Long-Term Conservation Tillage Practices Directly and Indirectly Affect Soil Micro-Food Web in a Chinese Mollisol
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Soil Sampling and Soil Physicochemical Property Analysis
2.3. DNA Extraction, PCR Amplification, and Sequencing
2.4. Quantitative PCR (qPCR)
2.5. Statistical Analysis
3. Results
3.1. Soil Physiochemical Properties
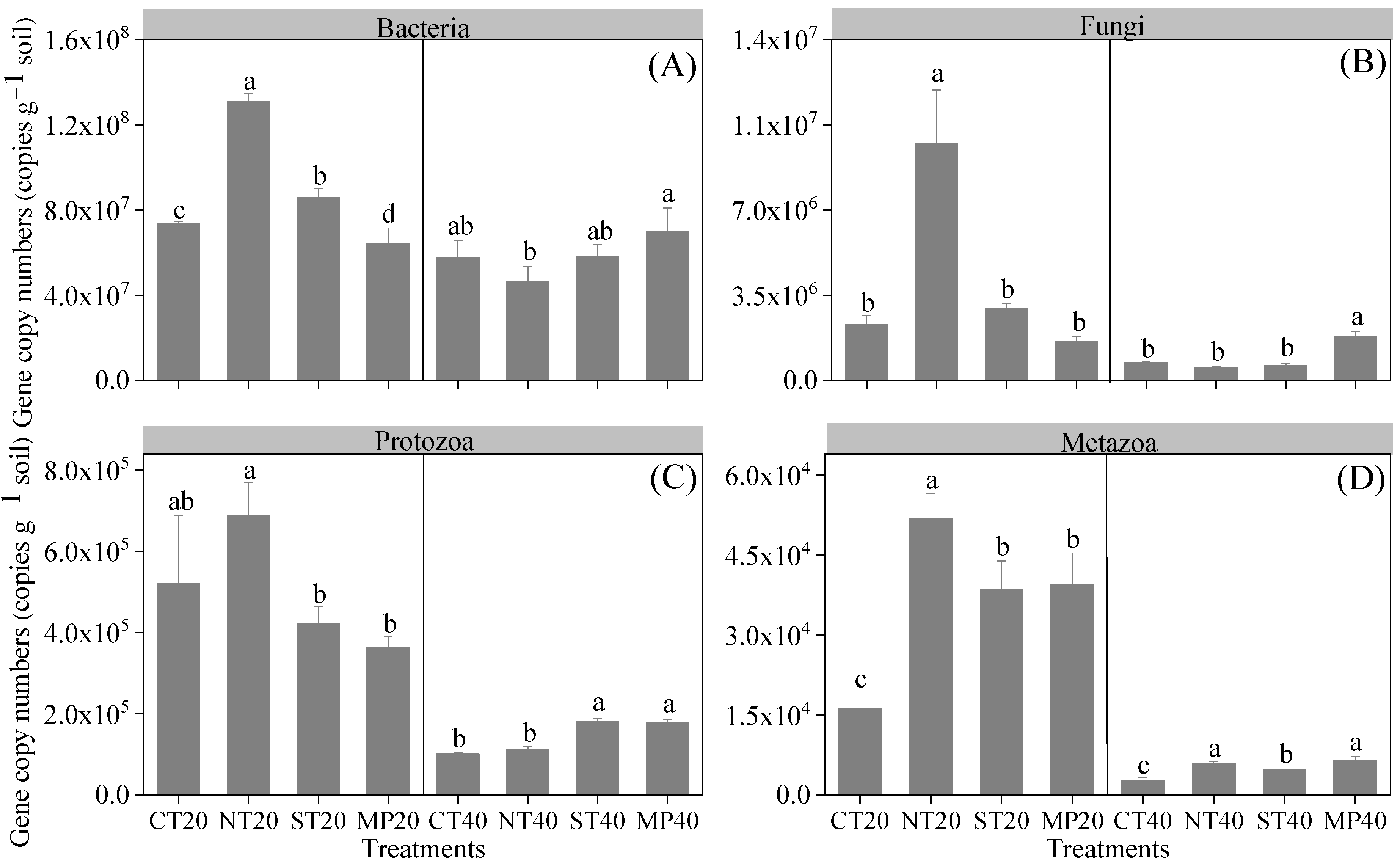
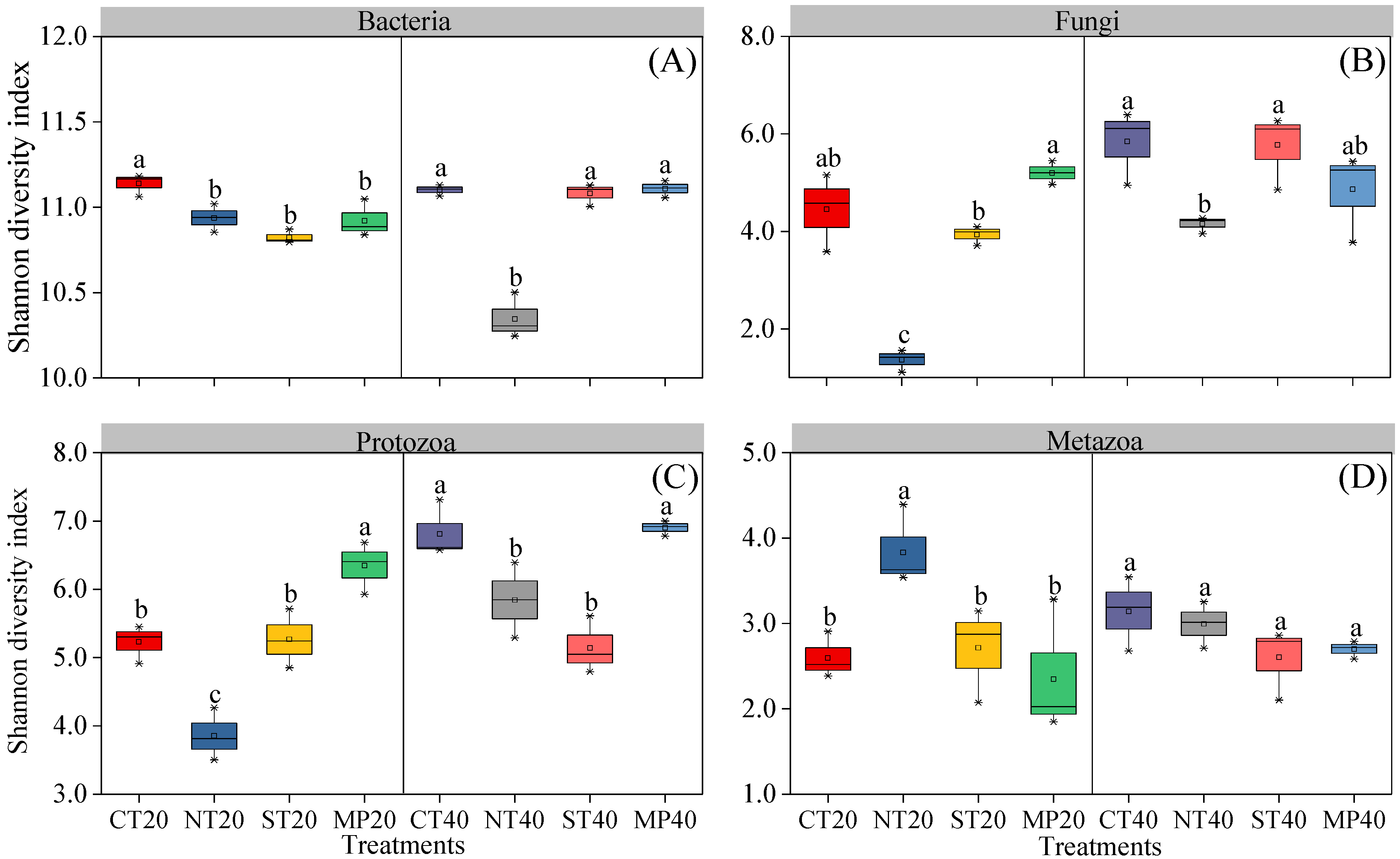
3.2. Soil Microbial and Faunal Communities
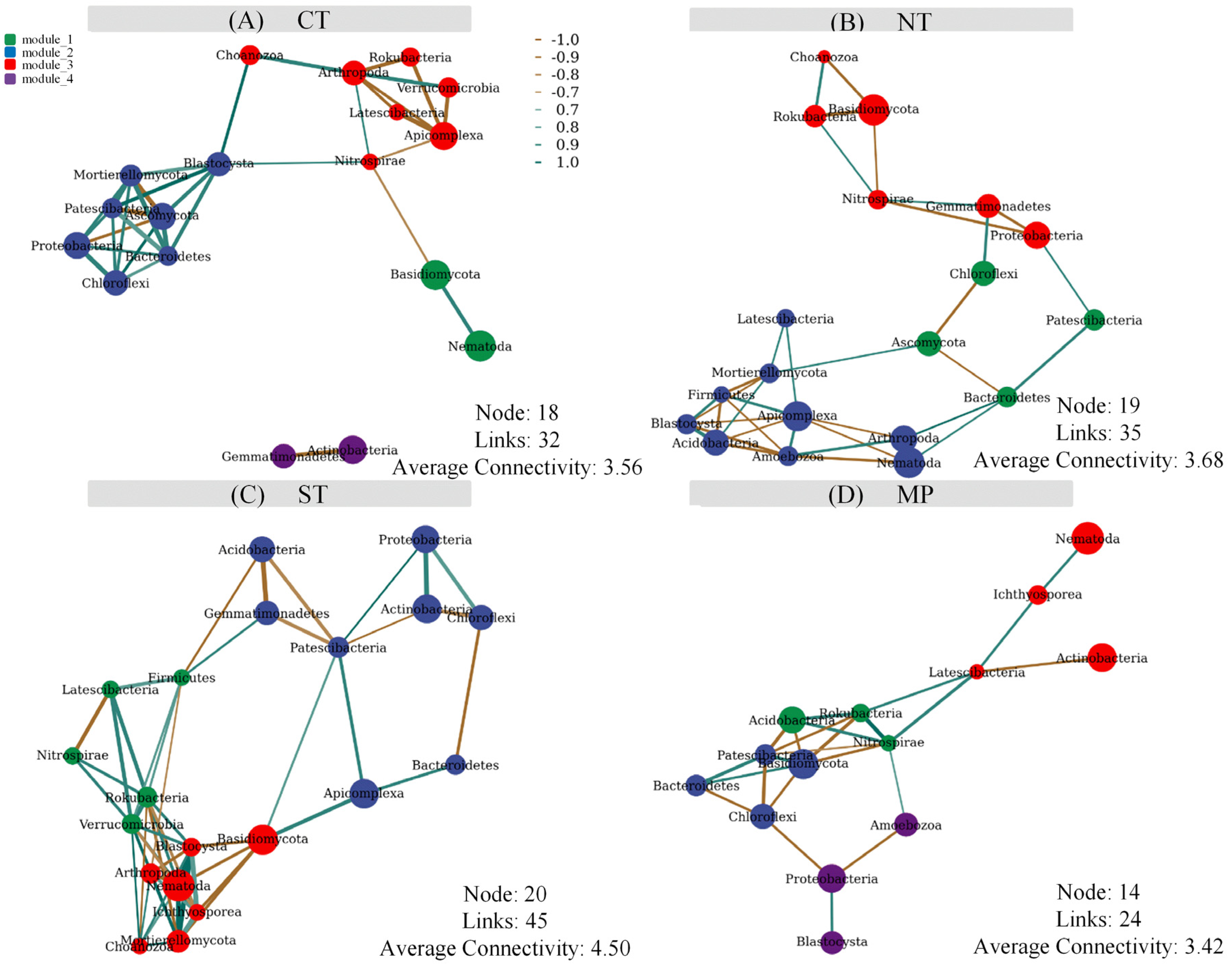
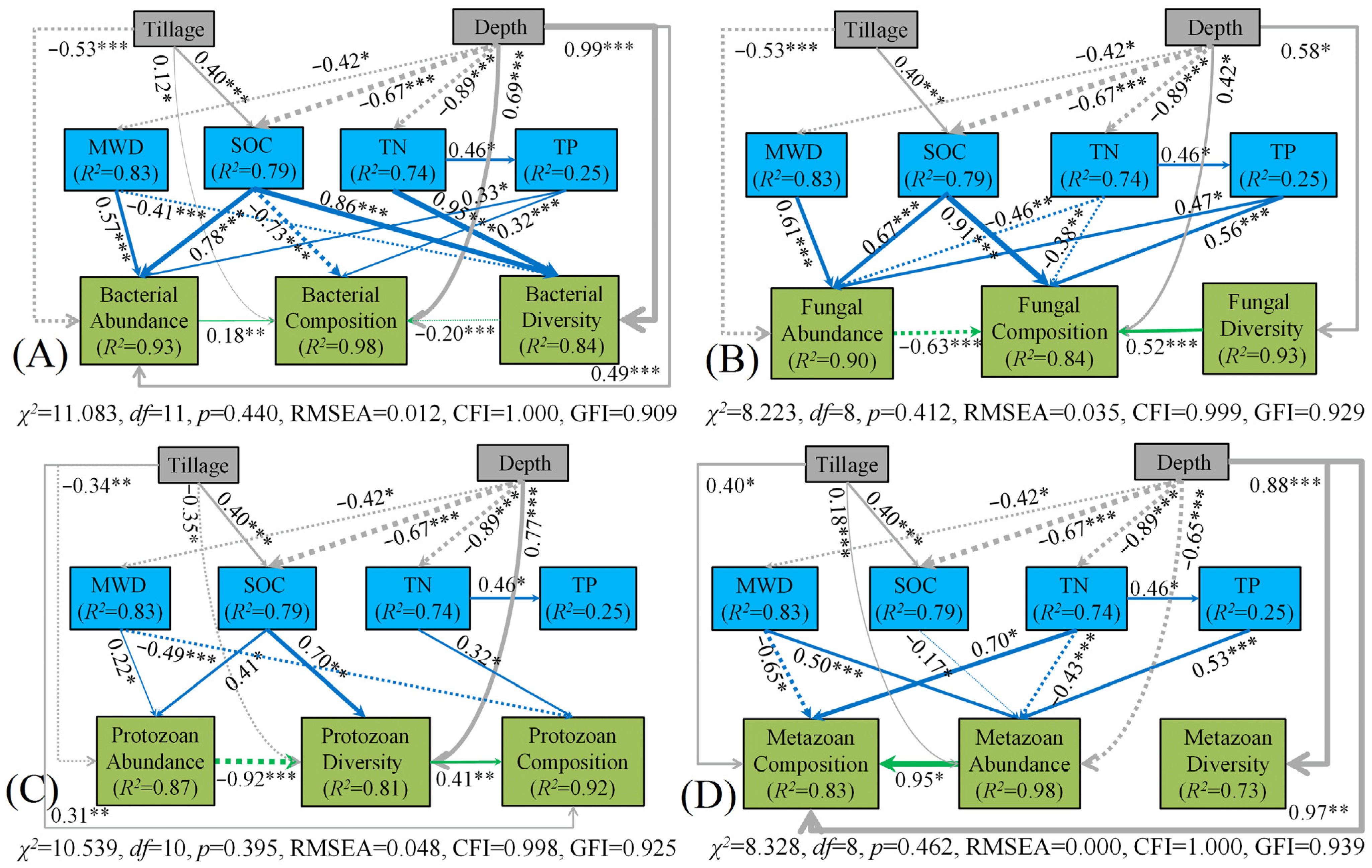
3.3. Soil Micro-Food Web Relationships
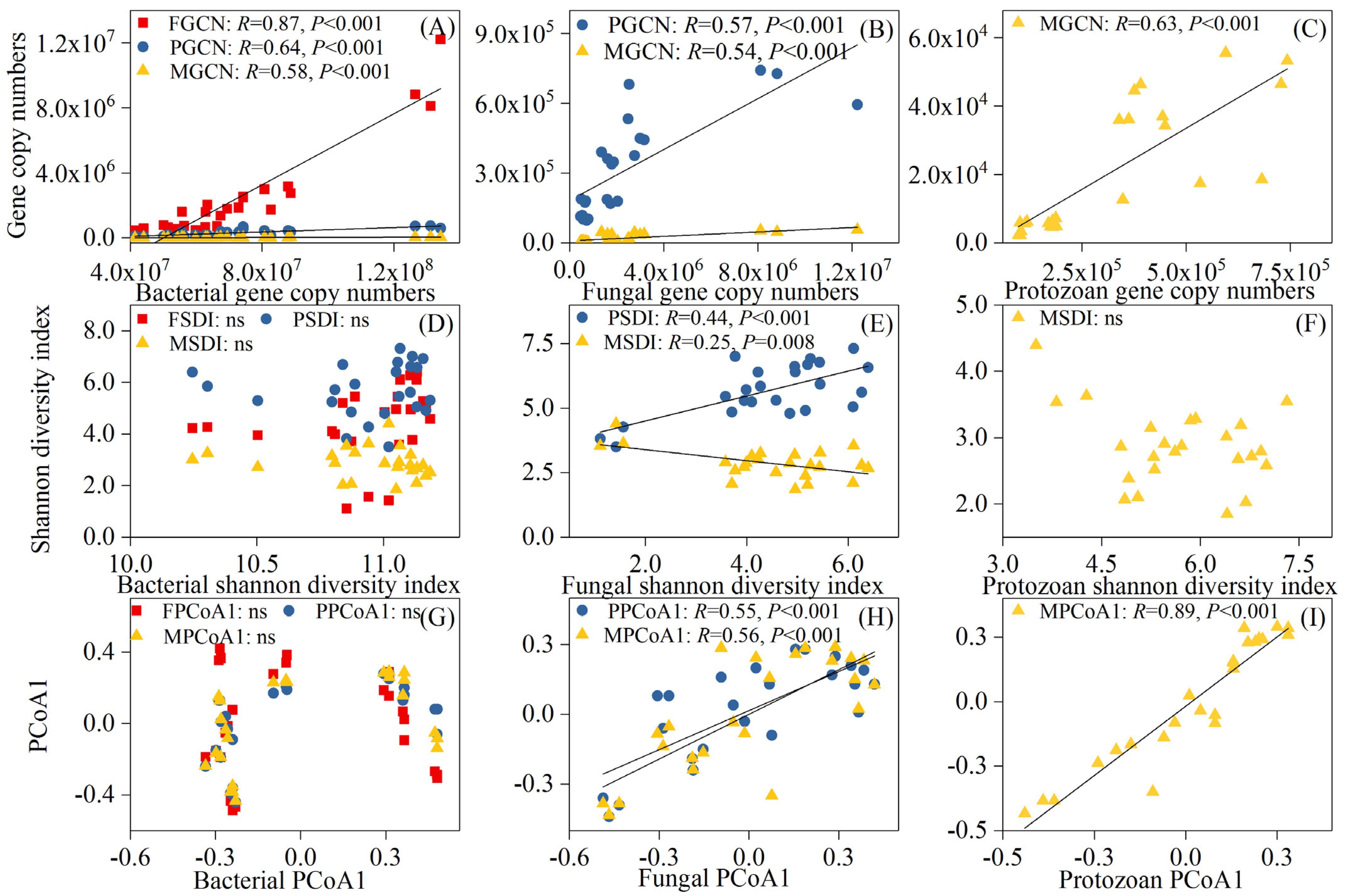
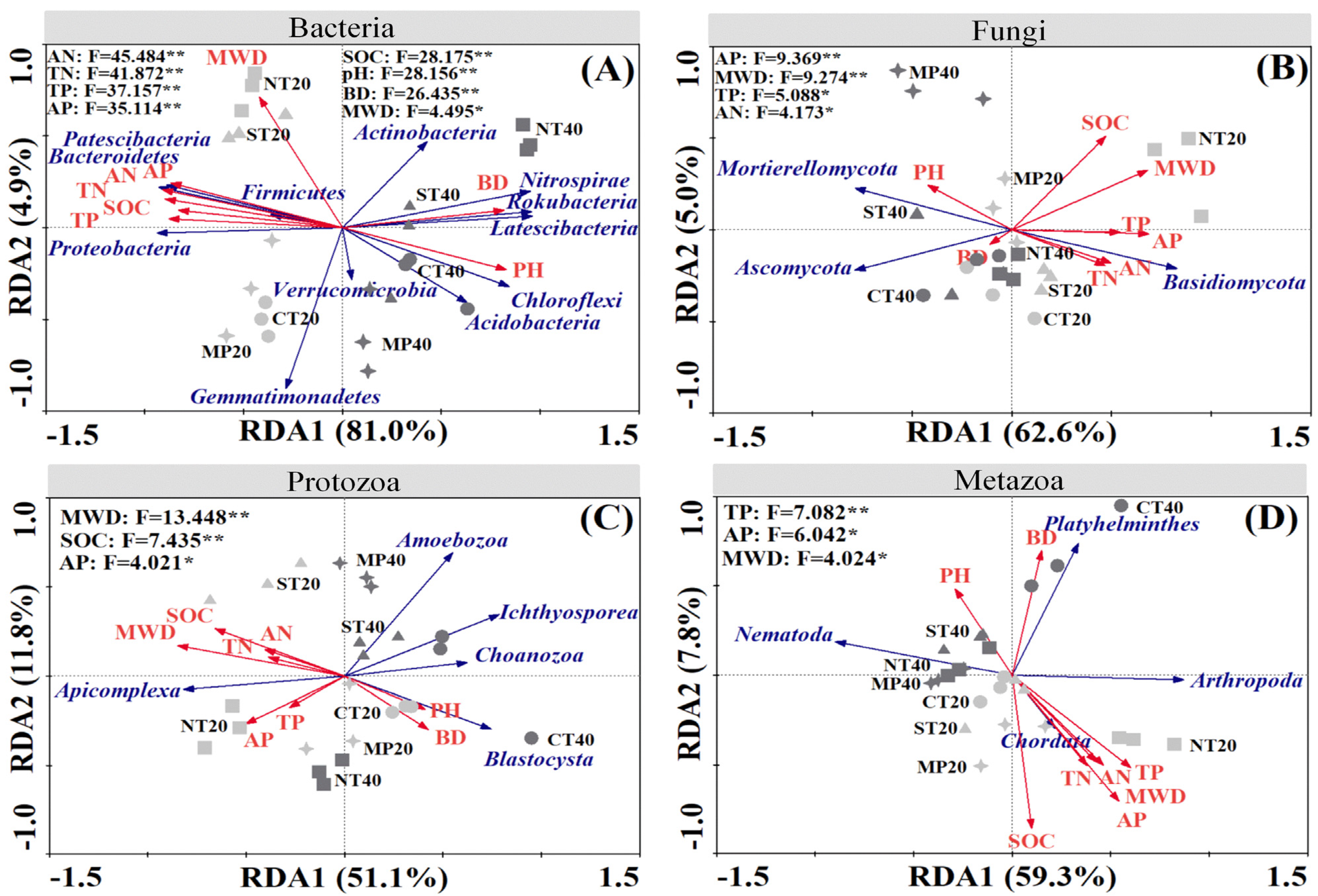
3.4. Relationship between Soil Microbial and Faunal Communities and Soil Properties
4. Discussion
4.1. Effect of Different Tillage Practices on Soil Microbial and Faunal Communities
4.2. Relationships between Soil Microbial and Faunal Communities
4.3. Control of Soil Properties on Microbial and Faunal Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, M.; He, P.; Guo, X.L.; Zhang, X.; Li, L.J. Fifteen-year no tillage of a Mollisol with residue retention indirectly affects topsoil bacterial community by altering soil properties. Soil Tillage Res. 2021, 205, 104804. [Google Scholar] [CrossRef]
- Ning, T.Y.; Liu, Z.; Hu, H.Y.; Li, G.; Kuzyakov, Y. Physical, chemical and biological subsoiling for sustainable agriculture. Soil Tillage Res. 2022, 223, 105490. [Google Scholar] [CrossRef]
- NRCS. Conservation Practices on Cultivated Cropland, a Comparison of CEAP I and CEAP II Survey Data and Modeling. 2022. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/main/national/technical/nra/ceap (accessed on 15 June 2022).
- Busari, M.A.; Kukal, S.S.; Kaur, A.; Bhatt, R.; Dulazi, A.A. Conservation tillage impacts on soil, crop and the environment. Int. Soil Water Conserv. 2015, 3, 119–129. [Google Scholar] [CrossRef]
- Gebhardt, M.R.; Daniel, T.C.; Schweizer, E.E.; Allmaras, R.R. Conservation tillage. Science 1985, 230, 625–630. [Google Scholar] [CrossRef]
- Wardle, D.A.; Verhoef, H.A.; Clarholm, M. Trophic relationships in the soil microfood–web: Predicting the responses to a changing global environment. Glob. Chang. Biol. 1998, 4, 713–727. [Google Scholar] [CrossRef]
- Wardle, D.A. Impacts of disturbance on detritus food webs in agro-ecosystems of contrasting tillage and weed management practices. Adv. Ecol. Res. 1995, 26, 107–185. [Google Scholar]
- Van den Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.A.; de Goede, R.G.M.; Adams, B.J.; Ahmad, W.; Andriuzzi, W.S.; et al. Soil nematode abundance and functional group composition at a global scale. Nature 2019, 572, 194–198. [Google Scholar] [CrossRef]
- Coleman, D.; Fu, S.L.; Hendrix, P.; Crossley, C.J. Soil foodwebs in agroecosystems: Impacts of herbivory and tillage management. Eur. J. Soil Biol. 2002, 38, 21–28. [Google Scholar] [CrossRef]
- Zhong, S.; Zeng, H.C.; Jin, Z.Q. Influences of different tillage and residue management systems on soil nematode community composition and diversity in the tropics. Soil Biol. Biochem. 2017, 107, 234–243. [Google Scholar] [CrossRef]
- Zhang, S.X.; Chang, L.; McLaughlin, N.B.; Cui, S.Y.; Wu, H.T.; Wu, D.H.; Liang, W.J.; Liang, A.Z. Complex soil food web enhances the association between N mineralization and soybean yield–a model study from long-term application of a conservation tillage system in a black soil of Northeast China. Soil 2021, 7, 71–82. [Google Scholar] [CrossRef]
- Zhang, S.X.; Li, Q.; Lü, Y.; Sun, X.M.; Jia, S.X.; Zhang, X.P.; Liang, W.J. Conservation tillage positively influences the microflora and microfauna in the black soil of Northeast China. Soil Tillage Res. 2015, 149, 46–52. [Google Scholar] [CrossRef]
- Kou, X.C.; Ma, N.N.; Zhang, X.K.; Xie, H.T.; Zhang, X.D.; Wu, Z.F.; Liang, W.J.; Li, Q.; Ferris, H. Frequency of stover mulching but not amount regulates the decomposition pathways of soil micro-foodwebs in a no-tillage system. Soil Biol. Biochem. 2020, 144, 107789. [Google Scholar] [CrossRef]
- Liu, J.Y.; Zhang, Z.X.; Xu, X.L.; Kuang, W.H.; Zhou, W.C.; Zhang, S.W.; Li, R.D.; Yan, C.Z.; Yu, D.S.; Wu, S.X.; et al. Spatial patterns and driving forces of land use change in China during the early 21st century. J. Geogr. Sci. 2010, 20, 483–494. [Google Scholar] [CrossRef]
- Li, H.L.; Shen, H.O.; Wang, Y.; Wang, Y.; Gao, Q. Effects of ridge tillage and straw returning on runoff and soil loss under simulated rainfall in the mollisol region of northeast China. Sustainability 2021, 13, 10614. [Google Scholar] [CrossRef]
- Wang, X.B.; Cai, D.X.; Hoogmoed, W.B.; Oenema, O.; Perdok, U.D. Developments in conservation tillage in rainfed regions of North China. Soil Tillage Res. 2007, 93, 239–250. [Google Scholar] [CrossRef]
- Jia, L.; Zhao, W.; Zhai, R.; Liu, Y.; Kang, M.; Zhang, X. Regional differences in the soil and water conservation efficiency of conservation tillage in China. Catena 2019, 175, 18–26. [Google Scholar] [CrossRef]
- Zheng, H.B.; Liu, W.R.; Zheng, J.Y.; Luo, Y.; Li, R.P.; Wang, H.; Qi, H. Effect of long–term tillage on soil aggregates and aggregate–associated carbon in black soil of Northeast China. PLoS ONE 2018, 28, e0199523. [Google Scholar] [CrossRef] [PubMed]
- Blake, G.R.; Hartage, K.H. Bulk Density. In Methods of Soil Analysis Part 1; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1986; pp. 364–366. [Google Scholar]
- Kemper, W.D.; Rosenau, R.C. Aggregate Stability and Size Distribution. In Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods–Agronomy Monograph 9; American Society of Agronomy: Madison, WI, USA, 1986; pp. 425–442. [Google Scholar]
- Olsen, S.; Sommers, L.; Page, A. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties of Phosphorus; ASA Monograph; Bloomsbury: London, UK, 1982; pp. 403–430. [Google Scholar]
- Dorich, R.A.; Nelson, D.W. Evaluation of Manual Cadmium Reduction Methods for Determination of Nitrate in Potassium Chloride Extracts of Soils. Soil Sci. Soc. Am. J. 1984, 48, 72. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B. Method to measure microbial phosphate in soils. Soil Biol. Biochem. 1982, 14, 377–385. [Google Scholar] [CrossRef]
- Motsara, M.R.; Roy, R.N. Guide to Laboratory Establishment for Plant Nutrient Analysis; FAO: Rome, Italy, 2008. [Google Scholar]
- Wang, Z.G.; Hu, Y.L.; Xu, W.H.; Liu, S.; Hu, Y.; Zhang, Y. Impacts of dimethyl phthalate on the bacterial community and functions in black soils. Front. Microbiol. 2015, 6, 405. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Awasthi, S.K.; Chen, H.; Liu, T.; Zhang, Z.; Zhang, L.; Awasthi, M.K.; Taherzadeh, M.J. Evaluating the impact of bamboo biochar on the fungal community succession during chicken manure composting. Bioresour. Technol. 2019, 272, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Hugerth, L.W.; Muller, E.E.L.; Hu, Y.O.O.; Lebrun, L.A.M.; Roume, H.; Lundin, D.; Wilmes, P.; Andersson, A.F.; Voolstra, C.R. Systematic design of 18S rRNA gene primers for determining eukaryotic diversity in microbial consortia. PLoS ONE 2014, 9, e95567. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, R.; Nicolaisen, M. High-throughput sequencing of nematode communities from total soil DNA extractions. BMC Ecol. 2015, 15, 3. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High–resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Li, X.; Wang, J.; Li, X.; Guo, Q.; Yu, Z.; Yang, T.; Zhang, H. Long–term no–tillage and different residue amounts alter soil microbial community composition and increase the risk of maize root rot in northeast China. Soil Tillage Res. 2020, 196, 104452. [Google Scholar] [CrossRef]
- Yu, H.L.; Ling, N.; Wang, T.T.; Zhu, C.; Wang, Y.; Wang, S.J.; Gao, Q. Responses of soil biological traits and bacterial communities to nitrogen fertilization mediate maize yields across three soil types. Soil Tillage Res. 2019, 185, 61–69. [Google Scholar] [CrossRef]
- Chen, T.; You, Y.; Xie, G.; Zheng, X.; Zhao, A.; Liu, J.; Jia, W. Strategy for an association study of the intestinal microbiome and brain metabolome across the lifespan of rats. Anal. Chem. 2018, 90, 2475–2483. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.W.; Liu, H.; Hu, Y.; Sun, Y.; Liu, Q.; Li, J.G.; Dong, Y.H. Shift in soil bacterial communities from K- to r-strategists facilitates adaptation to grassland degradation. Land Degrad. Dev. 2022, 33, 2076–2091. [Google Scholar] [CrossRef]
- Sui, P.X.; Tian, P.; Wang, Z.Y.; Lian, H.L.; Yang, Y.D.; Ma, Z.Q.; Jiang, Y.; Zheng, J.Y.; Qi, H. Soil properties and microbial communities of spring maize filed in response to tillage with straw incorporation and nitrogen fertilization in northeast China. PeerJ 2022, 10, e13462. [Google Scholar] [CrossRef]
- Brévault, T.; Bikay, S.; Maldes, J.M.; Naudin, K. Impact of a no-till with mulch soil management strategy on soil macrofauna communities in a cotton cropping system. Soil Tillage Res. 2007, 97, 140–149. [Google Scholar] [CrossRef]
- Manetti, P.L.; López, A.N.; Clemente, N.L.; Faberi, A.J. Tillage system does not affect soil macrofauna in southeastern Buenos Aires province, Argentina. Span. J. Agric. Res. 2010, 8, 377–384. [Google Scholar] [CrossRef]
- Becraft, E.D.; Woyke, T.; Jarett, J.; Ivanova, N.; Godoy-Vitorino, F.; Poulton, N.; Brown, J.M.; Brown, J.; Lau, M.; Onstott, T. Rokubacteria: Genomic giants among the uncultured bacterial phyla. Front. Microbiol. 2017, 8, 2264. [Google Scholar] [CrossRef]
- Wortman, S.E.; Drijber, R.A.; Francis, C.A.; Lindquist, J.L. Arable weeds, cover crops, and tillage drive soil microbial community composition in organic cropping systems. Appl. Soil Ecol. 2013, 72, 232–241. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Liu, J.; Liu, T.; Cheng, J.; Wei, G.; Lin, Y. Temporal and spatial succession and dynamics of soil fungal communities in restored grassland on the loess plateau in China. Land Degrad. Dev. 2019, 30, 1273–1287. [Google Scholar] [CrossRef]
- Del Campo, J.; Heger, T.J.; Rodríguez-Martínez, R.; Worden, A.Z.; Richards, T.A.; Massana, R.; Keeling, P.J. Assessing the diversity and distribution of apicomplexans in host and free-living environments using high-throughput amplicon data and a phylogenetically informed reference framework. Front. Microbiol. 2019, 10, 2373. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ni, T.; Xun, W.; Huang, X.; Huang, Q.; Ran, W.; Shen, Q. Influence of straw incorporation with and without straw decomposer on soil bacterial community structure and function in a rice-wheat cropping system. Appl. Microbiol. Biotechnol. 2017, 101, 4761–4773. [Google Scholar] [CrossRef]
- Rutere, C.; Knoop, K.; Posselt, M.; Ho, A.; Horn, M.A. Ibuprofen Degradation and Associated Bacterial Communities in Hyporheic Zone Sediments. Microorganisms 2020, 8, 1245. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Dong, W.; Hu, C.; Chen, S.; Zhang, Y. Tillage and residue management effects on soil carbon and CO2 emission in a wheat-corn double-cropping system. Nutr. Cycl. Agroecosyst. 2008, 83, 27–37. [Google Scholar] [CrossRef]
- Scheu, S.; Schaefer, M. Bottom–up control of the soil macro fauna community in a beech wood on limestone: Manipulation of food resources. Ecology 1998, 79, 1573–1585. [Google Scholar] [CrossRef]
- Wang, C.Y.; Yan, L.; Gao, Y.H.; Liang, A.Z.; Sui, B.; Zhao, L.P.; Liu, S.X. Long-term effects of tillage practices on soil bacterial community abundance and metabolic diversity of black soil from northeast China. Int. J. Agric. Biol. 2018, 20, 2753–2762. [Google Scholar]
- Li, Y.Z.; Song, D.P.; Liang, S.H.; Dang, P.F.; Qin, X.L.; Liao, Y.C.; Siddique, K.H.M. Effect of no-tillage on soil bacterial and fungal community diversity: A meta-analysis. Soil Tillage Res. 2020, 204, 104721. [Google Scholar] [CrossRef]
- Guan, Y.P.; Xu, B.; Zhang, X.M.; Yang, W. Tillage practices and residue management manipulate soil bacterial and fungal communities and networks in maize agroecosystems. Microorganisms 2022, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- Mougi, A.; Kondoh, M. Diversity of interaction types and ecological community stability. Science 2012, 337, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.X.; Gu, H.D.; Liang, A.Z.; Li, L.J.; Yao, Q.; Xu, Y.X.; Liu, J.J.; Jin, J.; Liu, X.B.; Wang, G.H. Conservation tillage regulates the assembly, network structure and ecological function of the soil bacterial community in black soils. Plant Soil 2022, 472, 207–223. [Google Scholar] [CrossRef]
- Jia, L.J.; Wang, Z.; Ji, L.; De Neve, S.; Struik, P.C.; Yao, Y.Q.; Lv, J.J.; Zhou, T.; Jin, K. Keystone microbiome in the rhizosphere soil reveals the effect of long-term conservation tillage on crop growth in the Chinese Loess Plateau. Plant Soil 2022, 473, 457–472. [Google Scholar] [CrossRef]
- Hu, X.J.; Liu, J.J.; Liang, A.Z.; Li, L.J.; Yao, Q.; Yu, Z.H.; Li, Y.S.; Jin, J.; Liu, X.B.; Wang, G.H. Conventional and conservation tillage practices affect soil microbial co-occurrence patterns and are associated with crop yields. Agric. Ecosyst. Environ. 2021, 319, 107534. [Google Scholar] [CrossRef]
- Geisen, S.; Koller, R.; Hünninghaus, M.; Dumack, K.; Urich, T.; Bonkowski, M. The soil food web revisited: Diverse and widespread mycophagous soil protists. Soil Biol. Biochem. 2016, 94, 10–18. [Google Scholar] [CrossRef]
- Thakur, M.P.; Geisen, S. Trophic regulations of the soil microbiome. Trends Microbiol. 2019, 27, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Beare, M.; Buckley, H.L.; Lear, G. Bacterial and fungal communities respond differently to varying tillage depth in agricultural soils. PeerJ 2017, 5, e3930. [Google Scholar] [CrossRef]
- Blankinship, J.C.; Fonte, S.J.; Six, J.; Schimel, J.P. Plant versus microbial controls on soil aggregate stability in a seasonally dry ecosystem. Geoderma 2016, 272, 39–50. [Google Scholar] [CrossRef]
- Frey, S.D.; Elliott, E.T.; Paustian, K. Bacterial and fungal abundance and biomass in conventional and no-tillage agroecosystems along two climatic gradients. Soil Biol. Biochem. 1999, 31, 573–585. [Google Scholar] [CrossRef]
- Zibilske, L.M.; Bradford, J.M.; Smart, J.R. Conservation tillage induced changes in organic carbon, total nitrogen and available phosphorus in a semi-arid alkaline subtropical soil. Soil Tillage Res. 2002, 66, 153–163. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Tisdall, J. How stubble affects organic matter, plants and animals in the soil. J. Dep. Agric. West. Aust. 1992, 33, 18–21. [Google Scholar]
- Wang, Z.T.; Li, T.; Li, Y.Z.; Zhao, D.Q.; Han, J.; Liu, Y.; Liao, Y.C. Relationship between the microbial community and catabolic diversity in response to conservation tillage. Soil Tillage Res. 2020, 196, 104431. [Google Scholar] [CrossRef]
- Corral-Fernández, R.; Parras-Alcántara, L.; Lozano-García, B. Stratification ratio of soil organic C, N and C:N in Mediterranean evergreen oak woodland with conventional and organic tillage. Agric. Ecosyst. Environ. 2013, 164, 252–259. [Google Scholar] [CrossRef]
- Moorhea, D.L.; Sinsabaugh, R.L. A theoretical model of litter decay and microbial interaction. Ecol. Monogr. 2006, 76, 151–174. [Google Scholar] [CrossRef]
- Liu, L.; Gundersen, P.; Zhang, T.; Mo, J.M. Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biol. Biochem. 2012, 44, 31–38. [Google Scholar] [CrossRef]
- DeForest, J.L.; Scott, L.G. Available organic soil phosphorus has an important influence on microbial community composition. Soil Sci. Soc. Am. J. 2010, 74, 2059–2066. [Google Scholar] [CrossRef]
- Kou, X.C.; Su, T.Q.; Ma, N.N.; Li, Q.; Wang, P.; Wu, Z.F.; Liang, W.J.; Cheng, W.X. Soil micro-food web interactions and rhizosphere priming effect. Plant Soil 2018, 432, 129–142. [Google Scholar] [CrossRef]
- Chen, X.; Han, X.Z.; You, M.Y.; Yan, J.; Lu, X.C.; Horwath, W.R.; Zou, W.X. Soil macroaggregates and organic-matter content regulate microbial communities and enzymatic activity in a Chinese Mollisol. J. Integr. Agric. 2019, 18, 2605–2618. [Google Scholar] [CrossRef]


| Soil Depth | Soil Properties | CT | NT | ST | MP |
|---|---|---|---|---|---|
| 0–20 cm | BD (g cm–3) | 1.36 ± 0.02 b | 1.43 ± 0.02 a | 1.30 ± 0.05 b | 1.32 ± 0.03 b |
| MWD (mm) | 0.98 ± 0.01 c | 1.19 ± 0.05 a | 1.17 ± 0.03 a | 1.06 ± 0.02 b | |
| SOC (g kg–1) | 13.31 ± 0.44 d | 17.10 ± 0.35 a | 14.52 ± 0.32 c | 15.55 ± 0.11 b | |
| TN (g kg–1) | 1.51 ± 0.02 b | 1.49 ± 0.03 b | 1.64 ± 0.09 a | 1.48 ± 0.01 b | |
| TP (g kg–1) | 0.54 ± 0.01 b | 0.58 ± 0.01 a | 0.48 ± 0.01 c | 0.55 ± 0.01 b | |
| AN (mg kg–1) | 135.62 ± 0.75 c | 141.23 ± 1.94 b | 158.70 ± 3.43 a | 135.09 ± 1.21 c | |
| AP (mg kg–1) | 24.69 ± 0.54 c | 31.83 ± 1.35 a | 19.95 ± 0.63 d | 27.17 ± 1.74 b | |
| pH | 5.48 ± 0.08 a | 5.45 ± 0.01 a | 5.15 ± 0.06 b | 5.48 ± 0.29 a | |
| 20–40 cm | BD (g cm–3) | 1.56 ± 0.02 a | 1.55 ± 0.03 a | 1.44 ± 0.04 b | 1.43 ± 0.03 b |
| MWD (mm) | 1.01 ± 0.01 b | 1.04 ± 0.01 a | 1.05 ± 0.01 a | 1.06 ± 0.01 a | |
| SOC (g kg–1) | 7.67 ± 0.33 c | 6.72 ± 0.20 d | 9.21 ± 0.45 b | 15.13 ± 0.40 a | |
| TN (g kg–1) | 0.94 ± 0.03 c | 0.76 ± 0.02 d | 1.21 ± 0.02 a | 1.08 ± 0.03 b | |
| TP (g kg–1) | 0.37 ± 0.02 b | 0.32 ± 0.02 c | 0.43 ± 0.02 a | 0.42 ± 0.01 a | |
| AN (mg kg–1) | 83.19 ± 0.89 c | 64.62 ± 1.74 d | 106.67 ± 2.39 a | 99.74 ± 0.55 b | |
| AP (mg kg–1) | 3.98 ± 0.17 c | 3.09 ± 0.18 d | 8.44 ± 0.23 a | 6.17 ± 0.17 b | |
| pH | 5.96 ± 0.01 c | 6.31 ± 0.05 a | 5.67 ± 0.06 d | 6.13 ± 0.04 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, P.; Li, R.; Zheng, H.; Wang, H.; Yuan, Y.; Luo, Y.; Zheng, J.; Liu, W. Long-Term Conservation Tillage Practices Directly and Indirectly Affect Soil Micro-Food Web in a Chinese Mollisol. Agronomy 2022, 12, 2356. https://doi.org/10.3390/agronomy12102356
Sui P, Li R, Zheng H, Wang H, Yuan Y, Luo Y, Zheng J, Liu W. Long-Term Conservation Tillage Practices Directly and Indirectly Affect Soil Micro-Food Web in a Chinese Mollisol. Agronomy. 2022; 12(10):2356. https://doi.org/10.3390/agronomy12102356
Chicago/Turabian StyleSui, Pengxiang, Ruiping Li, Hongbing Zheng, Hao Wang, Ye Yuan, Yang Luo, Jinyu Zheng, and Wuren Liu. 2022. "Long-Term Conservation Tillage Practices Directly and Indirectly Affect Soil Micro-Food Web in a Chinese Mollisol" Agronomy 12, no. 10: 2356. https://doi.org/10.3390/agronomy12102356
APA StyleSui, P., Li, R., Zheng, H., Wang, H., Yuan, Y., Luo, Y., Zheng, J., & Liu, W. (2022). Long-Term Conservation Tillage Practices Directly and Indirectly Affect Soil Micro-Food Web in a Chinese Mollisol. Agronomy, 12(10), 2356. https://doi.org/10.3390/agronomy12102356






