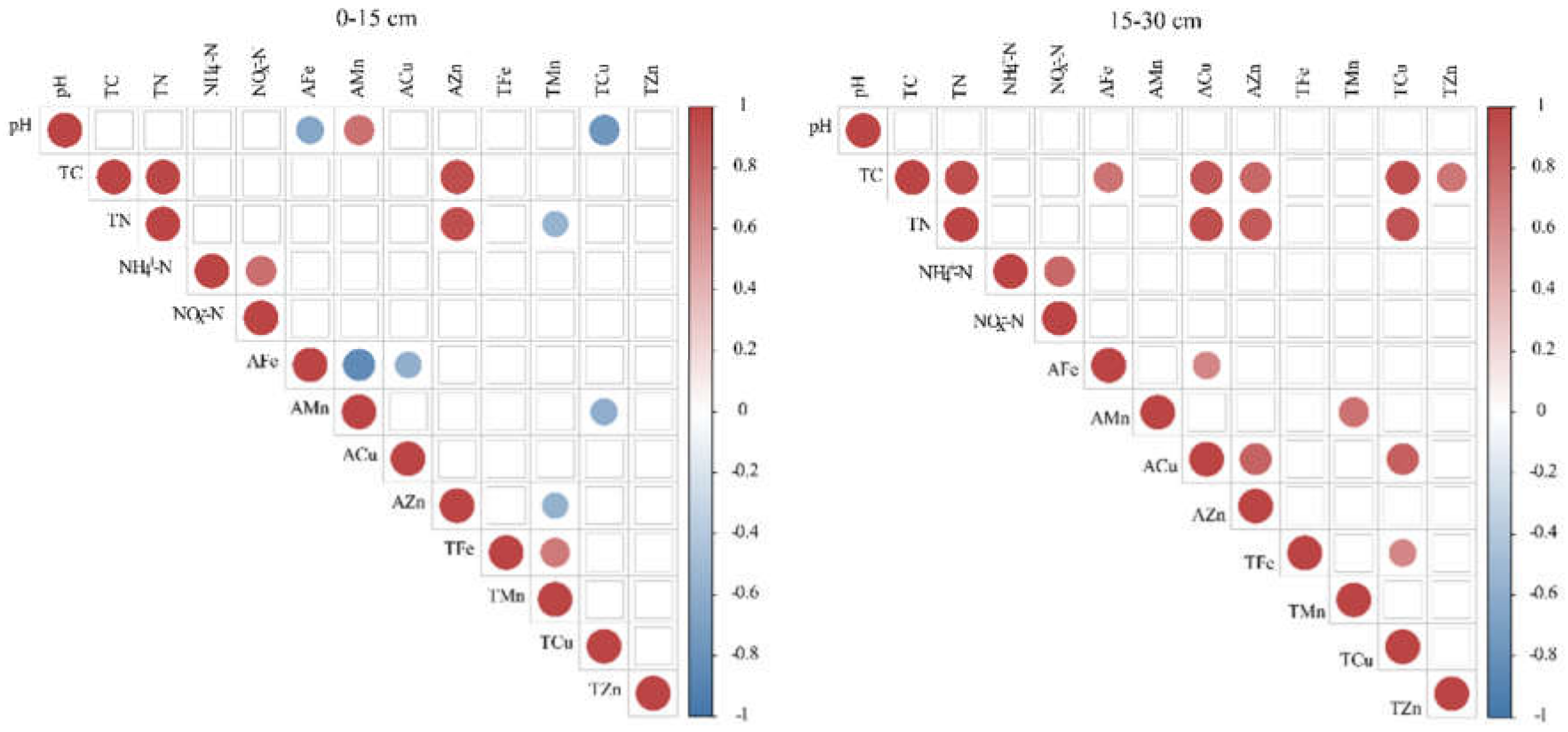
3.4. Correlation of Soil Micronutrients with Physicochemical Properties
4. Discussion
4.1. Long-Term Biochar Effect on Soil Fertility Status of Macro- and Micronutrients
4.2. Rice Yield and Grain Mineral Concentration
4.3. Long-Term Biochar Effect on Soil Carbon Accumulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ding, Y.; Liu, Y.G.; Liu, S.B.; Li, Z.W.; Tan, X.F.; Huang, X.X.; Zeng, G.M.; Zhou, L.; Zheng, B.H. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Kavitha, B.; Reddy, P.V.L.; Kim, B.; Lee, S.S.; Pandey, S.K.; Kim, K.H. Benefits and limitations of biochar amendment in agricultural soils: A review. J. Environ. Manag. 2018, 227, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Tisserant, A.; Cherubini, F. Potentials, limitations, co-benefits, and trade-offs of biochar applications to soils for climate change mitigation. Land 2019, 8, 179. [Google Scholar] [CrossRef]
- Buss, W.; Wurzer, C.; Manning, D.A.C.; Rohling, E.J.; Borevitz, J.; Mašek, O. Mineral-enriched biochar delivers enhanced nutrient recovery and carbon dioxide removal. Commun. Earth Environ. 2022, 3, 67. [Google Scholar] [CrossRef]
- Safian, M.; Motaghian, H.; Hosseinpur, A. Effects of sugarcane residue biochar and P fertilizer on P availability and its fractions in a Calcareous clay loam soil. Biochar 2020, 2, 357–367. [Google Scholar] [CrossRef]
- Alling, V.; Hale, S.E.; Martinsen, V.; Mulder, J.; Smebye, A.; Breedveld, G.D.; Cornelissen, G. The role of biochar in retaining nutrients in amended tropical soils. J. Plant. Nutr. Soil Sci. 2014, 177, 671–680. [Google Scholar] [CrossRef]
- Jin, L.; Wei, D.; Yin, D.W.; Zhou, B.K.; Ding, J.L.; Wang, W.; Zhang, J.M.; Qiu, S.J.; Zhang, C.J.; Li, Y.; et al. Investigations of the effect of the amount of biochar on soil porosity and aggregation and crop yields on fertilized black soil in northern China. PLoS ONE 2020, 15, e0238883. [Google Scholar] [CrossRef] [PubMed]
- Guedes, R.S.; Pinto, D.A.; Ramos, S.J.; Dias, Y.N.; Junior, C.F.C.; Gastauer, M.; Filho, P.W.M.E.S.; Fernandes, A.R. Biochar and conventional compost reduce hysteresis and increase phosphorus desorbability in iron mining waste. Rev. Bras. Cienc. Solo 2021, 45, e0200174. [Google Scholar] [CrossRef]
- Yan, T.T.; Xue, J.H.; Zhou, Z.D.; Wu, Y.B. Biochar-based fertilizer amendments improve the soil microbial community structure in a karst mountainous area. Sci. Total Environ. 2021, 794, 148757. [Google Scholar] [CrossRef]
- He, C.H.; Dong, W.X.; Hu, C.S.; Li, J.Z. Biochar’s effect on soil N2O consumption and the microbial mechanism. Chin. J. Eco-Agric. 2019, 27, 1301–1308. [Google Scholar]
- Xu, Q.; Xu, Q.Y.; Zhu, H.; Li, H.; Yin, W.Q.; Feng, K.; Wang, S.S.; Wang, X.Z. Does biochar application in heavy metal-contaminated soils affect soil micronutrient dynamics? Chemosphere 2022, 290, 133349. [Google Scholar] [CrossRef] [PubMed]
- Borchard, N.; Schirrmann, M.; Cayuela, M.; Kammann, C.; Wrage-Monnig, N.; Estavillo, J.; Fuertes-Mendizabal, T.; Sigua, G.; Spokas, K.; Ippolito, J.; et al. Biochar, soil and land-use interactions that reduce nitrate leaching and N2O Emissions: A meta-analysis. Sci. Total Environ. 2019, 651, 2354–2364. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Ata-Ul-Karim, S.T.; Singh, B.P.; Wang, H.L.; Wu, T.L.; Liu, C.; Fang, G.D.; Zhou, D.M.; Wang, Y.J.; Chen, W.F. A scientometric review of biochar research in the past 20 years (1998–2018). Biochar 2019, 1, 23–43. [Google Scholar] [CrossRef]
- Crombie, K.; Mašek, O.; Cross, A.; Sohi, S. Biochar-synergies and trade-offs between soil enhancing properties and C sequestration potential. GCB Bioenergy 2015, 7, 1161–1175. [Google Scholar] [CrossRef]
- Papageorgiou, A.; Azzi, E.S.; Enell, A.; Sundberg, C. Biochar produced from wood waste for soil remediation in Sweden: Carbon sequestration and other environmental impacts. Sci. Total Environ. 2021, 776, 145953. [Google Scholar] [CrossRef]
- Shin, J.D.; Park, D.G.; Hong, S.G.; Jeong, C.; Kim, H.; Chung, W. Influence of activated biochar pellet fertilizer application on greenhouse gas emissions and carbon sequestration in rice (Oryza sativa L.) production. Environ. Pollut. 2021, 285, 117457. [Google Scholar] [CrossRef]
- Wang, J.Y.; Xiong, Z.Q.; Kuzyakov, Y. Biochar stability in soil: Meta-analysis of decomposition and priming effects. GCB Bioenergy 2016, 8, 512–523. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L. Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ. Pollut. 2010, 158, 2282–2287. [Google Scholar] [CrossRef]
- Khan, S.; Chao, C.; Waqas, M.; Arp, H.P.H.; Zhu, Y.G. Sewage sludge biochar influence upon rice (Oryza sativa L.) yield, metal bioaccumulation and greenhouse gas emissions from acidic paddy soil. Environ. Sci. Technol. 2013, 47, 8624–8632. [Google Scholar] [CrossRef]
- Li, H.B.; Dong, X.L.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.S.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Bandara, T.; Herath, I.; Kumarathilaka, P.; Hseu, Z.Y.; Ok, Y.S.; Vithanage, M. Efficacy of woody biomass and biochar for alleviating heavy metal bioavailability in serpentine soil. Environ. Geochem. Health. 2017, 39, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Bogusz, A.; Oleszczuk, P.; Dobrowolski, R. Adsorption and desorption of heavy metals by the sewage sludge and biochar-amended soil. Environ. Geochem. Health. 2019, 41, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Namgay, T.; Singh, B.; Singh, B.P. Influence of biochar application to soil on the availability of As, Cd, Cu, Pb, and Zn to maize (Zea mays L.). Soil Res. 2010, 48, 638–647. [Google Scholar] [CrossRef]
- Doane, T.A.; Horwáth, W.R. Spectrophotometric determination of nitrate with a single reagent. Anal. Lett. 2003, 36, 2713–2722. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Gao, S.; Hoffman-Krull, K.; Bidwell, A.L.; DeLuca, T.H. Locally produced wood biochar increases nutrient retention and availability in agricultural soils of the San Juan Islands, USA. Agric. Ecosyst. Environ. 2016, 233, 43–54. [Google Scholar] [CrossRef]
- Sorrenti, G.; Ventura, M.; Toselli, M. Effect of biochar on nutrient retention and nectarine tree performance: A three-year field trial. J. Plant. Nutr. Soil Sci. 2016, 179, 336–346. [Google Scholar] [CrossRef]
- Hernandez-Soriano, M.C.; Kerré, B.; Goos, P.; Hardy, B.; Dufey, J.; Smolders, E. Long-term effect of biochar on the stabilization of recent carbon: Soils with historical inputs of charcoal. GCB Bioenergy 2016, 8, 371–381. [Google Scholar] [CrossRef]
- Weng, Z.; Zwieten, L.V.; Singh, B.-P.; Tavakkoli, E.; Kimber, S.W.L.; Morris, S.; Macdonald, L.M.; Cowie, A. The accumulation of rhizodeposits in organo-mineral fractions promoted biochar-induced negative priming of native soil organic carbon in Ferralsol. Soil Biol. Biochem. 2018, 118, 91–96. [Google Scholar] [CrossRef]
- Werner, S.; Kätzl, K.; Wichern, M.; Buerkert, A.; Steiner, C.; Marschner, B. Agronomic benefits of biochar as a soil amendment after its use as waste water filtration medium. Environ. Pollut. 2018, 233, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Abujabhah, I.S.; Doyle, R.; Bound, S.A.; Bowman, J.P. The effect of biochar loading rates on soil fertility, soil biomass, potential nitrification, and soil community metabolic profiles in three different soils. J. Soils Sediments 2016, 16, 2211–2222. [Google Scholar] [CrossRef]
- Efthymiou, A.; Grønlund, M.; Müller-Stöver, D.S.; Jakobsen, I. Augmentation of the phosphorus fertilizer value of biochar by inoculation of wheat with selected Penicillium strains. Soil Biol. Biochem. 2018, 116, 139–147. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Wei, Q.; Gou, J. Effects of short-term application of Moutai lees biochar on nutrients and fungal community structure in yellow soil of Guizhou. Environ. Sci. Pollut. Res. 2021, 28, 67404–67413. [Google Scholar] [CrossRef]
- Lehmann, J.; da Silva, J.P.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant. Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Graber, E.R.; Tsechansky, L.; Lew, B.; Cohen, E. Reducing capacity of water extracts of biochars and their solubilization of soil Mn and Fe. Eur. J. Soil Sci. 2014, 65, 162–172. [Google Scholar] [CrossRef]
- Olmo, M.; Alburquerque, J.A.; Barrón, V.; del Campillo, M.C.; Gallardo, A.; Fuentes, M.; Villar, R. Wheat growth and yield responses to biochar addition under Mediterranean climate conditions. Biol. Fertil. Soils 2014, 50, 1177–1187. [Google Scholar] [CrossRef]
- Houben, D.; Evrard, L.; Sonnet, P. Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar. Chemosphere 2013, 92, 1450–1457. [Google Scholar] [CrossRef]
- El-Naggar, A.; Shaheen, S.M.; Ok, Y.S.; Rinklebe, J. Biochar affects the dissolved and colloidal concentrations of Cd, Cu, Ni, and Zn and their phytoavailability and potential mobility in a mining soil under dynamic redox-conditions. Sci. Total Environ. 2018, 624, 1059–1071. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Stromberger, M.E.; Lentz, R.D.; Dungan, R.S. Hardwood biochar influences calcareous soil physicochemical and microbiological status. J. Environ. Qual. 2014, 43, 681–689. [Google Scholar] [CrossRef]
- Cai, Y.; Chang, S.X. Biochar effects on soil fertility and nutrient cycling. In Biochar: Production, Characterization and Applications, 1st ed.; Ok, Y.S., Uchimiya, S.M., Chang, S.X., Bolan, N., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 246–271. [Google Scholar]
- Schmidt, H.P.; Pandit, B.H.; Cornelissen, G.; Kammann, C.I. Biochar-Based Fertilization with Liquid Nutrient Enrichment: 21 Field Trials Covering 13 Crop Species in Nepal. Land Degrad. Dev. 2017, 28, 2324–2342. [Google Scholar] [CrossRef]
- Agegnehu, G.; Bass, A.M.; Nelson, P.N.; Bird, M.I. Benefits of biochar, compost and biochar-compost for soil quality, maize yield and greenhouse gas emissions in a tropical agricultural soil. Sci. Total Environ. 2016, 543, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Huang, S.; Wang, P.T.; Yamaji, N.; Ma, J.F. Plant Nutrition for Human Nutrition: Hints from Rice Research and Future Perspectives. Mol. Plant 2020, 13, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Nakandalage, N.; Seneweera, S. Micronutrients Use Efficiency of Crop-Plants Under Changing Climate. In Plant Micronutrient Use Efficiency: Molecular and Genomic Perspectives in Crop Plants; Hossain, M.A., Kamiya, T., Burritt, D.J., Phan Tran, L.-S., Fujiwara, T., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 209–224. [Google Scholar]
- Hartley, W.; Riby, P.; Waterson, J. Effects of three different biochars on aggregate stability, organic carbon mobility and micronutrient bioavailability. J. Environ. Manag. 2016, 181, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Bornø, M.L.; Müller-Stöver, D.S.; Liu, F. Biochar properties and soil type drive the uptake of macro- and micronutrients in maize (Zea mays L.). J. Plant Nutr. Soil Sci. 2019, 182, 149–158. [Google Scholar] [CrossRef]
- Weng, Z.; Van Zwieten, L.; Singh, B.P.; Tavakkoli, E.; Joseph, S.; Macdonald, L.M.; Rose, T.J.; Rose, M.T.; Kimber, S.W.L.; Morris, S.; et al. Biochar built soil carbon over a decade by stabilizing rhizodeposits. Nat. Clim. Chang. 2017, 7, 371–376. [Google Scholar] [CrossRef]
- Weng, Z.; Van Zwieten, L.; Singh, B.P.; Kimber, S.; Morris, S.; Cowie, A.; Macdonald, L.M. Plant-biochar interactions drive the negative priming of soil organic carbon in an annual ryegrass field system. Soil Biol. Biochem. 2015, 90, 111–121. [Google Scholar] [CrossRef]
- Fang, Y.Y.; Singh, B.P.; Singh, B. Temperature sensitivity of biochar and native carbon mineralisation in biochar-amended soils. Agric. Ecosyst. Environ. 2014, 191, 158–167. [Google Scholar] [CrossRef]
- Heitkötter, J.; Marschner, B. Is there anybody out there? Substrate availability controls microbial activity outside of hotspots in subsoils. Soil Syst. 2018, 2, 35. [Google Scholar] [CrossRef]

| Stalk | Biochar | Stalk | Biochar | ||
|---|---|---|---|---|---|
| pH | 7.02 | 9.70 | |||
| C (g kg−1) | 411.40 | 445.65 | N (g kg−1) | 11.11 | 19.38 |
| P (g kg−1) | 2.08 | 4.89 | AP (mg kg−1) | – | 800.56 |
| K (g kg−1) | 12.29 | 25.59 | AK (mg kg−1) | – | 14.71 |
| Fe (g kg−1) | 0.16 | 5.14 | AFe (mg kg−1) | – | 19.35 |
| Cu (mg kg−1) | 11.48 | 42.71 | ACu (mg kg−1) | – | 0.28 |
| Mn (mg kg−1) | 26.52 | 240.61 | AMn (mg kg−1) | – | 29.57 |
| Zn (mg kg−1) | 22.52 | 91.18 | AZn (mg kg−1) | – | 4.88 |
| Depth | Dose | pH | NH4+–N | NOx−–N | Total C | Total N |
|---|---|---|---|---|---|---|
| mg kg−1 | g kg−1 | |||||
| 0–15 cm | B0 | 6.48 a | 3.59 a | 2.67 a | 13.70 a | 1.25 a |
| B1 | 6.19 a | 25.20 b | 7.35 bc | 21.37 b | 1.66 a | |
| B2 | 6.38 a | 25.31 b | 9.46 c | 30.07 c | 2.17 b | |
| B3 | 6.40 a | 14.16 ab | 5.56 ab | 44.53 d | 2.66 c | |
| 15–30 cm | B0 | 7.18 a | 0.90 a | 1.89 a | 6.93 a | 0.66 a |
| B1 | 7.14 a | 14.88 b | 4.97 b | 8.53 a | 0.76 a | |
| B2 | 7.47 a | 12.42 b | 2.36 ab | 7.90 a | 0.73 a | |
| B3 | 7.25 a | 6.91 ab | 2.64 ab | 8.10 a | 0.70 a | |
| Two-way ANOVA | ||||||
| Dose | ns | **** | *** | **** | *** | |
| Depth | **** | *** | **** | **** | **** | |
| Dose × Depth | ns | ns | ** | **** | *** | |
| Dose | Yield | N | P | K | Fe | Cu | Mn | Zn |
|---|---|---|---|---|---|---|---|---|
| t ha−1 | g kg−1 | mg kg−1 | ||||||
| B0 | 7.21 a | 13.90 a | 4.57 a | 3.97 a | 14.67 a | 5.11 b | 37.85 a | 25.14 a |
| B1 | 7.49 a | 14.00 a | 4.56 a | 3.48 a | 14.84 a | 4.55 ab | 32.43 a | 25.90 a |
| B2 | 7.50 a | 13.93 a | 4.54 a | 2.83 a | 12.99 a | 4.11 a | 30.89 a | 20.97 a |
| B3 | 7.62 a | 13.75 a | 4.80 a | 2.99 a | 13.04 a | 4.38 a | 26.99 a | 25.18 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Wang, J.; Liu, Q.; Chen, Z.; Jin, P.; Du, J.; Fan, J.; Yin, W.; Xie, Z.; Wang, X. Long-Term Field Biochar Application for Rice Production: Effects on Soil Nutrient Supply, Carbon Sequestration, Crop Yield and Grain Minerals. Agronomy 2022, 12, 1924. https://doi.org/10.3390/agronomy12081924
Xu Q, Wang J, Liu Q, Chen Z, Jin P, Du J, Fan J, Yin W, Xie Z, Wang X. Long-Term Field Biochar Application for Rice Production: Effects on Soil Nutrient Supply, Carbon Sequestration, Crop Yield and Grain Minerals. Agronomy. 2022; 12(8):1924. https://doi.org/10.3390/agronomy12081924
Chicago/Turabian StyleXu, Qiao, Ji Wang, Qi Liu, Zhe Chen, Penghui Jin, Jiazhou Du, Jialu Fan, Weiqin Yin, Zubin Xie, and Xiaozhi Wang. 2022. "Long-Term Field Biochar Application for Rice Production: Effects on Soil Nutrient Supply, Carbon Sequestration, Crop Yield and Grain Minerals" Agronomy 12, no. 8: 1924. https://doi.org/10.3390/agronomy12081924
APA StyleXu, Q., Wang, J., Liu, Q., Chen, Z., Jin, P., Du, J., Fan, J., Yin, W., Xie, Z., & Wang, X. (2022). Long-Term Field Biochar Application for Rice Production: Effects on Soil Nutrient Supply, Carbon Sequestration, Crop Yield and Grain Minerals. Agronomy, 12(8), 1924. https://doi.org/10.3390/agronomy12081924







