Enhancement of Hyphae Growth and Medium Optimization for Pleurotus eryngii-3 under Submerged Fermentation
Abstract
:1. Introduction
2. Material and Methods
2.1. Microorganism
2.2. Medium Screening for Mycelium of P. eryngii-3
2.3. Medium Optimization by Single Factor and Orthogonal Experiments
2.4. Optimization of Fermentation Conditions
2.5. Optimization of Significant Variables Using Box–Behnken Design (BBD)
2.6. Analysis
2.6.1. Detection of Total Protein
2.6.2. Determination of Polysaccharide Content
2.6.3. Detection of Dry Cell Weight (DCW) for P. eryngii-3
2.6.4. Determination of Growth Rate and Diameter of Mycelium
2.6.5. Analysis of Laccase Activity
2.6.6. qRT-PCR for Laccase Genes
2.7. Statistical Analysis
3. Results
3.1. Screening of Culture Medium
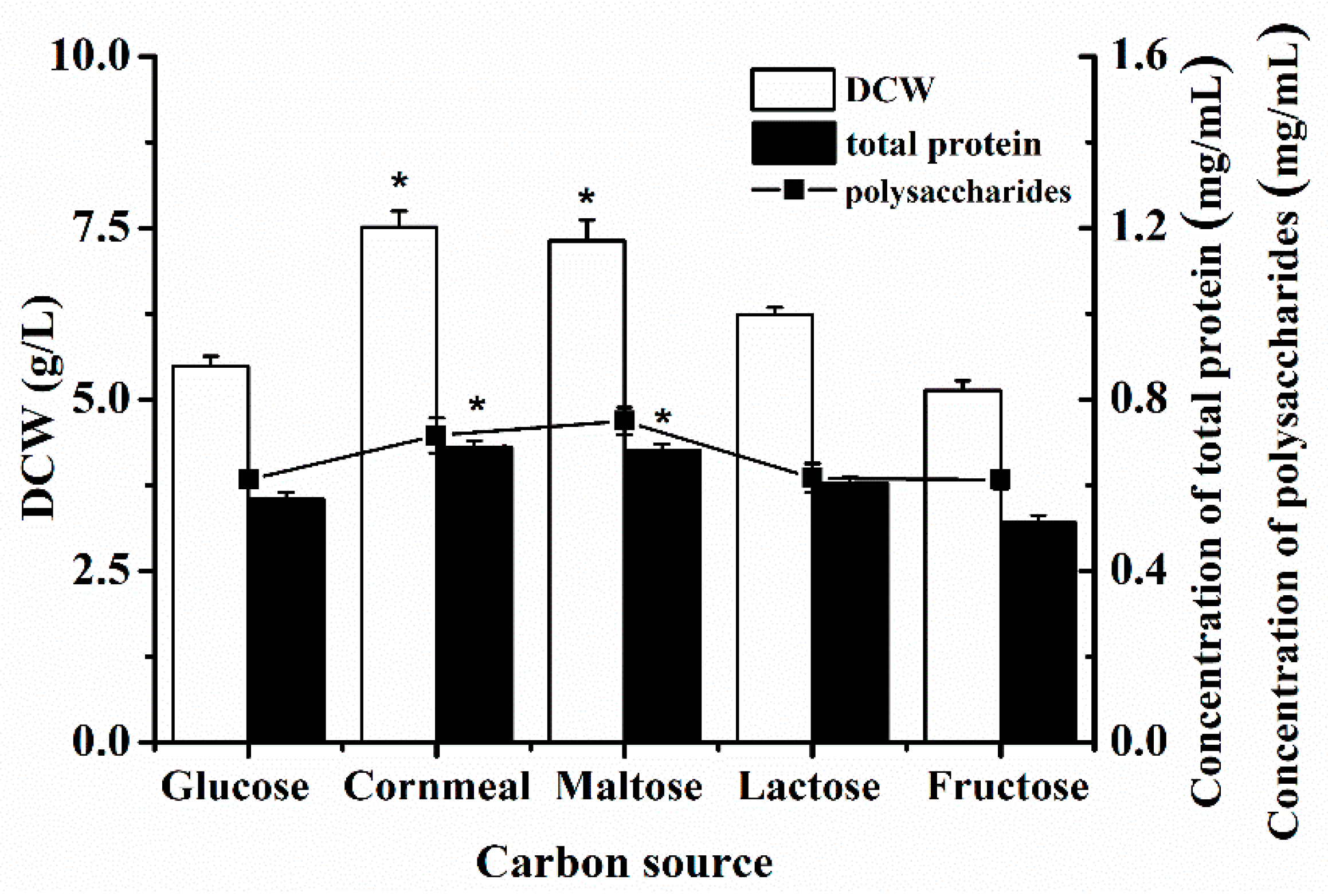
3.2. Optimization of Carbon and Nitrogen Sources
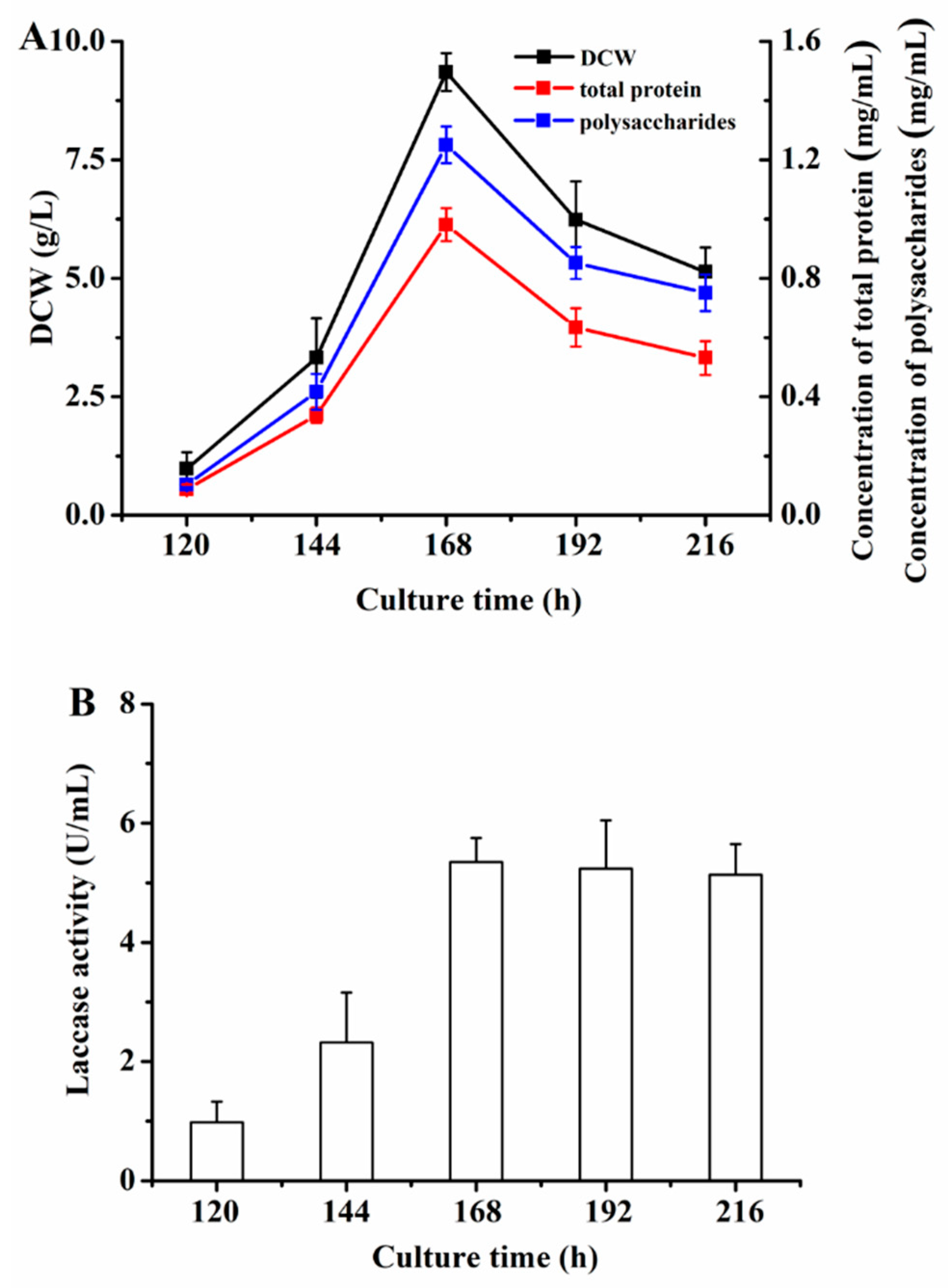
3.3. Determination of Culture Time by P. eryngii-3
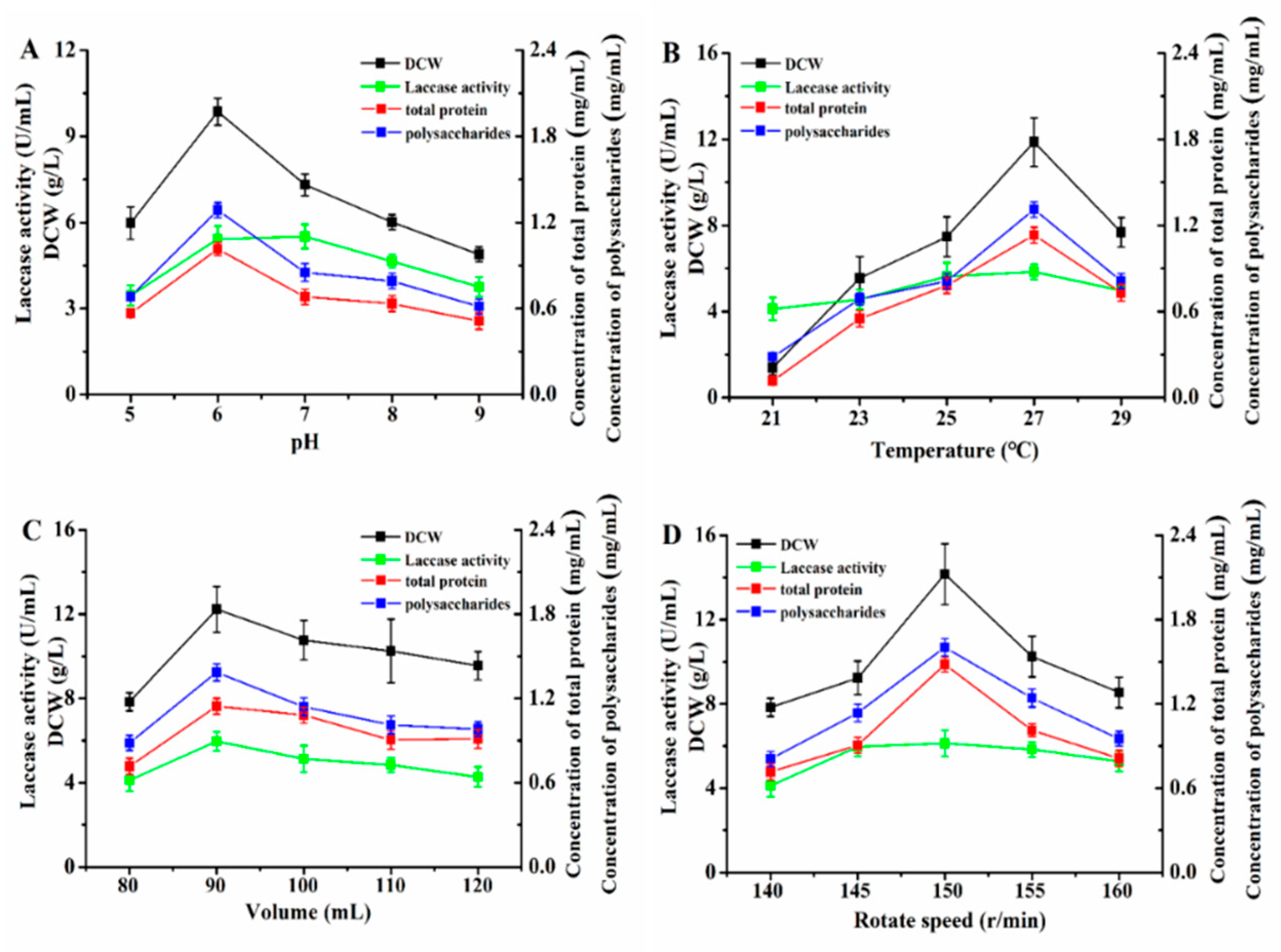
3.4. Optimization of Fermentation Conditions by P. eryngii-3
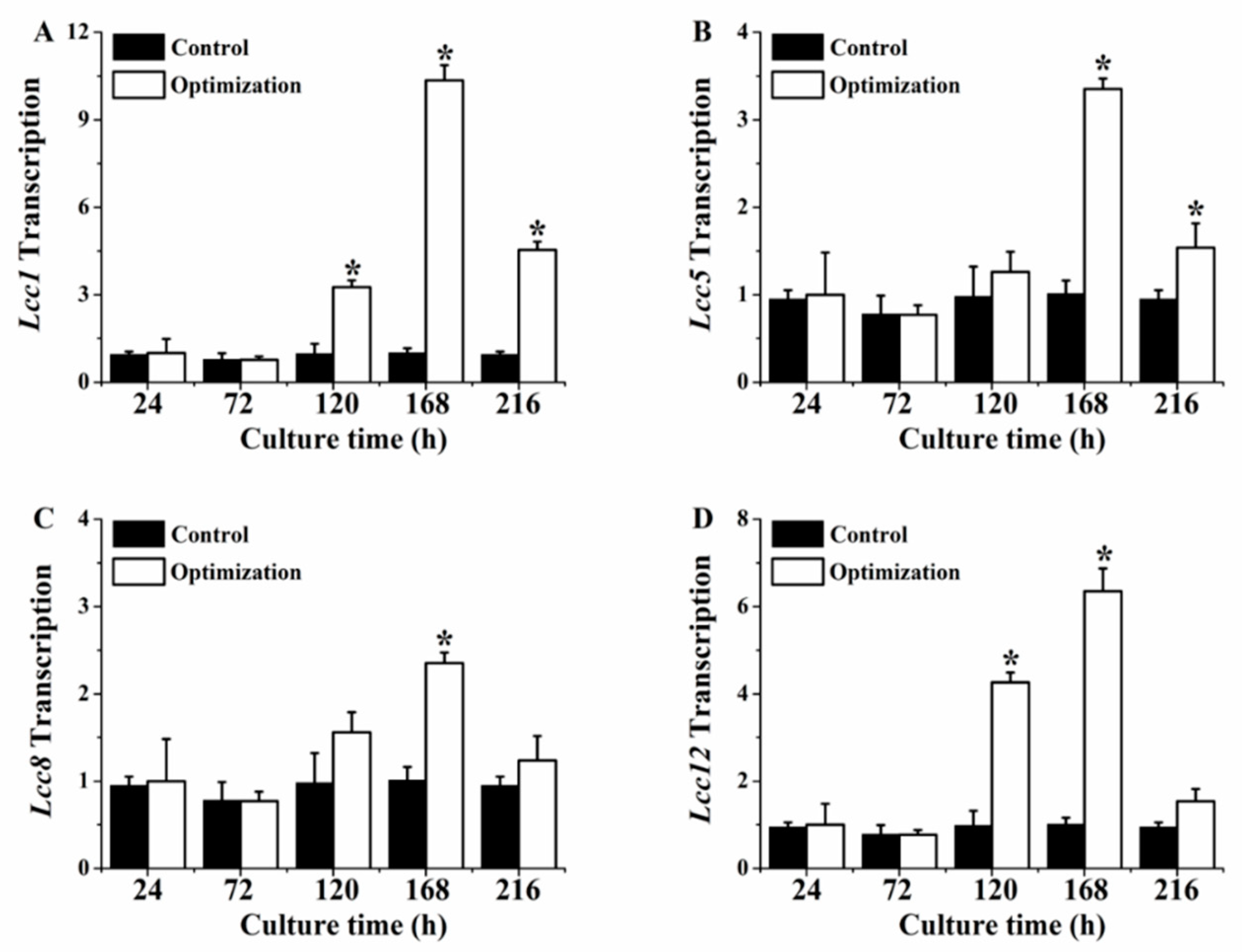
3.5. Transcription Profiling of P. eryngii-3 four Laccase Gene
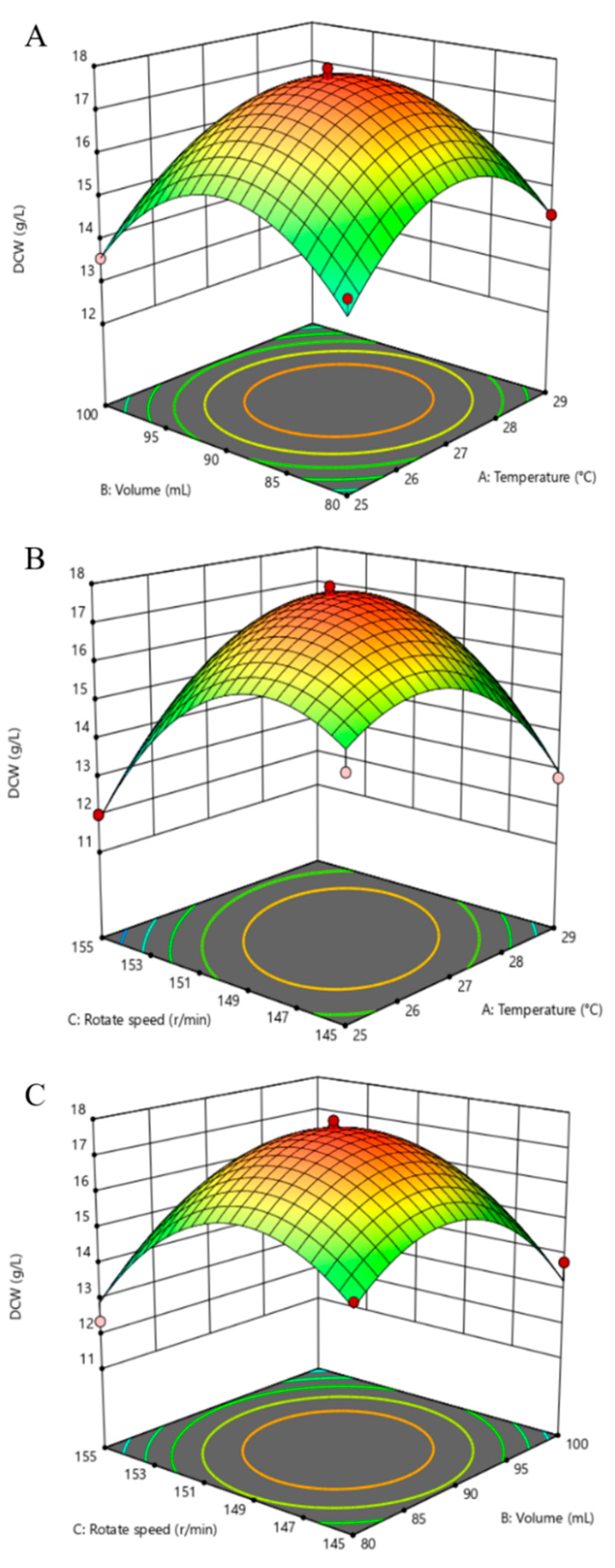
3.6. Optimization of Significant Variables by BBD
4. Discussion
4.1. Screening of Culture Medium by P. eryngii-3
4.2. Optimization of Carbon and Nitrogen Sources
4.3. Optimization of Fermentation Conditions by P. eryngii-3
4.4. Optimization of Fermentation by BBD
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Declarations
References
- Zervakis, G.I.; Ntougias, S.; Gargano, M.L.; Besi, M.I.; Polemis, E.; Typas, M.A.; Venturella, G. A reappraisal of the Pleurotus eryngii complex-New species and taxonomic combinations based on the application of a polyphasic approach, and an identification key to Pleurotus taxa associated with Apiaceae plants. Fungal. Biol. UK 2014, 118, 814–834. [Google Scholar] [CrossRef]
- Zervakis, G.I.; Venturella, G.; Papadopoulou, K. Genetic polymorphism and taxonomic infrastructure of the Pleurotus eryngii speciescomplex as determined by RAPD analysis, isozyme profiles and ecomorphological characters. Microbiology 2001, 147, 3183–3194. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.; Zhang, Z.; Gong, L.; Xun, H.; Li, J.; Qi, B.; Wang, Q.; Li, X.; Li, Y.; Liu, B. Transcriptome changes during major developmental transitions accompanied with little alteration of DNA methylome in two Pleurotus species. Genes 2019, 10, 465. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, R.; Mao, W.J.; Gong, M.; Gao, Y.N.; Tang, L.H.; Yang, R.F.; Li, Y.; Zhou, C.L.; Bao, D.P. A simple and efficient transformation system for the edible mushroom Pleurotus eryngii. Mycoscience 2016, 57, 356–360. [Google Scholar] [CrossRef]
- Deora, A.; Sharma, S.S.; Kumari, P.; Dahima, V.; Kumar, S.; Rohith, M. Cultivation of Kabul Dhingri (Pleurotus eryngii) mushroom by standardizing protocols in subtropical zones of world. Sci. Rep. 2021, 11, 14692–14702. [Google Scholar] [CrossRef]
- Niazi, A.R.; Ghafoor, A. Different ways to exploit mushrooms: A review. All Life 2021, 14, 450–460. [Google Scholar] [CrossRef]
- Hernández-Ortega, A.; Ferreira, P.; Martínez, A.T. Fungal aryl-alcohol oxidase: A peroxide-producing flavoenzyme involved in lignin degradation. Appl. Microbiol. Biotechnol. 2012, 93, 1395–1410. [Google Scholar] [CrossRef]
- Nayan, N.; Sonnenberg, A.S.M.; Hendriks, W.H.; Cone, J.W. Prospects and feasibility of fungal pretreatment of agricultural biomass for ruminant feeding. Anim. Feed Sci. Tech. 2020, 268, 114577. [Google Scholar] [CrossRef]
- Srikanth, M.; Sandeep, T.S.R.S.; Sucharitha, K.; Godi, S. Biodegradation of plastic polymers by fungi: A brief review. Bioresour. Bioprocess 2022, 9, 42. [Google Scholar] [CrossRef]
- Hock, O.G.; Lum, H.W.; Qin, D.D.; Kee, W.K.; Shing, W.L. The growth and laccase activity of edible mushrooms involved in plastics degradation. Appl. Curr. Top. Toxicol. 2019, 15, 57–62. [Google Scholar]
- Kapahi, M.; Sachdeva, S. Mycoremediation potential of Pleurotus species for heavy metals: A review. Bioresour. Bioprocess 2017, 4, 32. [Google Scholar] [CrossRef]
- Joo, J.H.; Hussein, K.A.; Hassan, S.H.A. Biosorptive capacity of Cd(II) and Pb(II) by lyophilized cells of Pleurotus eryngii. Korean J. Soil Sci. Fert. 2011, 44, 615–624. [Google Scholar] [CrossRef] [Green Version]
- Bederska-Łojewska, D.; Swiatkiewicz, S.; Muszynska, B. The use of Basidiomycota mushrooms in poultry nutrition—A review. Anim. Feed Sci. Tech. 2017, 230, 59–69. [Google Scholar] [CrossRef]
- Lee, T.T.; Ciou, J.Y.; Chen, C.N.; Yu, B. The effect of Pleurotus eryngii stalk residue dietary supplementation on layer performance, egg traits and oxidative status. Ann. Anim. Sci. 2015, 15, 447–461. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.H.; Ng, T.B.; Chan, H.H.L.; Liu, Q.; Man, G.C.W.; Zhang, C.Z.; Guan, S.; Ng, C.C.W.; Fang, E.F.; Wang, H.; et al. Mushroom extracts and compounds with suppressive action on breast cancer: Evidence from studies using cultured cancer cells, tumor-bearing animals, and clinical trials. Appl. Microbiol. Biot. 2020, 104, 4675–4703. [Google Scholar] [CrossRef]
- Giardina, P.; Faraco, V.; Pezzella, C.; Piscitelli, A.; Vanhulle, S.; Sannia, G. Laccases: A never-ending story. Cell. Mol. Life Sci. 2010, 67, 369–385. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Zhang, F.; Linhardt, R.J.; Zeng, G.; Zhang, A. Extraction, structure and bioactivities of the polysaccharides from Pleurotus eryngii: A review. Int. J. Biol. Macromol. 2020, 150, 1342–1347. [Google Scholar] [CrossRef]
- Leong, Y.K.; Ma, T.W.; Chang, J.S.; Yang, F.C. Recent advances and future directions on the valorization of spent mushroom substrate (SMS): A review. Bioresour. Technol. 2022, 344, 126157. [Google Scholar] [CrossRef]
- Correa, R.C.G.; Brugnari, T.; Bracht, A.; Peralta, R.M.; Ferreira, I.C.F.R. Biotechnological, nutritional and therapeutic uses of Pleurotus spp. (Oyster mushroom) related with its chemical composition: A review on the past decade findings. Trends Food Sci. Tech. 2016, 50, 103–117. [Google Scholar] [CrossRef] [Green Version]
- Palma, C.; Lloret, L.; Sepúlveda, L.; Contreras, E. Production of versatile peroxidase from Pleurotus eryngii by solid-state fermentation using agricultural residues and evaluation of its catalytic properties. Prep. Biochem. Biotech. 2016, 46, 200–207. [Google Scholar] [CrossRef]
- Stajic, M.; Vukojevic, J.; Duletic-Lausevic, S. Biology of Pleurotus eryngii and role in biotechnological processes: A review. Crit. Rev. Biotechnol. 2009, 29, 55–66. [Google Scholar] [CrossRef]
- Wei, H.; Yue, S.; Zhang, S.; Lu, L. Lipid-lowering effect of the Pleurotus eryngii (King Oyster Mushroom) polysaccharide from solid-state fermentation on both macrophage-derived foam cells and zebrafish models. Polymers 2018, 10, 492. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.Y. A review on the potential reuse of functional polysaccharides extracted from the by-products of mushroom processing. Food Bioprocess Tech. 2020, 13, 217–228. [Google Scholar] [CrossRef]
- Zeng, X.; Suwandi, J.; Fuller, J.; Doronila, A.; Ng, K. Antioxidant capacity and mineral contents of edible wild Australian mushrooms. Food Sci. Technol. Int. 2012, 18, 367–379. [Google Scholar] [CrossRef]
- Wyman, V.; Henríquez, J.; Palma, C.; Carvajal, A. Lignocellulosic waste valorisation strategy through enzyme and biogas production. Bioresource Technol. 2018, 247, 402–411. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, L.; Li, Y.; Wang, F.; Li, S.; Shi, G.; Ding, Z. Comparative transcriptomics and transcriptional regulation analysis of enhanced laccase production induced by co-culture of Pleurotus eryngii var. ferulae with Rhodotorula mucilaginosa. Appl. Microbiol. Biot. 2020, 104, 241–255. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, N.; Li, J.; Xu, R.; Wang, T.; Guo, L.; Ma, M.; Fan, M.; Wei, X. Selenium-enriched Lactobacillus plantarum improves the antioxidant activity and flavor properties of fermented Pleurotus eryngii. Food Chem. 2021, 345, 128770–128778. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Ranamukhaarachchi, S.L. Effect of different culture media, grain sources and alternate substrates on the mycelial growth of Pleurotus eryngii and Pleurotus ostreatus. Pak. J. Biol. Sci. 2020, 23, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Singh, U.; Gautam, A.; Singha, T.K.; Tiwari, A.; Tiwari, P.; Sahai, V.; Sharma, S. Mass production of Pleurotus eryngii mycelia under submerged culture conditions with improved minerals and vitamin D2. LWT-Food Sci. Technol. 2020, 131, 109665. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, R.; Lu, Y.; Yao, L. Improvement of chaetominine production by tryptophan feeding and medium optimization in submerged fermentation of Aspergillus fumigatus CY018. Bioresour. Bioprocess. 2016, 3, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Shenbhagaraman, R.; Jagadish, L.K.; Premalatha, K.; Kaviyarasan, V. Optimization of extracellular glucan production from Pleurotus eryngii and its impact on angiogenesis. Int. J Biol. Macromol. 2012, 50, 957–964. [Google Scholar] [CrossRef]
- Ge, X.; Huang, W.; Xu, X.; Lei, P.; Sun, D.; Xu, H.; Li, S. Production, structure, and bioactivity of polysaccharide isolated from Tremella fuciformis XY. Int. J Biol. Macromol. 2020, 148, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Bellettini, M.B.; Fiorda, F.A.; Maieves, H.A.; Teixeira, G.L.; Avila, S.; Hornung, P.S.; Junior, A.M.; Ribani, R.H. Factors affecting mushroom Pleurotus spp. Saudi J. Biol. Sci. 2019, 26, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Lai, P.; Weng, M.; Wu, L.; Li, Y. Optimization of submerged fermentation conditions for biosynthesis of ergothioneine and enrichment of selenium from Pleurotus eryngii 528. Food Sci. Tech. 2022, 42, e40022. [Google Scholar] [CrossRef]
- Singh, U.; Gautam, A.; Sharma, S. Enhancement of Vitamin D2 content through ultraviolet-B irradiation in submerged cultivated Pleurotus eryngii mycelia using response surface methodology. J. Appl. Biol. Biotechnol. 2021, 9, 121–126. [Google Scholar]
- Bourbonnais, R.; Paice, M.G. Oxidation of non-phenolic substrates: An expanded role for laccase in lignin biodegradation. FEBS 08563 1990, 267, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Cavallazzi, J.R.P.; Oliveira, M.G.D.A.; Kasuya, M.C.M. Laccase production by Lepista sordida. Braz. J. Microbiol. 2004, 35, 261–263. [Google Scholar] [CrossRef]
- Passarini, M.R.; Ottoni, C.A.; Santos, C.; Lima, N.; Sette, L.D. Induction, expression and characterisation of laccase genes from the marine-derived fungal strains Nigrospora sp. CBMAI 1328 and Arthopyrenia sp. CBMAI 1330. AMB Express 2015, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Yong, Y.; Xing, M.; Gu, Y.; Zhang, Z.; Zhang, S.; Lu, L. Characterization of polysaccharides with marked inhibitory effect on lipid accumulation in Pleurotus eryngii. Carbohyd. Polym. 2013, 97, 604–613. [Google Scholar] [CrossRef]
- Tong, A.; Lu, J.; Huang, Z.; Huang, Q.; Zhang, Y.; Farag, M.A.; Liu, B.; Zhao, C. Comparative transcriptomics discloses the regulatory impact of carbon/nitrogen fermentation on the biosynthesis of Monascus kaoliang pigments. Food Chem. X 2022, 13, 100250–100259. [Google Scholar] [CrossRef]
- Stajic, M.; Persky, L.; Friesem, D.; Hadar, Y.; Wasser, S.P.; Nevo, E.; Vukojevic, J. Effect of different carbon and nitrogen sources on laccase and peroxidases production by selected Pleurotus species. Enzym. Microb. Tech. 2006, 38, 65–73. [Google Scholar] [CrossRef]
- Archacka, M.; Celinska, E.; Białas, W. Techno-economic analysis for probiotics preparation production using optimized corn flour medium and spray-drying protective blends. Food Bioprod. Process. 2020, 123, 354–366. [Google Scholar] [CrossRef]
- Li, J.; Zhou, R.L.; Ren, Z.Q.; Fan, Y.W.; Hu, S.B.; Zhuo, C.F.; Deng, Z.Y. Improvement of protein quality and degradation of allergen in soybean meal fermented by Neurospora crassa. LWT Food Sci. Technol. 2019, 101, 220–228. [Google Scholar] [CrossRef]
- Melanouri, E.M.; Dedousi, M.; Diamantopoulou, P. Cultivating Pleurotus ostreatus and Pleurotus eryngii mushroom strains on agro-industrial residues in solid-state fermentation. Part I: Screening for growth, endoglucanase, laccase and biomass production in the colonization phase. Carbon Resour. Convers. 2022, 5, 61–70. [Google Scholar] [CrossRef]
- Kim, D.; Ko, Y.H.; Chung, H.C.; Han, G.D. Straightforward bacterial-fungal fermentation between Lactobacillus plantarum and Pleurotus eryngii for synergistic improvement of bioactivity. Food Sci. Biotechnol. 2015, 24, 607–610. [Google Scholar] [CrossRef]
- Mathur, P.; Sanyal, D.; Dey, P. Optimization of growth conditions for enhancing the production of microbial laccase and its application in treating antibiotic contamination in wastewater. 3 Biotechnology 2021, 11, 81–95. [Google Scholar] [CrossRef]
- Eibes, G.M.; Lu-Chau, T.A.; Ruiz-Duenas, F.J.; Feijoo, G.; Martinez, M.J.; Martinez, A.T.; Lema, J.M. Effect of culture temperature on the heterologous expression of Pleurotus eryngii versatile peroxidase in Aspergillus hosts. Bioproc. Biosyst. Eng. 2009, 32, 129–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.Y.; Lin, Y.P.; Wang, J.C.; Liang, Z.C. Effect of cultivation conditions on the production of mycelial biomass and exopolysaccharide by submerged culture of a rooting shank medicinal mushroom, Oudemansiella radicata (Relhan) singer (Agaricomycetideae). Int. J. Med. Mushrooms 2010, 12, 99–110. [Google Scholar] [CrossRef]
- Petre, M.; Petre, V.; Rusea, I. Microbial composting of fruit tree wastes through controlled submerged fermentation. Ital. J. Agron. 2014, 9, 610–614. [Google Scholar] [CrossRef] [Green Version]
- Vats, A.; Mishra, S. Laccase isoform diversity on basal medium in Cyathus bulleri and role in decolorization/detoxification of textile dyes and effluent. World, J. Microb. Biot. 2020, 36, 164. [Google Scholar] [CrossRef]
- Ahlawat, A.; Mishra, S. Structural, phylogenetic and expression analysis of genes producing lignin degrading enzymes in the basidiomycete Cyathus bulleri and their effect on the release of reducing sugars from agro-residues. Fuel 2022, 314, 122716. [Google Scholar] [CrossRef]
- Sato, T.; Suzuki, Y.; Naito, M.; Minami, A.; Suzuki, N.; Yaegashi, K.; Hirano, T. Overexpression of the laccase gene, lcc1, in Lentinula edodes using the pChG vector. Mycoscience 2019, 60, 246–249. [Google Scholar] [CrossRef]
- Yan, L.; Xu, R.; Bian, Y.; Li, H.; Zhou, Y. Expression Profile of Laccase Gene Family in White-Rot Basidiomycete Lentinula edodes under Different Environmental Stresses. Genes 2019, 10, 1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isham, N.K.M.; Mokhtar, N.; Fazry, S.; Lim, S.J. The development of an alternative fermentation model system for vinegar production. LWT-Food Sci. Technol. 2019, 100, 322–327. [Google Scholar] [CrossRef]
- Atly, B.; Yamac, M.; Yyldyz, Z.; Solener, M. Solid state fermentation optimization of Pleurotus ostreatus for lovastatin production. Pharm. Chem. J. 2019, 53, 858–864. [Google Scholar] [CrossRef]

| Glucose | Corn Flour | Wheat Bran | Yeast Extract | KH2PO4 | MgSO4 | Peptone | Soy Flour | Fe2(SO4)3 | VB1 | Potato | Sucrose | Soybean Meal Powder | Soybean Cake Powder | MnCl2 | pH | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M. 1 | 30 | 25 | 20 | 5 | 3 | 2 | ||||||||||
| M. 2 | 20 | 30 | 1.5 | 1 | 5 | 6.0–6.5 | ||||||||||
| M. 3 | 30 | 1 | 0.5 | 0.5 | 30 | 0.1 | 0.01 | |||||||||
| M. 4 | 30 | 0.85 | 1 | 1.4 | 100 | 27 | ||||||||||
| M. 5 | 20 | 0.5 | 0.5 | 3 | 0.01 | 60 | ||||||||||
| M. 6 | 60 | 0.5 | 0.5 | 5 | 0.01 | 60 | ||||||||||
| M. 7 | 10 | 0.5 | 0.5 | 5 | 0.01 | 60 | ||||||||||
| M. 8 | 1.5 | 1 | 30 | 5 | ||||||||||||
| M. 9 | 20 | 2.5 | 1 | 1 | 2.5 | 1 |
| No. | Corn Flour (g/L) | Soybean Meal (g/L) | DCW (g/L) | Total Protein (mg/mL) | Polysaccharides (mg/mL) |
|---|---|---|---|---|---|
| 1 | 20 | 20 | 7.8357 ± 0.0540 a | 0.7361 ± 0.0191 a | 0.8801 ± 0.0275 b |
| 2 | 20 | 30 | 8.7400 ± 0.0477 a | 0.8108 ± 0.0184 a | 1.0025 ± 0.1059 ab |
| 3 | 20 | 40 | 7.6326 ± 0.0442 a | 0.7045 ± 0.0121 a | 0.8713 ± 0.0304 b |
| 4 | 30 | 20 | 8.0269 ± 1.1181 a | 0.8105 ± 0.0152 a | 0.9457 ± 0.0254 b |
| 5 | 30 | 30 | 7.8382 ± 1.0456 a | 0.7163 ± 0.0178 a | 0.8667 ± 0.0401 b |
| 6 | 30 | 40 | 8.9400 ± 0.0212 a | 0.8094 ± 0.0125 a | 0.9014 ± 0.0325 b |
| 7 | 40 | 20 | 8.2488 ± 0.2156 a | 0.8216 ± 0.197 a | 0.9154 ± 0.0214 b |
| 8 | 40 | 30 | 8.9175 ± 0.0035 a | 0.8135 ± 0.0165 a | 1.0354 ± 0.1476 ab |
| 9 | 40 | 40 | 9.1782 ± 0.1105 a | 0.9201 ± 0.0184 a | 1.2456 ± 0.1327 a |
| Number | Culture Time (h) | pH | Temperature (°C) | Volume (mL) | Rotation Speed (r/min) |
|---|---|---|---|---|---|
| 1 | 120 | 5 | 21 | 80 | 140 |
| 2 | 144 | 6 | 23 | 90 | 145 |
| 3 | 168 | 7 | 25 | 100 | 150 |
| 4 | 192 | 8 | 27 | 110 | 155 |
| 5 | 216 | 9 | 29 | 120 | 160 |
| Number | Temperature (°C) | Volume (mL) | Rotation Speed (r/min) | DCW (g/L) |
|---|---|---|---|---|
| 1 | 25 | 90 | 155 | 12.0023 ± 1.1134 c |
| 2 | 29 | 80 | 150 | 14.3578 ± 2.3073 abc |
| 3 | 25 | 80 | 150 | 14.2535 ± 0.8412 abc |
| 4 | 29 | 100 | 150 | 13.4468 ± 0.9301 abc |
| 5 | 25 | 100 | 150 | 13.5591 ± 1.0642 abc |
| 6 | 27 | 100 | 145 | 13.7835 ± 1.2443 abc |
| 7 | 29 | 90 | 155 | 15.3645 ± 0.6463 abc |
| 8 | 27 | 90 | 150 | 17.5635 ± 1.0414 ab |
| 9 | 25 | 90 | 145 | 14.6941 ± 0.8503 abc |
| 10 | 27 | 90 | 150 | 17.8497 ± 1.4543 a |
| 11 | 27 | 90 | 150 | 17.5103 ± 0.4139 ab |
| 12 | 27 | 80 | 155 | 12.3636 ± 2.3576 c |
| 13 | 27 | 90 | 150 | 17.6689 ± 0.5201 ab |
| 14 | 27 | 100 | 155 | 13.2087 ± 1.6336 bc |
| 15 | 27 | 90 | 150 | 17.6945 ± 0.1203 a |
| 16 | 29 | 90 | 145 | 12.7845 ± 1.7735 c |
| 17 | 27 | 80 | 145 | 14.6925 ± 1.3436 abc |
| Genes | Primer Sequence | Amplicon Length (bp) | |
|---|---|---|---|
| Forward Primer (5’-3’) | Reverse Primer (5’-3’) | ||
| Lcc1 | ATGGTTGAGACCGATTTGCAC | AAGAAGGACGGGAACATTAGGG | 159 |
| Lcc5 | GGCAGGAGGTGCTGACTACAA | ACGACCTTATTGCGGGCTAAC | 195 |
| Lcc8 | TCAAATTCCACTTCCCCTACCA | GGACCAAAATAAGAGAGCCAGG | 199 |
| Lcc12 | GAAGTCCACGCCTATGATGAA | CAGGGTTGGCAATACTAACGA | 103 |
| GAPDH | GTTCAAGTACGATTCCGTCCA | TTCTCAGCGAAGACGGTGAC | 102 |
| Parameters | DCW (g/L) | Total Protein (mg/mL) | Polysaccharides (mg/mL) | Regrowth Rate (mm/d) | Diameter of Mycelium (mm) | |
|---|---|---|---|---|---|---|
| Types | ||||||
| 1 | 3.8250 ± 1.1061 bcd | 0.3283 ± 0.0011 cd | 0.3727 ± 0.0634 bc | 0.6725 ± 0.0106 a | 1.5750 ± 0.3536 ab | |
| 2 | 0.5918 ± 0.0400 f | 0.0119 ± 0.0021 e | 0.0768 ± 0.0098 e | 0.5150 ± 0.0000 b | 0.2450 ± 0.0102 cd | |
| 3 | 5.9863 ± 0.3518 a | 0.4811 ± 0.0642 a | 0.51230 ± 0.0001 a | 0.5075 ± 0.0601 b | 2.0700 ± 0.3137 a | |
| 4 | 2.6950 ± 0.0088 de | 0.2565 ± 0.0267 d | 0.2034 ± 0.0004 d | 0.0750 ± 0.0354 d | 0.9550 ± 0.5861 bcd | |
| 5 | 4.5600 ± 0.1379 bc | 0.4320 ± 0.0332 ab | 0.4536 ± 0.0161 ab | 0.2375 ± 0.0177 c | 0.5400 ± 0.2828 bcd | |
| 6 | 4.8450 ± 0.0124 ab | 0.4674 ± 0.0088 a | 0.4376 ± 0.0016 ab | 0.2250 ± 0.0354 c | 1.0900 ± 0.3267 abc | |
| 7 | 3.3950 ± 0.0141 cd | 0.3697 ± 0.0015 bc | 0.2988 ± 0.0115 cd | 0.2000 ± 0.0000 c | 1.1300 ± 0.2054 abc | |
| 8 | 1.5513 ± 0.0141 ef | 0.0389 ± 0.0147 e | 0.2303 ± 0.0651 d | 0.2000 ± 0.0707 cd | 0.4900 ± 0.1414 cd | |
| 9 | 0.3513 ± 0.0424 f | 0.0151 ± 0.0008 e | 0.0124 ± 0.0018 e | 0.2000 ± 0.0000 c | 0.0350 ± 0.0077 d | |
| Source | Sum of Squares | df | Mean Square | F-Value | P-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 64.92 | 9 | 7.21 | 31.36 | <0.0001 | ** |
| A-Temperature | 0.2628 | 1 | 0.2628 | 1.14 | 0.3205 | |
| B-Volume | 0.3444 | 1 | 0.344 | 1.50 | 0.2606 | |
| C-Rotation speed | 1.13 | 1 | 1.13 | 4.92 | 0.0620 | |
| AB | 0.0121 | 1 | 0.0121 | 0.0526 | 0.8251 | |
| AC | 6.94 | 1 | 6.94 | 30.19 | 0.0009 | ** |
| BC | 0.7744 | 1 | 0.7744 | 3.37 | 0.1091 | |
| A2 | 13.32 | 1 | 13.32 | 57.93 | 0.0001 | ** |
| B2 | 16.44 | 1 | 16.44 | 71.50 | <0.0001 | ** |
| C2 | 19.90 | 1 | 19.90 | 86.51 | <0.0001 | ** |
| Residual | 1.61 | 7 | 0.2300 | |||
| Lack of Fit | 1.53 | 3 | 0.5108 | 26.33 | 0.0043 | ** |
| Pure Error | 0.0776 | 4 | 0.0194 | |||
| Cor. Total | 66.53 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Feng, J.; Wang, S.; Cao, Y.; Shen, Y. Enhancement of Hyphae Growth and Medium Optimization for Pleurotus eryngii-3 under Submerged Fermentation. Agronomy 2022, 12, 2413. https://doi.org/10.3390/agronomy12102413
Liu C, Feng J, Wang S, Cao Y, Shen Y. Enhancement of Hyphae Growth and Medium Optimization for Pleurotus eryngii-3 under Submerged Fermentation. Agronomy. 2022; 12(10):2413. https://doi.org/10.3390/agronomy12102413
Chicago/Turabian StyleLiu, Changqing, Jiajie Feng, Simin Wang, Yi Cao, and Yuxiang Shen. 2022. "Enhancement of Hyphae Growth and Medium Optimization for Pleurotus eryngii-3 under Submerged Fermentation" Agronomy 12, no. 10: 2413. https://doi.org/10.3390/agronomy12102413
APA StyleLiu, C., Feng, J., Wang, S., Cao, Y., & Shen, Y. (2022). Enhancement of Hyphae Growth and Medium Optimization for Pleurotus eryngii-3 under Submerged Fermentation. Agronomy, 12(10), 2413. https://doi.org/10.3390/agronomy12102413