Local Agricultural Management Filters Morphological Traits of the South American Palm Weevil (Rhynchophorus palmarum L.; Coleoptera: Curculionidae) in Ornamental Palm Plantations
Abstract
:1. Introduction
2. Materials and Methods
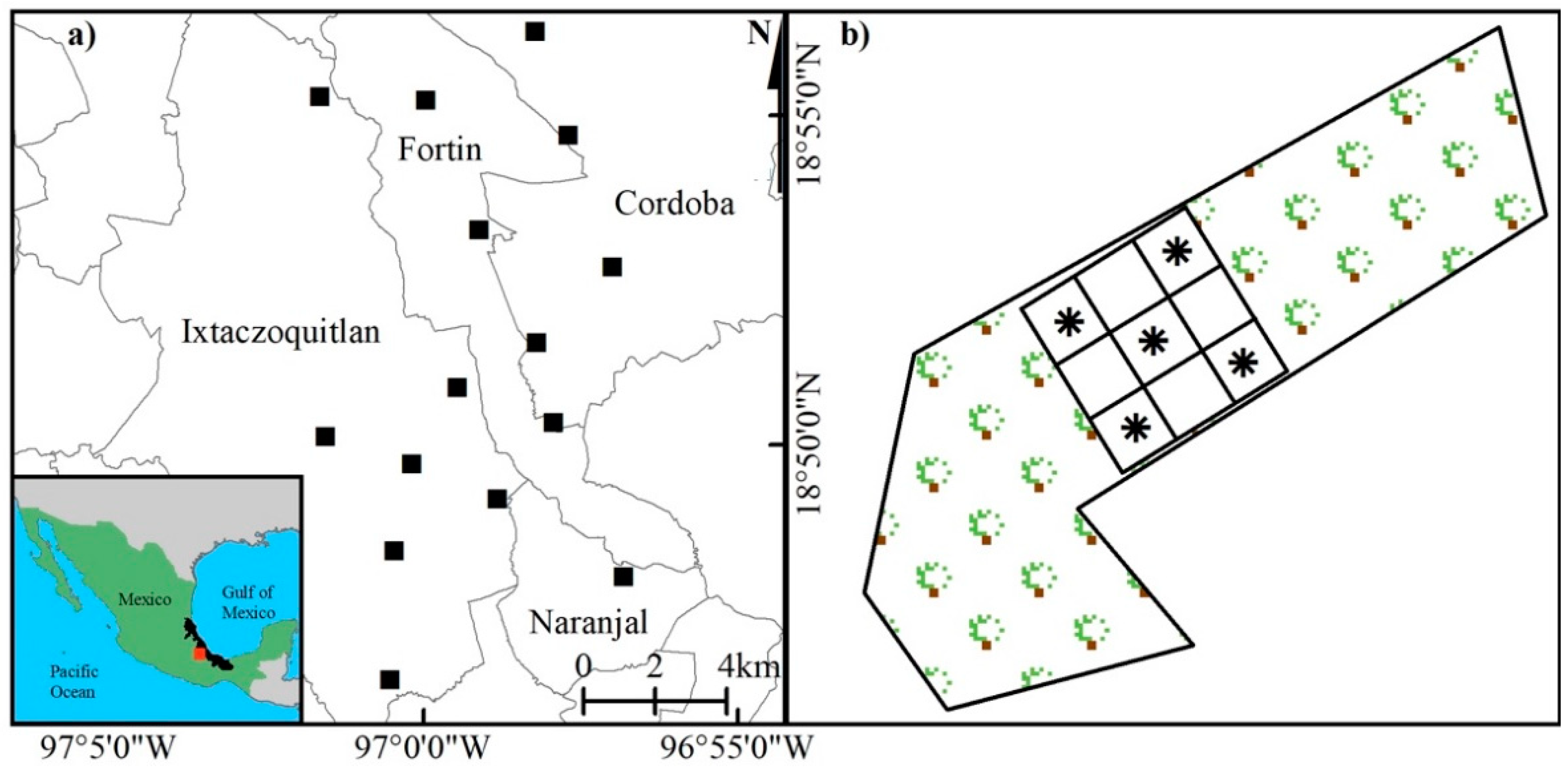
2.1. Study Area
2.2. Sampling Plot Selection and Design
2.3. Local Agricultural Management Characterization
2.4. Population Adult Survey
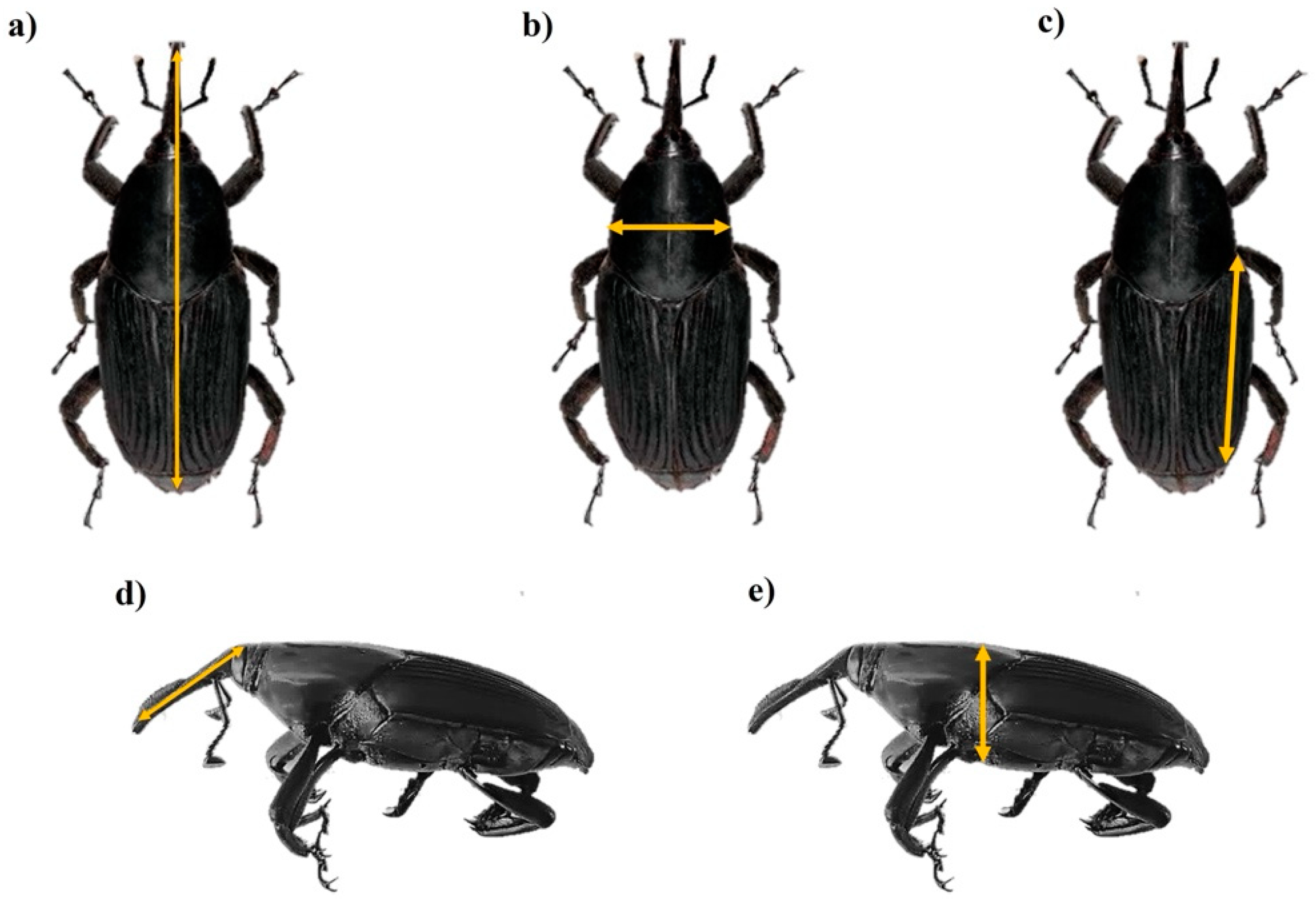
2.5. Morphometric Trait Measurements
2.6. Data Analysis
3. Results
3.1. Variation in the Morphological Traits
3.2. Local Management Characteristics
3.3. Local Filters of Morphological Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emery, S.E.; Jonsson, M.; Silva, H.; Ribeiro, A.; Mills, N.J. High agricultural intensity at the landscape scale benefits pests, but low intensity practices at the local scale can mitigate these effects. Agric. Ecosyst. Environ. 2021, 306, 107199. [Google Scholar] [CrossRef]
- Hernández-Martínez, G. Clasificación agroecológica. In Agroecosistemas Cafetaleros de Veracruz: Biodiversidad, Manejo y Conservación; Manson, R.H., Hernández-Ortiz, V., Gallina, S., Mehltreter, K., Eds.; Instituto de Ecología A.C. (INECOL) e Instituto Nacional de Ecología (INE-SEMARNAT): Xalapa, Mexico, 2008; pp. 15–34. ISBN 970-709-112-6. [Google Scholar]
- Vaidya, C.; Cruz, M.; Kuesel, R.; Gonthier, D.J.; Iverson, A.; Ennis, K.K.; Perfecto, I. Local and landscape constraints on coffee leafhopper (Hemiptera: Cicadellidae) diversity. J. Insect Sci. 2017, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Hajian-Forooshani, Z.; Gonthier, D.J.; Marín, L.; Iverson, A.L.; Perfecto, I. Changes in species diversity of arboreal spiders in Mexican coffee agroecosystems: Untangling the web of local and landscape influences driving diversity. PeerJ 2014, 2, e623. [Google Scholar] [CrossRef] [PubMed]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity—Ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Gallé, R.; Geppert, C.; Földesi, R.; Tscharntke, T.; Batáry, P. Arthropod functional traits shaped by landscape-scale field size, local agri-environment schemes and edge effects. Basic Appl. Ecol. 2020, 48, 102–111. [Google Scholar] [CrossRef]
- Elahi, E.; Weijun, C.; Zhang, H.; Nazeer, M. Agricultural intensification and damages to human health in relation to agrochemicals: Application of artificial intelligence. Land Use Policy 2019, 83, 461–474. [Google Scholar] [CrossRef]
- Bernal, J.S.; Medina, R.F. Agriculture sows pests: How crop domestication, host shifts, and agricultural intensification can create insect pests from herbivores. Curr. Opin. Insect Sci. 2018, 26, 76–81. [Google Scholar] [CrossRef]
- Jasrotia, P.; Bhardwaj, A.K.; Katare, S.; Yadav, J.; Kashyap, P.L.; Kumar, S.; Singh, G.P. Tillage intensity influences insect-pest and predator dynamics of wheat crop grown under different conservation agriculture practices in rice-wheat cropping system of Indo-gangetic plain. Agronomy 2021, 11, 1087. [Google Scholar] [CrossRef]
- Larsen, A.E.; Noack, F. Impact of local and landscape complexity on the stability of field-level pest control. Nat. Sustain. 2021, 4, 120–128. [Google Scholar] [CrossRef]
- Gong, P.; Li, X.; Wang, C.; Zhu, S.; Li, Q.; Zhang, Y.; Li, X.; Li, G.; Liu, E.; Gao, H.; et al. The sensitivity of field populations of metopolophium dirhodum (Walker) (hemiptera: Aphididae) to seven insecticides in Northern China. Agronomy 2021, 11, 1556. [Google Scholar] [CrossRef]
- Presa-Parra, E.; Llarena-Hernandez, C.; Serna-Lagunes, R.; Briones-Ruiz, G.; Herrera-Solano, A.; Nuñez-Pastrana, R.; Garcia-Martinez, M.A. Effects of concentrations of azadirachtin oil on mortality and post-exposure time of Atta mexicana leaf-cutter worker ants. Southwest. Entomologist. 2021, 46, 83–94. [Google Scholar] [CrossRef]
- Murguía-González, J.; Presa-Parra, E.; Serna-Lagunes, R.; Andres-Meza, P.; Rosas-Mejia, M.; Garcia-Martinez, M.A. Low Concentration of azadirachtin has the same toxic effect as imidacloprid + lambda-cyhalothrin in workers of two species of leaf-cutter ants. Southwest. Entomol. 2022, 47, 313–323. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Hassan, E.; Lee, K.Y. Methyl benzoate as a promising, environmentally safe insecticide: Current status and future perspectives. Agriculture 2022, 12, 378. [Google Scholar] [CrossRef]
- Tarifa, R.; Martínez, C.; Valera, F.; González, J.P.; Salido, V.T.; Rey, P.J. Agricultural intensification erodes taxonomic and functional diversity in Mediterranean olive groves by filtering out rare species. J. Appl. Ecol. 2021, 58, 2266–2276. [Google Scholar] [CrossRef]
- Brévault, T.; Clouvel, P. Pest management: Reconciling farming practices and natural regulations. Crop Prot. 2019, 115, 1–6. [Google Scholar] [CrossRef]
- Pigliucci, M.; Murren, C.J.; Schlichting, C.D. Phenotypic plasticity and evolution by genetic assimilation. J. Exp. Biol. 2006, 209, 2362–2367. [Google Scholar] [CrossRef]
- Sazali, S.N.; Nasarudin, N.A. Variation of the pronotal markings in Rhynchophorus (Coleoptera: Curculionidae) species from Kuala Terengganu, Terengganu. Borneo J. Resour. Sci. Technol. 2022, 12, 135–140. [Google Scholar] [CrossRef]
- Habel, J.C.; Ulrich, W.; Biburger, N.; Seibold, S.; Schmitt, T. Agricultural intensification drives butterfly decline. Insect Conserv. Divers. 2019, 12, 289–295. [Google Scholar] [CrossRef]
- Rolim, G.G.; Coelho, R.R.; Antonino, J.D.; Arruda, L.S.; Rodrigues, A.S.; Barros, E.M.; Torres, J.B. Field-evolved resistance to beta-cyfluthrin in the boll weevil: Detection and characterization. Pest Manag. Sci. 2021, 77, 4400–4410. [Google Scholar] [CrossRef]
- Ng, K.; Barton, P.S.; Blanchard, W.; Evans, M.J.; Lindenmayer, D.B.; Macfadyen, S.; McIntyre, S.; Driscoll, D.A. Disentangling the effects of farmland use, habitat edges, and vegetation structure on ground beetle morphological traits. Oecologia 2018, 188, 645–657. [Google Scholar] [CrossRef]
- Alves, V.M.; Hernández, M.I.M. Morphometric modifications in Canthon quinquemaculatus castelnau 1840 (coleoptera: Scarabaeinae): Sublethal effects of transgenic maize? Insects 2017, 8, 115. [Google Scholar] [CrossRef]
- Soares, J.A.C.; dos Santos, A.V.F.; Farias, P.R.S.; Medeiros, L.R.; Gomes, A.A.C. Detection of diseases in oil palm plantations in the Brazilian Amazon through orbital image. J. Agric. Sci. 2020, 12, 138. [Google Scholar] [CrossRef]
- Raine, E.H.; Gray, C.L.; Mann, D.J.; Slade, E.M. Tropical dung beetle morphological traits predict functional traits and show intraspecific differences across land uses. Ecol. Evol. 2018, 8, 8686–8696. [Google Scholar] [CrossRef]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- Guamani-Quimis, L.A.; Solís-Bowen, A.L.; Portalanza, D.; Garcés-Fiallos, F.R. Can mathematical models describe spear rot progress in oil palm trees? A five-year black weevil-disease assessment from Ecuador. Agriculture 2022, 12, 257. [Google Scholar] [CrossRef]
- Hagley, E.A.C. On the life history and habits of the palm weevil, Rhynchophorus palmarium. Ann. Entomol. Soc. Am. 1965, 58, 22–28. [Google Scholar] [CrossRef]
- Panduro-Pisco, G.; Salinas-Pimentel, N.M.; Cotrina-Barrueta, J.; Arbaiza-Peña, Á.K.; Plaza-Castro, J.; Iannacone, J. Characteristics of oil palm trunks for rearing of Rhynchophorus palmarum (Coleoptera: Curculionidae) in the Peruvian Amazon. Rev. Chapingo Ser. Cienc. For. Ambiente 2018, 24, 91–100. [Google Scholar] [CrossRef]
- Magalhães, J.A.S.; De Moraes Neto, A.H.A.; Miguens, F.C. Nematodes of Rhynchophorus palmarum L. (Coleoptera: Curculionidae), vector of the Red Ring disease in coconut plantations from the north of the Rio de Janeiro State. Parasitol. Res. 2008, 102, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Plata-Rueda, A.; Martínez, L.C.; Fernandes, F.L.; De Sousa Ramalho, F.; Zanuncio, J.C.; Serrão, J.E. Interactions between the bud rot disease of oil palm and Rhynchophorus palmarum (Coleoptera: Curculionidae). J. Econ. Entomol. 2016, 109, 962–965. [Google Scholar] [CrossRef]
- EPPO. Rhynchophorus palmarum. EPPO Bull. 2020, 35, 468–471. [CrossRef]
- Centre for Agriculture and Bioscience International (CABI). Invasive Species Compendium, Centre for Agriculture and Biosciences International Invasive Species Compendium. Rhynchophorus palmarum (South American Palm Weevil). Available online: https://www.cabi.org/isc/datasheet/47473 (accessed on 1 July 2022).
- Hagley, E.A.C. The role of the palm weevil, Rhynchophorus palmarum, as a vector of red ring disease of coconuts. I. results of preliminary investigations. J. Econ. Entomol. 1963, 56, 375–380. [Google Scholar] [CrossRef]
- Griffth, R. Red ring disease of coconut palm. Plant Dis. 1987, 71, 193–196. [Google Scholar]
- Broschat, T.K.; Elliott, M.L.; Hodel, D.R. Ornamental palms: Biology and horticulture. In Horticultural Reviews; Janick, J., Ed.; Wiley Blackwell: Hoboken, NJ, USA, 2014; Volume 42, pp. 1–120. ISBN 978-1-118-91679-7. [Google Scholar]
- Ramírez-Rojas, J.L.; Sandoval-Vega, C.A.; Ramírez-Vázquez, Y. Diagnóstico de las condiciones de operación y manejo administrativo de la producción de palmas ornamentales en las unidades productoras de los municipios de Actopan, Emiliano Zapata, Paso de Ovejas y Puente Nacional. Cienc. Adm. 2011, 11, 56–63. [Google Scholar]
- Murguía-González, J.; Landero-Torres, I.; Leyva-Ovalle, O.R.; Galindo-Tovar, M.E.; Llarena-Hernández, R.C.; Presa-Parra, E.; García-Martínez, M.A. Efficacy and cost of trap–bait combinations for capturing Rhynchophorus palmarum L. (Coleoptera: Curculionidae) in ornamental palm polycultures. Neotrop. Entomol. 2018, 47, 302–310. [Google Scholar] [CrossRef]
- Martínez, L.C.; Plata-Rueda, A.; Rodríguez-Dimaté, F.A.; Mendonça-Campos, J.; Dos Santos-junior, C.V.; Da Silva-Rolim, G.; Lemes-Fernandes, F.; Meloni-Silva, W.; Federico-Wilcken, C.; Cola-Zanuncio, J.; et al. Exposure to Insecticides Reduces Populations of Rhynchophorus palmarum in oil palm plantations with bud rot disease. Insects 2019, 10, 111. [Google Scholar] [CrossRef]
- Landero-Torres, I.; Galindo-Tovar, M.E.; Leyva-Ovalle, O.R.; Murguía-Gonzalez, J.; Presa-Parra, E.; Garcia-Martinez, M.A. Evaluación de cebos para el control de Rhynchophorus palmarum L. (Coleoptera: Curculionidae) en cultivos de palmas ornamentales. Entomol. Mex. 2015, 2, 112–118. [Google Scholar]
- Haseeb, M.; Dosunmu, O.G.; Kanga, L.H.B.; O’Brien, C.W.; Zhang, R. Development of a training program to identify invasive weevils in the Caribbean basin and the United States. Florida Entomol. 2019, 102, 469–474. [Google Scholar] [CrossRef]
- Landero-Torres, I.; Presa-Parra, E.; Galindo-Tovar, M.E.; Leyva-Ovalle, O.R.; Murguía-González, J.; Valenzuela-González, J.E.; García-Martínez, M.A. Temporal and spatial variation of the abundance of the black weevil (Rhynchophorus palmarum L., Coleoptera: Curculionidae) in ornamental palm crops from central Veracruz, Mexico. Southwest. Entomol. 2015, 40, 179–188. [Google Scholar] [CrossRef]
- Esparza-Díaz, G.; Olguin, A.; Carta, L.K.; Skantar, A.M.; Villanueva, R.T. Detection of Rhynchophorus palmarum (Coleoptera: Curculionidae) and identification of associated nematodes in South Texas. Florida Entomol. 2013, 96, 1513–1521. [Google Scholar] [CrossRef]
- Löhr, B.; Vásquez-Ordóñez, A.A.; Lopez-Lavalle, L.A.B. Rhynchophorus palmarum in disguise: Undescribed polymorphism in the “black” palm weevil. PLoS ONE 2015, 10, e0143210. [Google Scholar] [CrossRef]
- Wattanapongsiri, A. A revision of the genera Rhynchophorus and Dynamis (Coleoptera: Curculionidae). Sci. Bull. Dep. Agric. Thailand. 1966, 1, 1–328. [Google Scholar]
- Moczek, A.P.; Emlen, D.J. Proximate determination of male horn dimorphism in the beetle Onthophagus taurus (Coleoptera: Scarabaeidae). J. Evol. Biol. 1999, 12, 27–37. [Google Scholar] [CrossRef]
- Dalbon, V.A.; Acevedo, J.P.M.; Ribeiro Junior, K.A.L.; Ribeiro, T.F.L.; da Silva, J.M.; Fonseca, H.G.; Santana, A.E.G.; Porcelli, F. Perspectives for synergic blends of attractive sources in south american palm weevil mass trapping: Waiting for the red palm weevil Brazil invasion. Insects 2021, 12, 828. [Google Scholar] [CrossRef]
- García, E. Modificaciones al Sistema Climático de Köppen; Instituto de Geografía, UNAM: Ciudad de México, México, 1973. [Google Scholar]
- Vargas-Rueda, A.F.; Rivera-Hernández, J.E.; Álvarez-Aquino, C.; Salas-Morales, S.H.; Alcántara-Salinas, G.; Pérez-Sato, J.A. Composición florística del bosque mesófilo de montaña perturbado y sus ecotonos en el Parque Nacional Cañón del Río Blanco, Veracruz, México. Acta Bot. Mex. 2020, 128, 1715. [Google Scholar] [CrossRef]
- Landero-Torres, I.; Madrid-Ñeco, I.; Valenzuela-González, J.E.; Galindo-Tovar, M.E.; Leyva-Ovalle, O.R.; Murguía-González, J.; Lee-Espinosa, H.E.; Garcia-Martinez, M.Á. Myrmecofauna from three ornamental agroecosystems with different management and a forest remnant in Ixtaczoquitlán, Veracruz, Mexico. Southwest. Entomol. 2014, 39, 783–796. [Google Scholar] [CrossRef]
- Chinchilla, C.M.; Oehlschlager, C.A. Comparación de trampas para capturar adultos de Rhynchophorus palmarum utilizando la feromona de agregación producida por el macho. ASD Oil Palm Pap. 1992, 5, 9–14. [Google Scholar]
- Huang, Y.; Ao, Y.; Jiang, M.; Way, M.O. Variation of body size in rice water weevil (Coleoptera: Curculionidae) and its associations with population biology. J. Insect Sci. 2018, 18, 4. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, G.; Zhang, X.; Li, X.; Yu, Z.; Liu, Y.; Liang, H. Ground Beetle (Coleoptera: Carabidae) Diversity and body-size variation in four land use types in a mountainous area near Beijing, China. Coleopt. Bull. 2017, 71, 402–412. [Google Scholar] [CrossRef]
- Luzyanin, S.; Saveliev, A.; Ukhova, N.; Vorobyova, I.; Solodovnikov, I.; Anciferov, A.; Shagidullin, R.; Teofilova, T.; Nogovitsyna, S.; Brygadyrenko, V.; et al. Modeling sexual differences of body size variation in ground beetles in geographical gradients: A case study of Pterostichus melanarius (Illiger, 1798) (Coleoptera, Carabidae). Life 2022, 12, 112. [Google Scholar] [CrossRef]
- Damron, E.P.; Momcilovitch, A.N.S.; Jo, D.; Belk, M.C. No evidence for increased fitness of offspring from multigenerational effects of parental size or natal carcass size in the burying beetle Nicrophorus marginatus. PLoS ONE 2021, 16, e0253885. [Google Scholar] [CrossRef]
- Zhang, G.; Guo, W.; Wang, X.; Wang, Q.; Cui, J.; Wen, J. Structural comparison of the rostra of two species of weevils coexisting on Ailanthus altissima: The response to ecological demands of egg deposition. BMC Ecol. Evol. 2021, 21, 101. [Google Scholar] [CrossRef] [PubMed]
- Tanyi Tambe, J.; Riolo, P.; Okolle, J.N.; Isidoro, N.; Fanciulli, P.P.; Dallai, R. Sexual size differences and colour polymorphism of Rhynchophorus phoenicis in the southwest region of Cameroon. Bull. Insectol. 2013, 66, 67–73. [Google Scholar]
- García-Martínez, M.; Valenzuela-González, J.E.; Escobar-Sarria, F.; López-Barrera, F.; Castaño-Meneses, G. The surrounding landscape influences the diversity of leaf-litter ants in riparian cloud forest remnants. PLoS ONE 2017, 12, e0172464. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.M.; Brown, J.A.; Jiménez-Valverde, A.; Real, R. modEvA: Model Evaluation and Analysis 2014. Available online: http://modeva.r-forge.r-project.org/ (accessed on 1 July 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R. Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. R Core Team Nlme: Linear and Nonlinear Mixed Effects Models. Available online: https://cran.r-project.org/package=nlme (accessed on 1 July 2022).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Al-Ayedh, H. Evaluation of date palm cultivars for rearing the red date palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Florida Entomol. 2008, 91, 353–358. [Google Scholar] [CrossRef]
- Broschat, T.K. Palm nutrition and fertilization. Horttechnology 2009, 19, 690–694. [Google Scholar] [CrossRef] [Green Version]
- Lohr, B. Manual de cría del Picudo Negro de las Palmas Rhynchophorus Palmarum; Corporación Colombiana de Investigación Agropecuaria: Mosquera, Colombia, 2016. [Google Scholar]
- García-Hernández, J.L.; Beltrán-Morales, L.F.; Loya-Ramírez, J.G.; Morales-Cota, J.R.; Troyo-Diégue, E.; Beltrán-Morales, F. Primer informe del Rhynchophorus palmarum (Coleoptera: Dryophthoridae) en baja California Sur. Folia Entomol. Mex. 2003, 42, 415–417. [Google Scholar]
- Schlickmann-Tank, J.A.; Enciso-Maldonado, G.A.; Haupenthal, D.I.; Luna-Alejandro, G.; Badillo-López, S.E. Detección y variación temporal de Rhynchophorus palmarum (Linnaeus) (Coleoptera: Dryophthoridae) en cultivos de Acrocomia aculeata (Jacq.) Lodd. ex Mart. en Itapúa, Paraguay. Rev. Chil. Entomol. 2020, 8994, 163–169. [Google Scholar] [CrossRef]
- Cocco, A.; Pusceddu, M.; Lentini, A.; Floris, I. Can increasing infestations by Rhynchophorus ferrugineus threaten endemic Chamaerops humilis in Sardinia (Italy)? EPPO Bull. 2019, 49, 405–413. [Google Scholar] [CrossRef]
- Beillouin, D.; Ben-Ari, T.; Malézieux, E.; Seufert, V.; Makowski, D. Positive but variable effects of crop diversification on biodiversity and ecosystem services. Glob. Chang. Biol. 2021, 27, 4697–4710. [Google Scholar] [CrossRef]
- Ince, S.; Porcelli, F. Egg laying and egg laying behavior of Red Palm Weevil, Rhynchophorus ferrugineus (Olivier) 1790 (Coleoptera: Curculionidae). Agric. Biol. J. N. Am. 2011, 11, 1368–1374. [Google Scholar] [CrossRef]
- Ribera, I.; Dolédec, S.; Downie, I.S.; Foster, G.N. Effect of land disturbance and stress on species traits of ground beetle assemblages. Ecology 2001, 82, 1112–1129. [Google Scholar] [CrossRef]
- Osorio-Osorio, R.; Cibrián-Tovar, J.; López-Collado, J.; Cortéz-Madrigal, H.; Cibrián-Tovar, D. Exploración de factores para incrementar la eficiencia de captura de Rhynchophorus palmarum (Coleoptera: Dryophthoridae). Folia Entomol. Mex. 2003, 42, 27–36. [Google Scholar]
- Cheng, L.; Tong, Y.; Zhao, Y.; Sun, Z.; Wang, X.; Ma, F.; Bai, M. Study on the relationship between richness and morphological diversity of higher taxa in the darkling beetles (Coleoptera: Tenebrionidae). Diversity 2022, 14, 60. [Google Scholar] [CrossRef]
- Evans, M.E.G. Locomotion in the Coleoptera Adephaga, especially Carabidae. J. Zool. 1977, 181, 189–226. [Google Scholar] [CrossRef]
- Manseguiao, M.R.S.; Demayo, C.G. Morphological variations on the pronotum of the hispid beetle P. reichei from eight mindanao populations. Adv. Environ. Biol. 2015, 9, 46–55. [Google Scholar]
- dos Santos, S.; da Silva Júnior, V.A.; Broglio, S.M.F.; Negrisoli Junior, A.S.; Guzzo, E.C. Effect of plant protection products on Rhynchophorus palmarum L. (Coleoptera: Curculionidae) larvae in laboratory. Arq. Inst. Biol. 2018, 85, 1–4. [Google Scholar] [CrossRef]
- Löhr, B.; Negrisoli, A.; Molina, J.P. Billaea rhynchophorae, a palm weevil parasitoid with global potential. Arab J. Plant Prot. 2019, 37, 101–108. [Google Scholar] [CrossRef]
- Gaviria, J.; Löhr, B. Parasitismo de Billaea claripalpis (Diptera: Tachinidae) Sobre larvas de Rhynchophorus palmarum (Coleoptera: Dryophthoridae). Av. Investig. Agropecu. 2020, 24, 67–80. [Google Scholar]
- Quezada, J.R.; Amaya, C.A.; Herman, L.H. Xanthopygus cognatus Sharp (Coleoptera: Staphylinidae), an enemy of the coconut weevil, Rhynchophorus palmarum L. (Coleoptera: Curculionidae) in El Salvador. J. N. Y. Entomol. Soc. 1969, 77, 264–269. [Google Scholar]
- López-Luján, L.M.; Ramírez-Restrepo, S.; Bedoya-Pérez, J.C.; Salazar-Yepes, M.; Arbeláez-Agudelo, N.; Granada-García, D. Bioactivity of fungi isolated from coconut growing areas against Rhynchophorus palmarum. Pesqui. Agropecuária Bras. 2022, 57, 1–8. [Google Scholar] [CrossRef]
- Costa, F.; Sacramento, J.; Melo, L.; Goebel, V.; Le Coustour, N.; Lummerzheim, M.; Lacerda, J.; Motta, R. Mass trapping and biological control of Rhynchophorus palmarum L.: A hypothesis based on morphological evidences. EntomoBrasilis 2011, 4, 49–55. [Google Scholar]
- Gaviria, J.; Montoya-Lerma, J.; Armbrecht, I.; Löhr, B.; Vásquez-Ordóñez, A.A. Dynamis borassi (Coleoptera: Curculionidae), a new potential pest to the palms (Arecaceae): An early warning for the palm producers. Florida Entomol. 2021, 104, 107–116. [Google Scholar] [CrossRef]
- Ouyang, F.; Hui, C.; Ge, S.; Men, X.Y.; Zhao, Z.H.; Shi, P.J.; Zhang, Y.S.; Li, B.L. Weakening density dependence from climate change and agricultural intensification triggers pest outbreaks: A 37- year observation of cotton bollworms. Ecol. Evol. 2014, 4, 3362–3374. [Google Scholar] [CrossRef] [PubMed]

| Morphometric Measuments (mm) | Males | Females | Both Sexes |
|---|---|---|---|
| Body length | 38.32 ± 0.61 | 36.09 ± 1.13 | 37.21 ± 1.44 |
| Pronotum width | 12.45 ± 1.44 | 9.61 ± 1.81 | 11.02 ± 2.16 |
| Elytron length | 20.95 ± 0.16 | 20.27 ± 0.49 | 20.61 ± 0.49 |
| Rostrum length | 13.31 ± 0.31 | 12.43 ± 0.53 | 12.87 ± 0.61 |
| Mesothorax depth | 6.11 ± 0.14 | 5.69 ± 0.31 | 5.9 ± 0.31 |
| Abundance (individuals) | 3077 | 1895 | 4972 |
| Management Descriptor | Mean ± SE | Range | Mode |
|---|---|---|---|
| (a) Biophysical structure | |||
| Palm density (palms/ha) | 1508.87 ± 115.77 | 943–2331 | 1260 |
| Palm diversity (palm species/ha) | 4.93 ± 0.51 | 1–8 | 6 |
| S. romanzoffiana abundance (individuals) | 724.67 ± 99.85 | 0–1602 | 0 |
| P. roebelenii abundance (individuals) | 295.20 ± 77.75 | 0–1242 | 135 |
| (b) Agrochemical use | |||
| Insecticides (1-L-bottles/year) | 27.33 ± 8.80 | 0–88 | 0 |
| Herbicides (1-L-bottles/year) | 25.73 ± 8.22 | 0–86 | 0 |
| Fungicides (1-L-bottles/year) | 21.47 ± 6.50 | 0–88 | 0 |
| Fertilizers (10-kg-bags/year) | 28.20 ± 8.89 | 0–104 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponce-Méndez, M.; García-Martínez, M.A.; Serna-Lagunes, R.; Lasa-Covarrubias, R.; Presa-Parra, E.; Murguía-González, J.; Llarena-Hernández, C. Local Agricultural Management Filters Morphological Traits of the South American Palm Weevil (Rhynchophorus palmarum L.; Coleoptera: Curculionidae) in Ornamental Palm Plantations. Agronomy 2022, 12, 2371. https://doi.org/10.3390/agronomy12102371
Ponce-Méndez M, García-Martínez MA, Serna-Lagunes R, Lasa-Covarrubias R, Presa-Parra E, Murguía-González J, Llarena-Hernández C. Local Agricultural Management Filters Morphological Traits of the South American Palm Weevil (Rhynchophorus palmarum L.; Coleoptera: Curculionidae) in Ornamental Palm Plantations. Agronomy. 2022; 12(10):2371. https://doi.org/10.3390/agronomy12102371
Chicago/Turabian StylePonce-Méndez, Moises, Miguel A. García-Martínez, Ricardo Serna-Lagunes, Rodrigo Lasa-Covarrubias, Ehdibaldo Presa-Parra, Joaquin Murguía-González, and Carlos Llarena-Hernández. 2022. "Local Agricultural Management Filters Morphological Traits of the South American Palm Weevil (Rhynchophorus palmarum L.; Coleoptera: Curculionidae) in Ornamental Palm Plantations" Agronomy 12, no. 10: 2371. https://doi.org/10.3390/agronomy12102371
APA StylePonce-Méndez, M., García-Martínez, M. A., Serna-Lagunes, R., Lasa-Covarrubias, R., Presa-Parra, E., Murguía-González, J., & Llarena-Hernández, C. (2022). Local Agricultural Management Filters Morphological Traits of the South American Palm Weevil (Rhynchophorus palmarum L.; Coleoptera: Curculionidae) in Ornamental Palm Plantations. Agronomy, 12(10), 2371. https://doi.org/10.3390/agronomy12102371







