Cultivating Improved Varieties of Maize Does Not Guarantee Sufficient Dietary Provision of Fe and Zn in a Maize–Bean Intercropping System in Eastern Uganda: Evaluating Effects of Climate and Soil Types
Abstract
:1. Introduction
- i.
- Assess the effect of soil types, climate variables and seasons on yield and nutrient (Fe, Zn, starch and essential amino acids) quantities in maize–grains.
- ii.
- Identify sites where the intercropping system leads to an increase in grain-yield, Fe and Zn concentrations and amino acids’ content across the Kyoga basin.
2. Methods and Materials
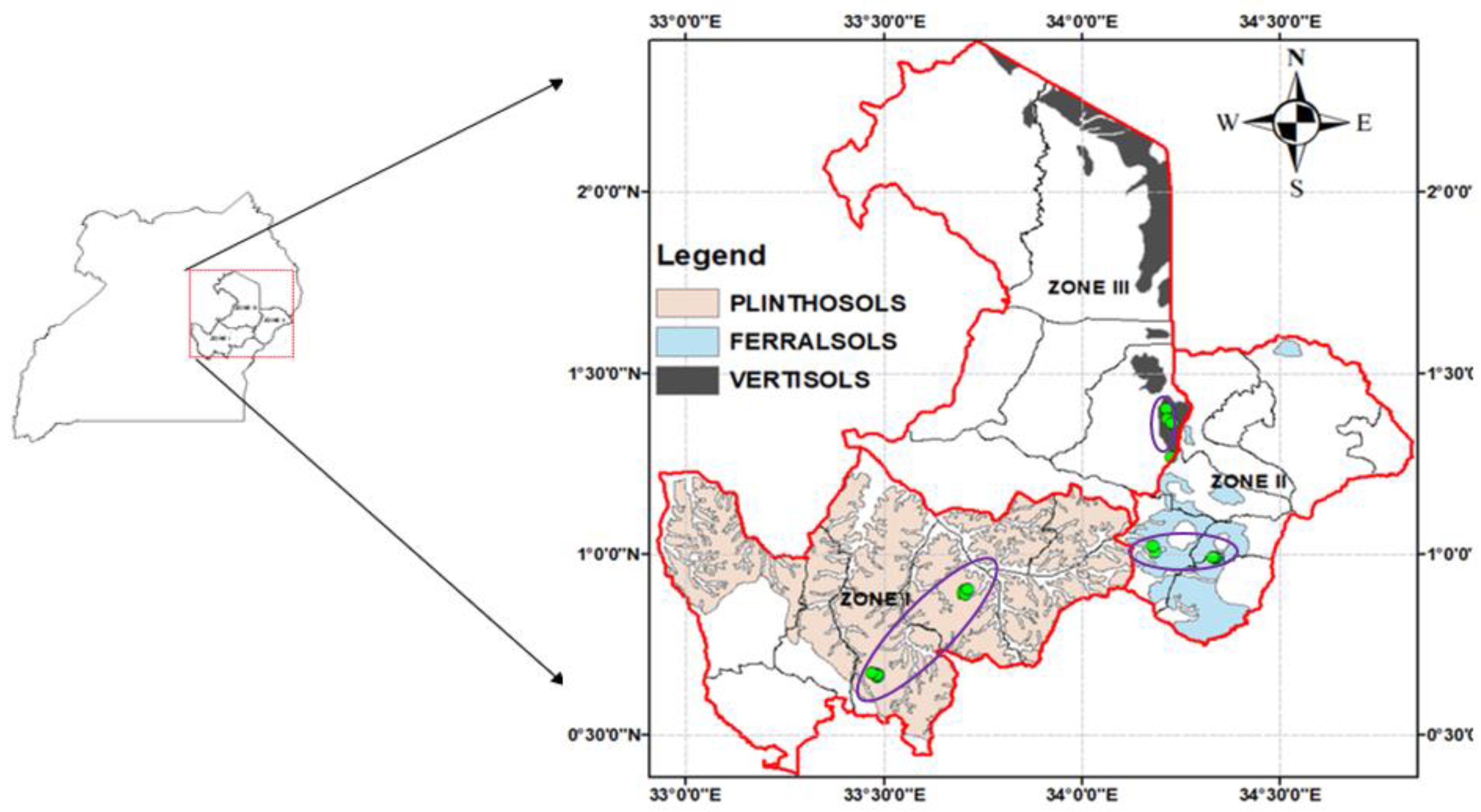
2.1. Study Area and Experimental Setup
2.2. Soil Characteristics
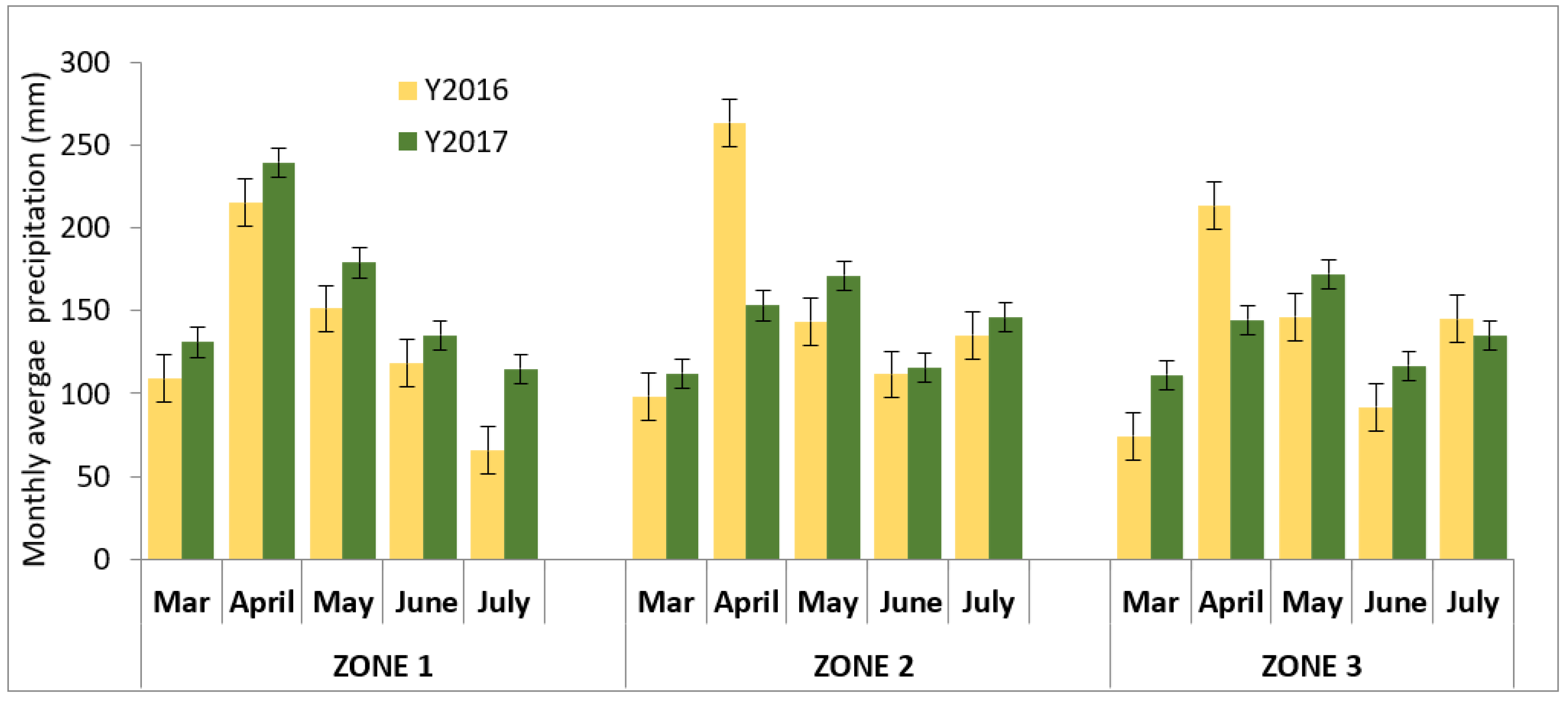
2.3. Weather Data
2.4. Crop Parameters
2.5. Statistical Analyses
3. Results
3.1. Characterization of Soil and Climate Conditions of Kyoga Basin
3.1.1. Soil Conditions
3.1.2. Climatic Conditions
3.2. Maize Grain Yield
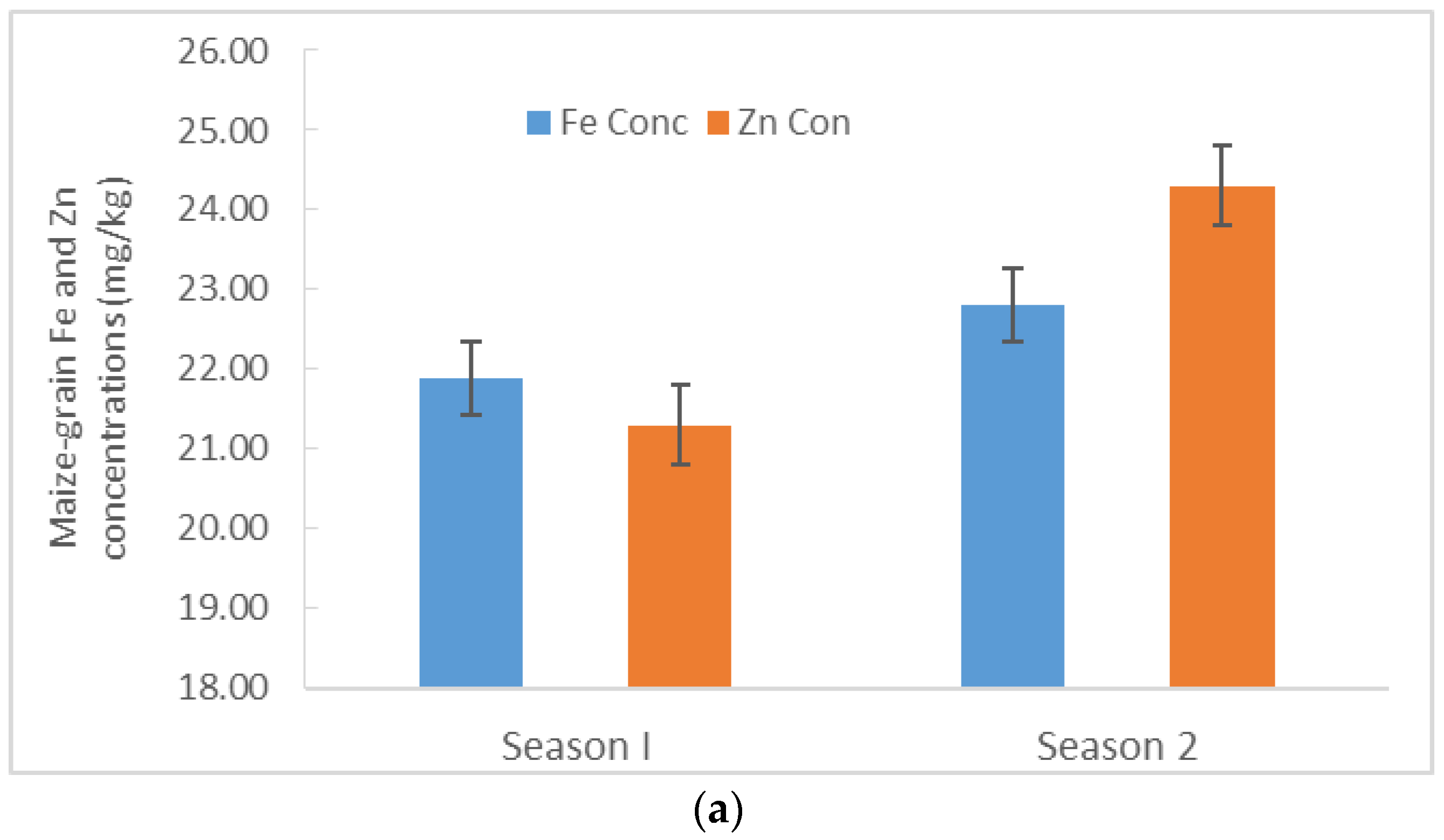
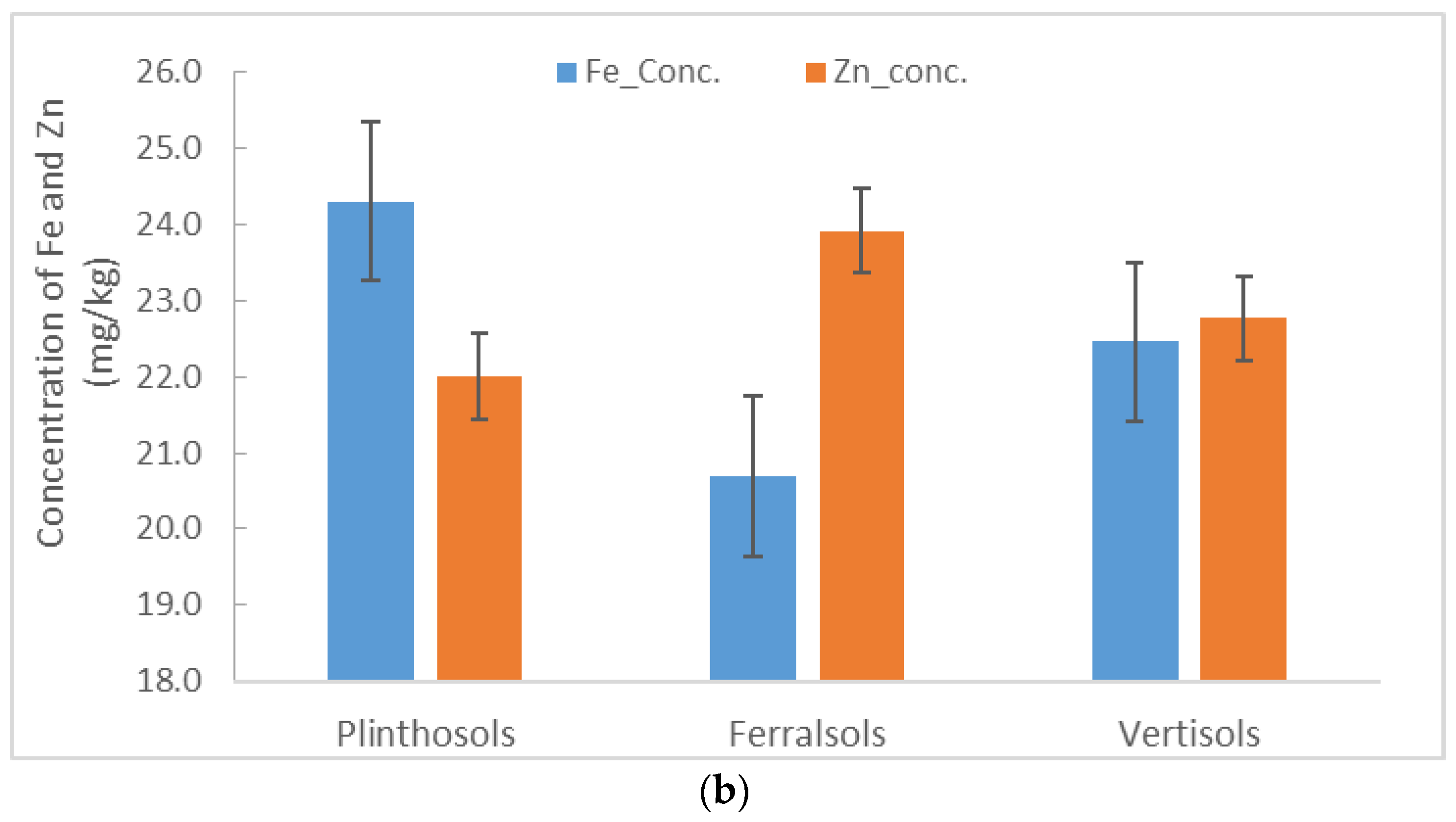
3.3. Maize–Grain Iron (Fe) and Zinc (Zn) Concentrations
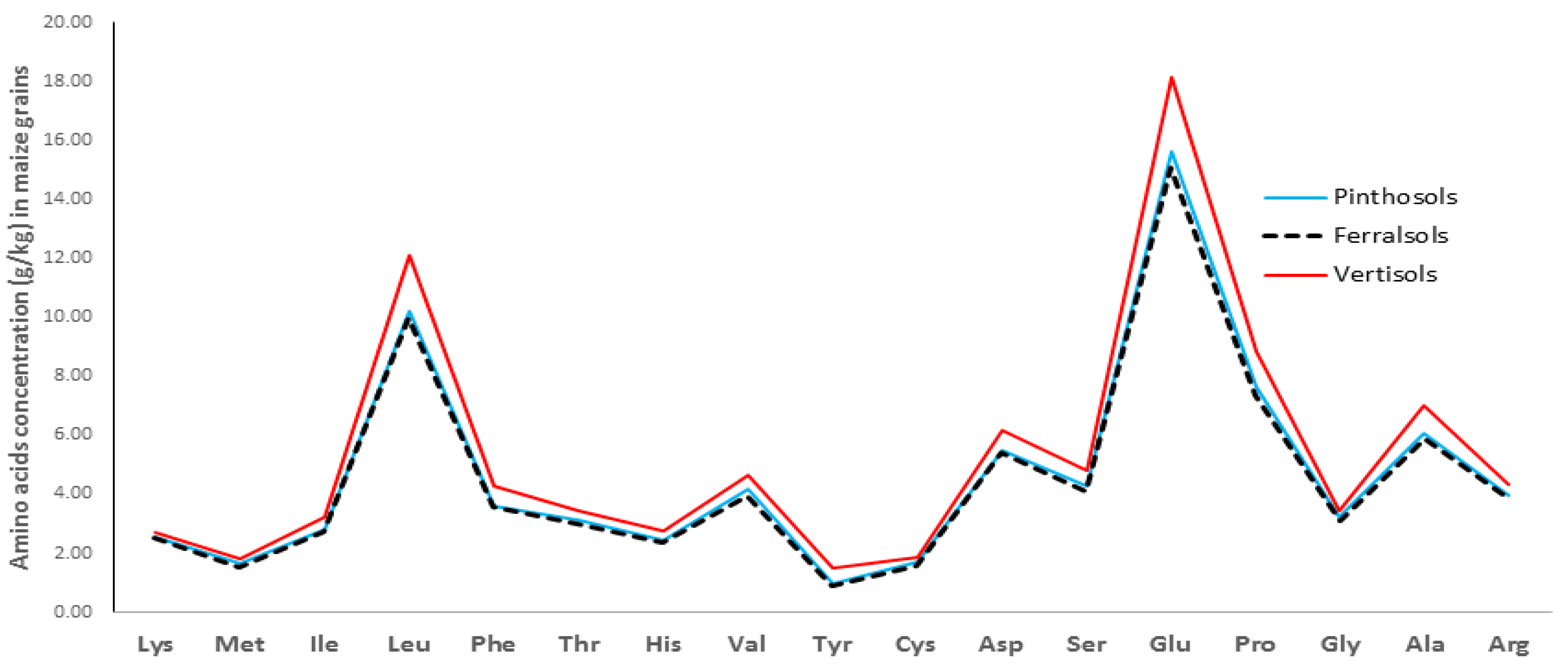
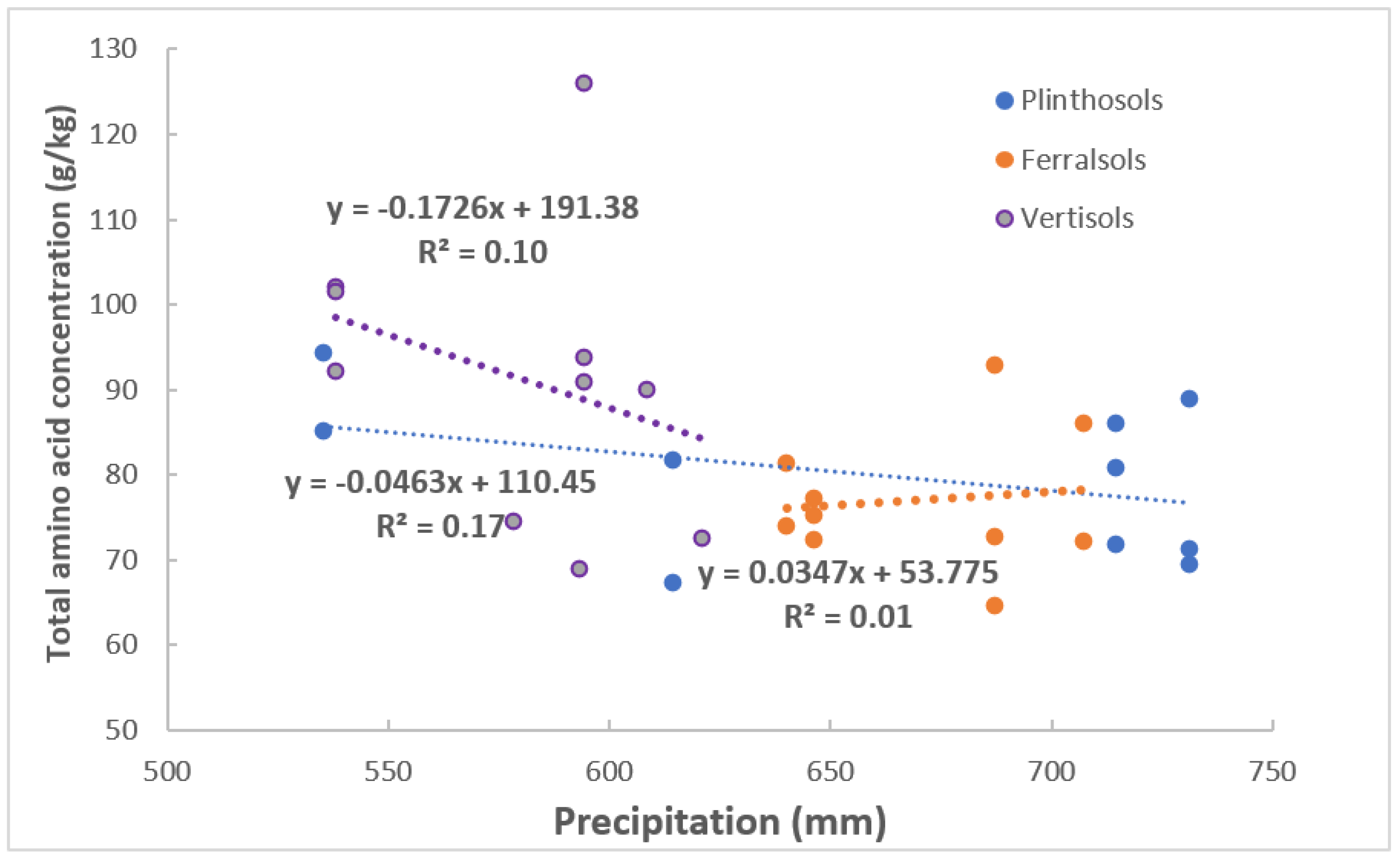
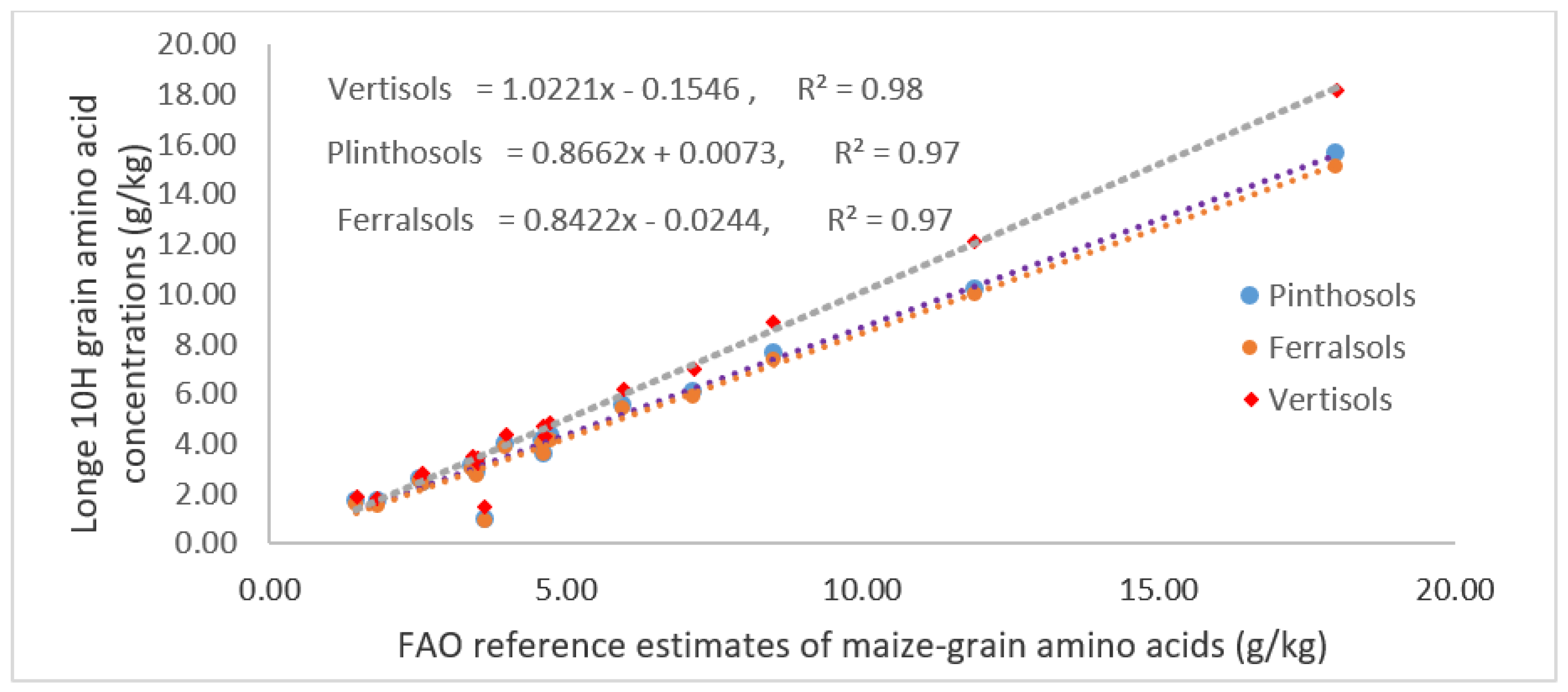
3.4. Maize–Grain Starch, Crude and Total Proteins Content
4. Discussion
4.1. Maize–Grain Yield
4.2. Iron and Zn Concentrations and Uptake in Maize Grains
4.3. Starch, CP and TP Content in Maize–Grains
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouis, H.E.; Welch, R.M. Biofortification—A Sustainable Agricultural Strategy for Reducing Micronutrient Malnutrition in the Global South. Crop Sci. 2010, 50 (Suppl. S1), S-20–S-32. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2007, 302, 1–17. [Google Scholar] [CrossRef]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Welch, R.M.; Graham, R.D. Breeding for micronutrients in staple food crops from a human nutrition perspective. J. Exp. Bot. 2004, 55, 353–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galili, G.; Amir, R. Fortifying plants with the essential amino acids lysine and methionine to improve nutritional quality. Plant Biotechnol. J. 2013, 11, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Ufaz, S.; Galili, G. Improving the content of essential amino acids in crop plants: Goals and opportunities. Plant Physiol. 2008, 147, 954–961. [Google Scholar] [CrossRef] [Green Version]
- Gupta, H.S.; Hossain, F.; Nepolean, T.; Vignesh, M.; Mallikarjuna, M.G. Understanding Genetic and Molecular Bases of Fe and Zn Accumulation Towards Development of Micronutrient-Enriched Maize. In Nutrient Use Efficiency: From Basics to Advances; Springer: New Delhi, India, 2015; pp. 255–282. [Google Scholar]
- Šimić, D.; Sudar, R.; Ledenčan, T.; Jambrović, A.; Zdunić, Z.; Brkić, I.; Kovačević, V. Genetic variation of bioavailable iron and zinc in grain of a maize population. J. Cereal Sci. 2009, 50, 392–397. [Google Scholar] [CrossRef]
- Briat, J.F.; Dubos, C.; Gaymard, F. Iron nutrition, biomass production, and plant product quality. Trends Plant Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef]
- Shewry, P.R. Improving the protein content and composition of cereal grain. J. Cereal Sci. 2007, 46, 239–250. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2017; Building Resilience for Peace and Food Security. Rome, FAO. 2017. Available online: http://www.fao.org/state-of-food-security-nutrition (accessed on 18 September 2018).
- Harton, S.; Alderman, H.; Rivera, J.A. The Challenge of Hunger and Malnutrition; Copenhagen Consensus 2008 Project, Challenge Paper. 2008. Available online: http://www.who.int/pmnch/topics/nutrition/hunger/en/ (accessed on 5 September 2018).
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Sec. 2017, 12, 49–58. [Google Scholar] [CrossRef]
- de Valença, A.W.; Bake, A.; Brouwer, I.D.; Giller, K.E. Agronomic biofortification of crops to fight hidden hunger in sub-Saharan Africa. Glob. Food Secur. 2017, 12, 8–14. [Google Scholar] [CrossRef]
- Grusak, M.A.; DellaPenna, D. Improving the nutrient composition of plants to enhance human nutrition and health. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 133–161. [Google Scholar] [CrossRef]
- Zuo, Y.M.; Zhang, F.S.; Li, X.L.; Cao, Y.P. Studies on the improvement in iron nutrition of peanut by intercropping maize on a Calcareous soils. Plant Soil 2000, 220, 13–35. [Google Scholar] [CrossRef]
- Das, J.K.; Salam, R.A.; Kumar, R.; Bhutta, Z.A. Micronutrients fortification of food and its impact on woman and Child health: A systematic review. Syst. Rev. 2013, 2, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, S.; Arjun, F.M.; Anjum, M.A. Micronutrients fortification of Wheat flour: Recent development and strategies. Food Res. Int. 2011, 44, 652–659. [Google Scholar] [CrossRef]
- Gomez-Galera, S.; Rojas, E.; Christou, P. Critical evaluation of strategies for mineral fortification of staple Food crops. Transgenic Res. 2010, 19, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Zhang, F. Iron and Zinc biofortication strategies in dicot plants by intercropping with gramineous species. A review. Agron. Sustain. Dev. 2009, 29, 63–71. [Google Scholar] [CrossRef]
- Prasad, R.; Shivay, Y.S.; Kumar, D. Agronomic Biofortification of Cereal Grains with Iron and Zinc; Elsevier: Amsterdam, The Netherlands, 2014; pp. 55–91. [Google Scholar]
- Manzeke, G.M.; Mapfumo, P.; Mtambanengwe, F.; Chikowo, R.; Tendayi, T.; Cakmak, I. Soil fertility management effects on maize productivity and grain zinc content in smallholder farming systems of Zimbabwe. Plant Soil 2012, 361, 57–69. [Google Scholar] [CrossRef]
- Manzeke, G.M.; Mtambanengwe, F.; Nezomba, H.; Mapfumo, P. Zinc fertilization influence on maize productivity and grain nutritional quality under integrated soil fertility management in Zimbabwe. Field Crops Res. 2014, 166, 128–136. [Google Scholar] [CrossRef]
- Singh, B.R.; Timsina, Y.N.; Lind, O.C.; Cagno, S.; Janssens, K. Zinc and Iron Concentration as Affected by Nitrogen Fertilization and Their Localization in Wheat Grain. Front. Plant Sci. 2018, 9, 307. [Google Scholar] [CrossRef] [Green Version]
- Kermah, M.; Franke, A.C.; Adjei-Nsiah, S.; Ahiabor, B.D.K.; Abaidoo, R.C.; Giller, K.E. Maize-grain legume intercropping for enhanced resource use efficiency and crop productivity in the Guinea savanna of northern Ghana. Field Crops Res. 2017, 213, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Yue, S.; Zhang, W.; Liu, D.; Cui, Z.; Chen, X.; Ye, Y.; Zou, C. Zinc, Iron, Managanese and Copper uptake requirements in response to Nitrogen supply and the increased grain yield of summer maize. PLoS ONE 2014, 9, e93895. [Google Scholar] [CrossRef] [PubMed]
- Layek, J.; Das, A.; Mitran, T.; Nath, C.; Meena, R.S.; Yodav, G.S.; Shivakumar, B.G.; Kumar, S.; Lal, R. Cereal+legumes intercropping: An option for improving Productivity and sustainable soil health. In Legumes for Soil Health and Sustainable Management; Meena, R.S., Ed.; Springer Nature: Singapore, 2018. [Google Scholar]
- Pandian, S.S.; Robin, S.; Vinod, K.K.; Rajeswari, S.; Manonmani, S.; Subramanian, K.S.; Saraswathi, R.; Kirubhakaran, A.P.M. Influence of intrinsic soil factors on genotype-by-environment interactions governing micronutrient content of milled rice grains. Aust. J. Crop Sci. 2011, 5, 1737–1744. [Google Scholar]
- Yang, X.E.; Chen, W.-R.; Feng, X. Improving human micronutrient nutrition through biofortification in the soil-plant system: China as case study. Environ. Geochem. Health 2007, 29, 413–428. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, B.D.; Bouldin, D.R.; Urey, G.H.; Kizito, F. Modeling cropping strategies to improve human nutrition in Uganda. Agric. Syst. 2001, 67, 105–120. [Google Scholar] [CrossRef]
- Lugoi, L.P.; Bamutaze, Y.; Martinsen, V.; Dick, Ø.B.; Almås, Å.R. Ecosystem productivity response to environmental forcing, prospect for improved rain-fed cropping productivity in lake Kyoga Basin. Appl. Geogr. 2019, 102, 1–11. [Google Scholar] [CrossRef]
- Kaizzi, K.C.; Nansamba, A.; Musisi, K.F. Optimizing Fertilizer Use within the Context of Integrated Soil Fertility in Uganda. In Fertilizer Use Optimization in Sub-Saharan Africa; Wortmann, C.S., Sones, K.R., Eds.; CABI: Oxfordshire, UK, 2017. [Google Scholar]
- WRB, I.W. World Reference Base for Soil Resources 2006; World Soil Resources Reports No. 103; FAO: Rome, Italy, 2006. [Google Scholar]
- NaCRRI. National Crop Resources Research Institute. Ministry of Agriculture, Animal Industry and Fisheries, Gov of Uganda. 2009. Available online: https://tasai.org.info_portal (accessed on 8 September 2016).
- Ranst, E.v.; Verloo, M.; Demeyer, A.; Pauwels, J. Manual for the soil chemistry and fertility laboratory: Analytical methods for soils and plants equipment, and management of consumables. Lab Manual. 1999.
- Gibson, R.B. The Determination of Nitrogen by the Kjeldahl Method. J. Am. Chem. Soc. 1904, 26, 105–110. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Chang, S.K.C.; Zhang, Y. Protein Analysis. In Food Analysis; Neilsen, S.S., Ed.; Food Science Text Series; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 4 June 2018).
- Foster, H.L. Rapid routine soil and plant analysis without automatic equipment I. East Afr. Agric. For. J. 1971, 37, 11. [Google Scholar] [CrossRef]
- Dangour, A.D.; Dodhia, S.K.; Hayter, A.; Allen, E.; Lock, K.; Uauy, R. Nutritional quality of organic foods: A systematic review. Am. J. Clin. Nutr. 2009, 90, 680–685. [Google Scholar] [CrossRef] [Green Version]
- Van Ittersum, M.K.; Van Bussel, L.G.J.; Wolf, J.; Grassini, P.; Van Wart, J.; Guilpart, N.; Claessens, L.; De Groot, H.; Wiebe, K. Can Sub-Saharan Africa Feed itself? Proc. Natl. Acad. Sci. USA 2016, 113, 14964–14969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaizzi, K.C.; Byalebeka, J.; Semalulu, O.; Alou, I.; Zimwanguyizza, W.; Nansamba, A.; Musinguzi, P.; Ebanyat, P.; Hyuha, T.; Wortmann, C.S. Maize Response to Fertilizer and Nitrogen Use Efficiency in Uganda. Agron. J. 2012, 104. [Google Scholar] [CrossRef]
- Mloza-Banda, H.R.; Makwiza, C.N.; Mloza-Banda, M.L. Soil properties after conversion to conservation agriculture from ridge tillage in Southern Malawi. J. Arid. Environ. 2016, 127, 7–16. [Google Scholar] [CrossRef]
- Martinsen, V.; Shitumbanuma, V.; Mulder, J.; Ritz, C.; Cornelissen, G. Effects of hand-hoe tilled conservation farming on soil quality and carbon stocks under on-farm conditions in Zambia. Agric. Ecosyst. Environ. 2017, 241, 168–178. [Google Scholar] [CrossRef]
- Vanlauwe, B.; Tittonell, P.; Mukalama, J. Within-farm soil fertility gradients affect response of maize to fertiliser application in western Kenya. Nutr. Cycl. Agroecosystems 2006, 76, 171–182. [Google Scholar] [CrossRef]
- Nkonya, E.; Kaizzi, C.; Pender, J. Determinants of nutrient balances in a maize farming system in eastern Uganda. Agric. Syst. 2005, 85, 155–182. [Google Scholar] [CrossRef]
- Nyemba, R.C.; Dakora, F.D. Evaluating nitrogen fixation by food legumes in farmers’ fields in three agro ecological zones of Zambia using 15N natural abundance. Biol. Fertil. Soils 2010, 46, 461–470. [Google Scholar] [CrossRef]
- Njira, K.O.W.; Nalivata, P.C.; Kanyama-Phiri, G.Y.; Lowole, M.W. Biological Nitrogen Fixation in sole and doubled-up legume cropping systems in the sandy soils of Kasungu, Central Malawi. J. Soil Sci. Environ. Manag. 2012, 3, 224–230. [Google Scholar]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier/Academic Press: Cambridge, CA, USA, 2012. [Google Scholar]
- Grujcic, D.; Hansen, T.H.; Husted, S.; Drinic, M.; Singh, B.R. Effect of nitrogen and zinc fertilization on zinc and iron bioavailability and chemical speciation in maize silage. J. Trace Elem. Med. Biol. 2018, 49, 269–275. [Google Scholar] [CrossRef]
- Colombo, C.; Palumbo, G.; He, J.Z.; Pinton, R.; Cesco, S. Review on Iron availability in soil: Interaction of Fe minerals, plants and microbes. J. Soils Sediments 2014, 14, 538–548. [Google Scholar] [CrossRef]
- Blume, H.P.; Brummer, G.W.; Fleige, H.; Horn, R.; Kandeler, E.; Knabner, I.K.; Kretzschmar, R.; Stahr, K.; Wilke, B.M. Scheffer/Schachtschabel Soil Science, 16th ed.; Springer-Cerlag: Berlin, Germany, 2016; p. 618. [Google Scholar]
- Almås, Å.R.; Lofts, S.; Tipping, E.; Mulder, J. Solubility of major cations and Cu, Zn and Cd in soil extracts of some contaminated agricultural soils near a zinc smelter in Norway: Modelling with a multisurface extension of WHAM. Eur. J. Soil Sci. 2007, 58, 1074–1086. [Google Scholar] [CrossRef] [Green Version]
- FAO. Amino Acid Content of Foods and Biological Data on Proteins. FAO Nutritional Studies No. 4 Rome—Italy. 1981. Available online: www.fao.org/docrep/005/AC854T/AC854T00.htm#TOC (accessed on 6 November 2017).
- Clemens, S. Zn and Fe biofortification: The right chemical environment for human bioavailability. Plant Sci. 2014, 225, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.S.; Perlas, L.; Hotz, C. Improving the bioavailability of nutrients in plant foods at the household level. Proc. Nutr. Soc. 2006, 65, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Bänziger, M.; Long, J. The potential for increasing the iron and zinc density of maize through plant-breeding. Food Nutr. Bull. 2000, 21, 397–400. [Google Scholar] [CrossRef] [Green Version]
- Oikeh, S.O.; Menkir, A.; Dixon, B.M.; Welch, R.; Glahn, R.P. Assessment of Concentrations of Iron and Zinc and Bioavailable Iron in Grains of Early-Maturing Tropical Maize Varieties. J. Agric. Food Chem. 2003, 51, 3688–3694. [Google Scholar] [CrossRef]
- Maziya-Dixon, B.; Kling, J.G.; Menkir, A.; Dixon, A. Genetic variation in total carotene, iron, and zinc contents of maize and cassava genotypes. Food Nutr. Bull. 2000, 21, 419–422. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Mazumdar, S.; Chakraborti, M.; Hossain, F.; Manjaiah, K.M.; Agrawal, P.K.; Guleria, S.K.; Gupta, H.S. Genetic variability and genotype year interactions for Kernel iron and zinc concentration in maize (Zea mays L.). Indian J. Agric. Sci. 2011, 81, 704–711. [Google Scholar]
- Agrawal, P.K.; Jaiswal, S.K.; Prasanna, B.M.; Hossain, F.; Saha, S.; Guleria, S.K.; Gupta, H.S. Genetic variability and stability for kernel iron and zinc concentration in maize (Zea mays L.) Genotypes. Indian J. Genet. Breed 2012, 72, 421–428. [Google Scholar]
- Chen, F.; Chun, L.; Song, J.; Mi, G. Heterosis and genetic analysis of iron concentration in grains and leaves of maize. Plant Breed 2007, 126, 107–109. [Google Scholar] [CrossRef]
- Berardo, N.; Mazzinelli, G.; Valoti, P.; Lagana, P.; Redaelli, R. Characterization of Maize Germplasm for the chemical composition of the grain. J. Agric. Food Chem. 2009, 57, 2378–2384. [Google Scholar] [CrossRef] [PubMed]
- Uribelarrea, M.; Below, F.E.; Moose, S.P. Grain Composition and Productivity of Maize Hybrids Derived from the Illinois Protein Strains in Response to Variable Nitrogen Supply. Crop Sci. 2004, 44, 1593–1600. [Google Scholar] [CrossRef]
- Nuss, E.T.; Tanumihardjo, S.A. Quality protein maize for Africa: Closing the protein inadequacy gap in vulnerable populations. Adv. Nutr. 2011, 2, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Soil Parameter | Zone 1 (Plinthosols) | Zone 2 (Ferralsols) | Zone 3 (Vertisols) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Avg | Max | Min | Avg | Max | Min | Avg | Max | |
| pH (1:25) | 5.42 | 6.22 | 7.32 | 4.93 | 5.65 | 6.35 | 5.46 | 6.13 | 6.65 |
| SOM (%) | 1.28 | 2.18 | 3.07 | 1.79 | 3.54 | 4.86 | 1.54 | 2.2 | 3.07 |
| TotN (%) | 0.07 | 0.13 | 0.18 | 0.09 | 0.21 | 0.28 | 0.09 | 0.13 | 0.18 |
| Pav(mg/kg) | 2.38 | 6.89 | 33.81 | 5.01 | 17.57 | 33.92 | 3.75 | 13.33 | 31.01 |
| Kav(mg/kg) | 0.13 | 0.36 | 0.7 | 0.18 | 0.43 | 0.75 | 0.14 | 0.65 | 1.67 |
| Feav (mg/kg) | 89.35 | 115.45 | 141.12 | 79.05 | 102.69 | 132.54 | 56.38 | 68.3 | 83.29 |
| Fetot(mg/kg) | 3800 | 21,580 | 41,000 | 12,000 | 59,920 | 100,000 | 9500 | 18,920 | 27,000 |
| Zntot (mg/kg) | 10 | 30 | 60 | 20 | 120 | 230 | 10 | 30 | 70 |
| Seasons | Soil Type | Yield (t/ha) | Fe Conc (mg/kg) | Fe_Uptake (g/ha) | Zn Conc (mg/kg) | Zn_Uptake (g/ha) |
|---|---|---|---|---|---|---|
| Season 1 | Plinthosols | 1.05 ± 0.3 a | 22.18 ± 5.1 | 23.29 ± 1.6 a | 21.09 ± 2.6 | 22.14 ± 1.30 a |
| Ferralsols | 0.99 ± 0.5 a | 20.36 ± 4.7 | 20.15 ± 2.3 a | 22.36 ± 2.7 | 22.13 ± 1.3 a | |
| Vertisols | 1.74 ± 0.9 a | 24.29 ± 5.4 | 42.26 ± 2.3 a | 20.71 ± 1.9 | 36.00 ± 1.7 a | |
| Season 2 | Plinthosols | 2.41 ± 0.6 a | 25.2 ± 7.6 | 60.73 ± 4.2 a | 25.2 ± 2.6 a | 60.73 ± 1.3 a |
| Ferralsols | 2.95 ± 0.5 a | 22 ± 5.3 | 64.9 ± 2.3 a | 24.73 ± 2.1 a | 72.95 ± 1.3 a | |
| Vertisols | 3.58 ± 1.3 a | 20.3 ± 2.5 | 72.67 ± 4.9 a | 25.17 ± 1.0 a | 90.11 ± 1.7 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lugoi, L.P.; Martinsen, V.; Almås, Å.R. Cultivating Improved Varieties of Maize Does Not Guarantee Sufficient Dietary Provision of Fe and Zn in a Maize–Bean Intercropping System in Eastern Uganda: Evaluating Effects of Climate and Soil Types. Agronomy 2022, 12, 2383. https://doi.org/10.3390/agronomy12102383
Lugoi LP, Martinsen V, Almås ÅR. Cultivating Improved Varieties of Maize Does Not Guarantee Sufficient Dietary Provision of Fe and Zn in a Maize–Bean Intercropping System in Eastern Uganda: Evaluating Effects of Climate and Soil Types. Agronomy. 2022; 12(10):2383. https://doi.org/10.3390/agronomy12102383
Chicago/Turabian StyleLugoi, Lazarus Pierentino, Vegard Martinsen, and Åsgeir Rossebø Almås. 2022. "Cultivating Improved Varieties of Maize Does Not Guarantee Sufficient Dietary Provision of Fe and Zn in a Maize–Bean Intercropping System in Eastern Uganda: Evaluating Effects of Climate and Soil Types" Agronomy 12, no. 10: 2383. https://doi.org/10.3390/agronomy12102383






