The Effects of the Tree Structure of Zaosu Pear on the Transport and Distribution of Photosynthetic Assimilates and Fruit Quality under Desert-Area Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Determination of Tree Shape Parameters and Chlorophyll Content
2.3. Determination of the Canopy Characteristics of the Five Tree Shapes
2.4. Determination of Photosynthesis Parameters
2.5. Determination of the Fruit Quality Index
2.6. Determination of Fruit Colour
2.7. Statistical Analysis
3. Results
3.1. Different Tree Shapes Have Different Tree Parameters
3.2. Different Tree Shapes Have Different Canopy Structure Indices
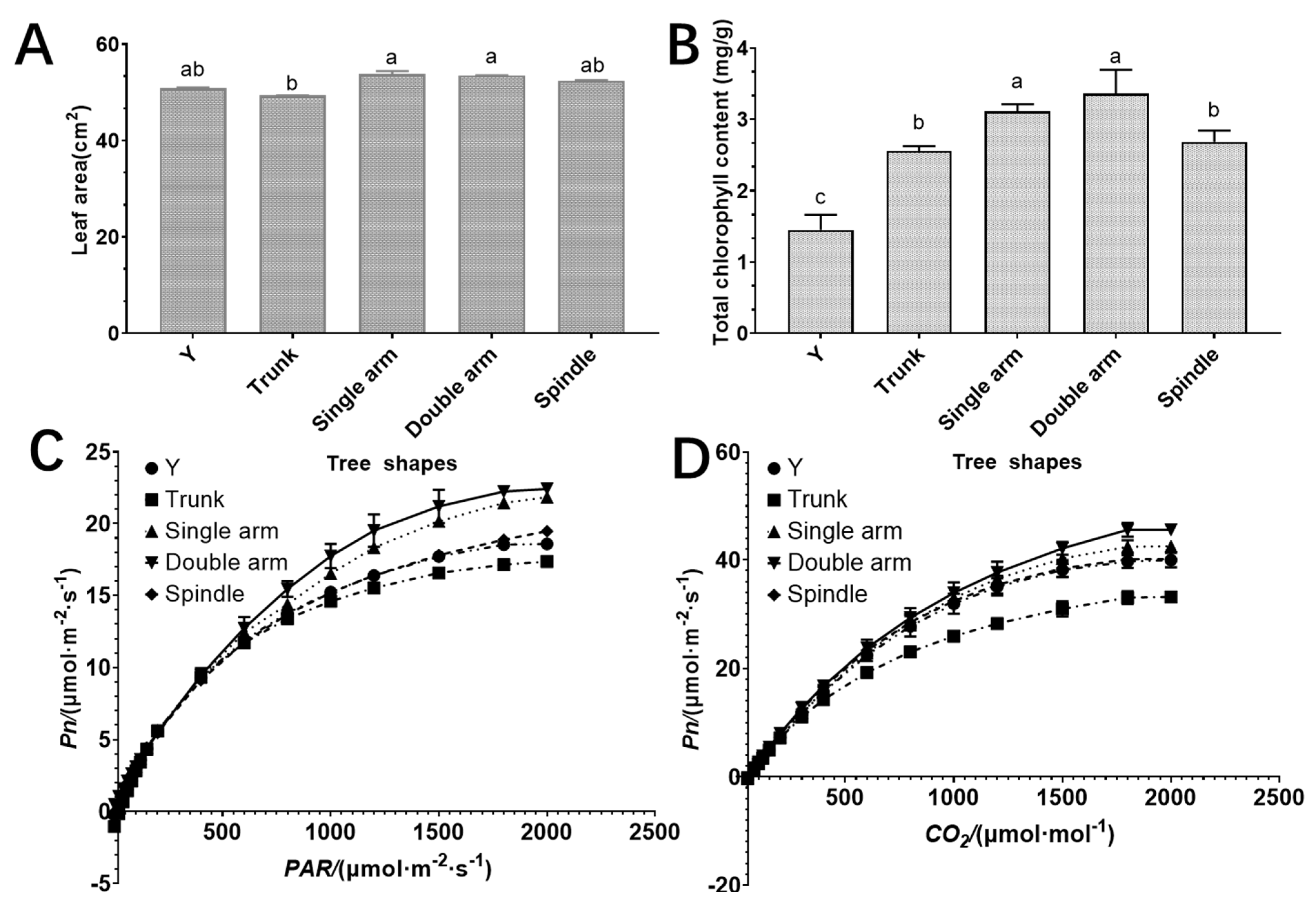
3.3. Effects of Different Tree Shapes on Leaf Area, Chlorophyll Content, Net Photosynthetic Rate, Light Intensity, and CO2
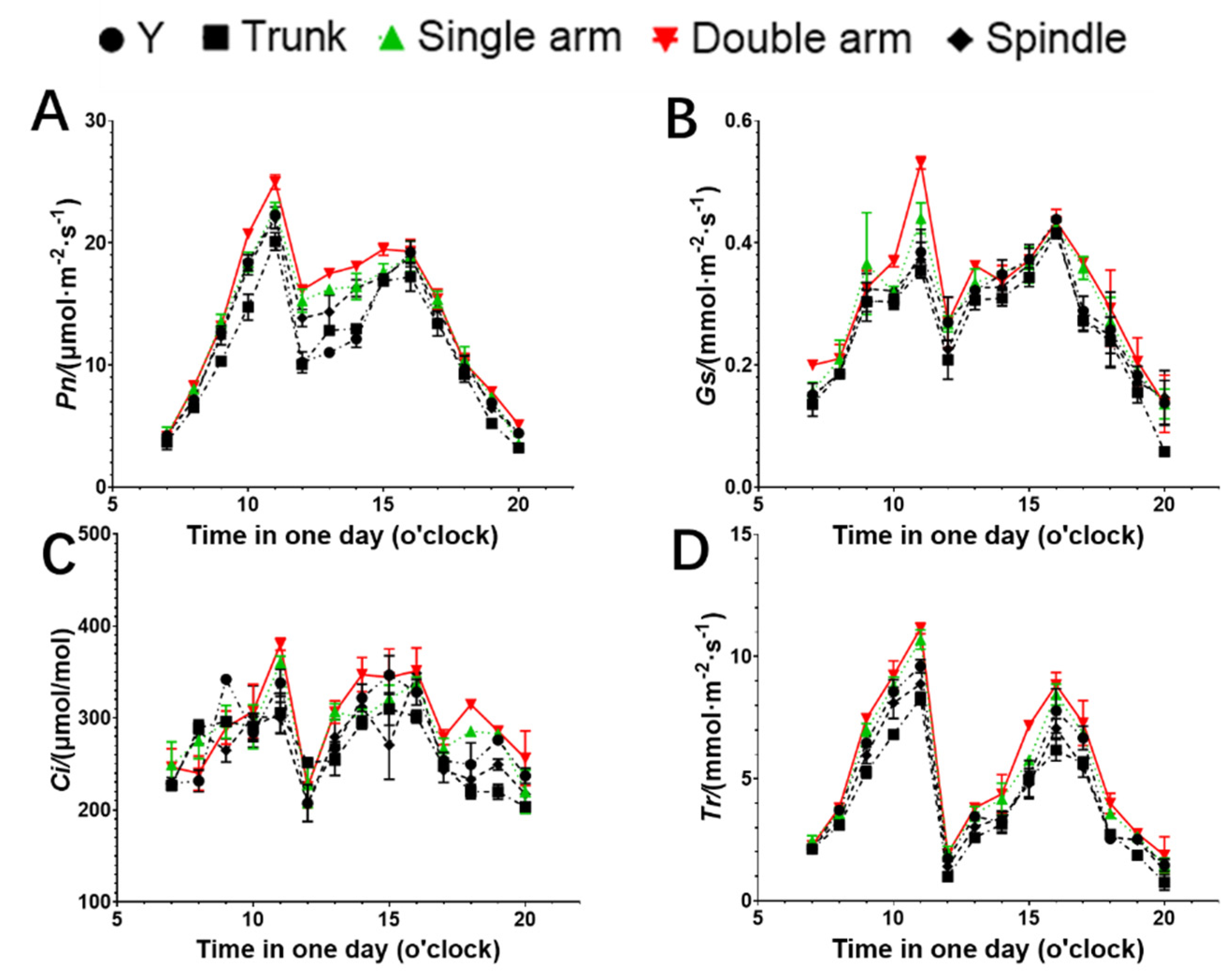
3.4. Effects of Different Tree Shapes on Photosynthesis Parameters
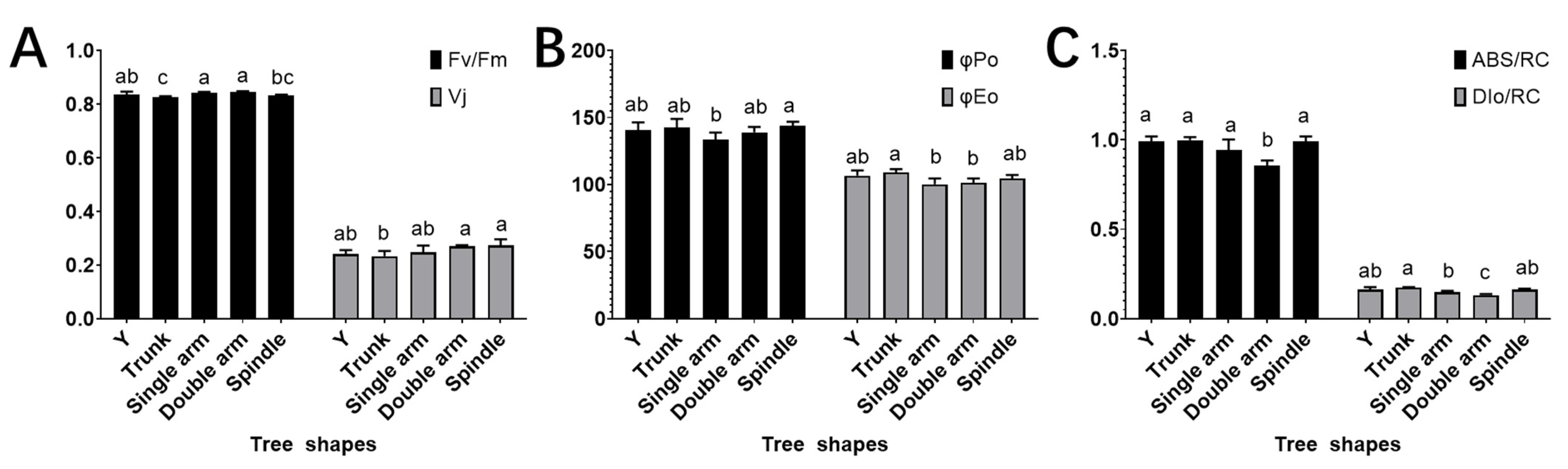
3.5. The Effects of Different Tree Shapes on Chlorophyll Fluorescence Parameters, Quantum Yield, and Activity of the PSII Reaction Centre
3.6. The Effects of Different Tree Shapes on the Picking Time and Number of Fruits
3.7. Effects of Different Tree Shapes on Fruit Firmness, Shape, and Colour
3.8. The Effects of Different Tree Shapes on Fruit Internal Quality
4. Discussion
4.1. Effects of the Tree Shape on Photosynthetic Parameters
4.2. Effects of Tree Shapes on Fruit Quality
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reuscher, S.; Fukao, Y.; Morimoto, R.; Otagaki, S.; Oikawa, A.; Isuzugawa, K.; Shiratake, K. Quantitative proteomics-based reconstruction and identification of metabolic pathways and membrane transport proteins related to sugar accumulation in developing fruits of pear (pyrus communis). Plant Cell Physiol. 2016, 57, 505–518. [Google Scholar] [CrossRef]
- Liu, J.L.; Zhang, C.X.; Li, T.T.; Liang, C.L.; Yang, Y.J.; Li, D.; Cui, Z.H.; Wang, R.; Song, J.K. Phenotype and mechanism analysis of plant dwarfing in pear regulated by abscisic acid. J. Integr. Agric. 2022, 21, 1346–1356. [Google Scholar] [CrossRef]
- Lordan, J.; Francescatto, P.; Dominguez, L.I.; Robinson, T.L. Long-term effects of tree density and tree shape on apple orchard performance, a 20 year study—part 1, agronomic analysis. Sci. Hortic. 2018, 238, 303–317. [Google Scholar] [CrossRef]
- Palmer, J. Annual dry matter production and partitioning over the first 5 years of a bed system of crispin/m. 27 apple trees at four spacings. J. Appl. Ecol. 1988, 25, 569–578. [Google Scholar] [CrossRef]
- Palmer, J.W. Changing concepts of efficiency in orchard systems. Acta Hortic. 2011, 903, 41–49. [Google Scholar] [CrossRef]
- Calatayud, A.; Roca, D.; Gorbe, E.; Martínez, P.F. Light acclimation in rose (rosa hybrida cv. Grand gala) leaves after pruning: Effects on chlorophyll a fluorescence, nitrate reductase, ammonium and carbohydrate. Sci. Hortic. 2007, 111, 152–159. [Google Scholar] [CrossRef]
- Li, K.; Lakso, A.; Piccioni, R.; Robinson, T.J. Summer pruning effects on fruit size, fruit quality, return bloom and fine root survival in apple trees. Hortic. Sci. Biotechnol. 2003, 78, 7. [Google Scholar] [CrossRef]
- Li, K.; Lasko, A. Photosynthesis characteristics of apple spur leaves after summer pruning to improve exposure to light. Hortscience 2004, 39, 969–972. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.; Sharma, R. Pruning alters fruit quality of mango cultivars (mangifera indica L.) under high density planting. J. Trop. Agric. 2010, 48, 55–57. [Google Scholar]
- Singh, S.; Singh, S.; Sharma, R.; Patel, V. Influence of pruning intensity on flowering, fruit yields and floral malformation in three mango cultivars planted under high density. Indian J. Hortic. 2010, 67, 84–89. [Google Scholar]
- Robinson, T.; Lakso, A.; Ren, Z. Modifying apple tree canopies for improved production efficiency. HortScience 1991, 26, 1005–1012. [Google Scholar] [CrossRef]
- Cheng, X.; Liao, K.; Li, N.; Zhao, S.; Sun, H.; Mansur, N.; Liu, J. Analysis on the composition and structure of branches of two kinds of tree shapes in korla fragrant pear. Agric. Sci. Tech. 2013, 14, 954–958. [Google Scholar]
- Lakso, A.N.; Robinson, T.L. Sunlight, yield, and productivity of apples. N. Y. Fruit Q. 2014, 22, 5–7. [Google Scholar]
- Lakso, A.; Corelli Grappadelli, L. Implications of pruning and training practices to carbon partitioning and fruit development in apple. Acta Hortic. 1991, 322, 231–240. [Google Scholar] [CrossRef]
- Oliveira, I.V.M.O.; Lopes, P.R.C.; Silva-Matos, R.R.S. Phenology evaluation of ‘triumph’ pear trees grown in semi-arid climate in the northeast brazil in 2012 season. Rev. Bras. De Frutic. 2015, 37, 261–266. [Google Scholar] [CrossRef][Green Version]
- Zhao, M.X.; Zhang, J.H.; Sun, W.T.; Cao, G.; Wang, W.; Cao, S.F.; Li, H.X. Effects of different tree canopy structures on the yield and quality of ‘zaosu’ pear. J. Fruit Trees 2016, 33, 1076–1083. [Google Scholar]
- Jiang, X.Y.; Li, J.C.; Wang, J.Z.; Sha, S.F.; Cai, Z.M.; Li, H.J. Effects of different tree shapes on photosynthetic characteristics, tree growth and fruit quality of early crisp pear. Jiangsu Agric. Sci. 2022, 50, 163–166. [Google Scholar]
- Kappel, K.; Brownlee, R. Early performance of ‘conference’ pear on four training systems. Hortscience 2001, 36, 69–71. [Google Scholar] [CrossRef]
- Sobierajski, G.; Silva, T.; Hernandes, J.; Pedro Júnior, M. Y-Shaped and Fruiting wall Peach Orchard Training System in Subtropical Brazil. Available online: Http://www.Scielo.Br/scielo.Php?Pid=s0006-87052019005007102&script=sci_arttext (accessed on 12 November 2018).
- Liu, J.R. Studies on The Pollination Varieties Pear of Yunnan Red Skinned Pyrus. Master’s Thesis, Northwest Sci-Tech University of Agriculture and Forestry, Xianyang, China, 2003. [Google Scholar]
- Torres, E.; Recasens, I.; Lordan, J.; Alegre, S. Combination of strategies to supply calcium and reduce bitter pit in ‘golden delicious’ apples. Sci. Hortic. 2017, 217, 179–188. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Zhang, S.L.; Qiao, Y.J.; Tao, S.T.; Miao, Y.C.; Cao, H.L. Effect of bagging with different types of bags on fruit quality of dang shan su pear cultivar J. Fruit Sci. 2006, 23, 510–514. [Google Scholar]
- Voss, D.H. Relating colorimeter measurement of plantcolour to the royal horticultural society colour chart. Hortic. Sci. 1992, 27, 1256–1260. [Google Scholar]
- Wang, L.X.; Zhang, X.J.; Liu, Y.L.; Shi, X.Y.; Wang, Y.J.; Zhang, C.F.; Zhao, Z.Y. The effect of fruit bagging on the color, phenolic compounds and expression of the anthocyanin biosynthetic and regulatory genes on the ‘granny smith’ apples. Eur. Food Res. Technol. 2013, 237, 875–885. [Google Scholar] [CrossRef]
- Lauri, P.E.; Costes, E.; Regnard, J.L.; Brun, L.; Simon, S.; Monney, P.; Sinoquet, H. Does knowledge on fruit tree architecture and its implications for orchard management improve horticultural sustainability? An overview of recent advances in the apple. Acta Hortic. 2009, 817, 243–250. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, Y.Q.; Su, S.C.; Yang, S.Y.; Ma, L.; Zhang, L.Y.; Wang, X.N. Effects of tree shape on the microclimate and fruit quality parameters of camellia oleifera abel. Forests 2019, 10, 563. [Google Scholar] [CrossRef]
- Buler, Z.; Mika, A. The influence of canopy architecture on light interception and distribution in ‘sampion’ apple trees. J. Fruit Ornam. Plant Res. 2009, 27, 45–52. [Google Scholar]
- Chen, X.L. An Experiemtnal Study of Light Interception and Photosynthesis Product for 3D Virtual Tall-Shape Apple Canopy in Loess Plateau Region of China. Master’s Thesis, Northwest A&F University, Xianyang, China, 2013. [Google Scholar]
- Jiang, Z. The Research on Classification Transformation Technology of Low Yield Stands of Camellia Oleifera. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 2012. [Google Scholar]
- Hao, J. The Effect of Topiary Work on the Growth Characteristics and Photosynthetic Physiology of Camellia Sapling. Master’s Thesis, Jiangxi Agricultural University, Nanchang, China, 2011. [Google Scholar]
- Gao, Z.; Li, Z. Light Use Efficiency Distribution as a Function of Different Tree Shapes in Apple. J. Anim. Plant. Sci. 2015, 25, 247–253. [Google Scholar]
- Yang, T.F. Effect of Different Shoot Amount after Pruning on Growth and Fruiting on fuji Apple Trees at Full Bearing Stage in Shaanxi Weibei Loess Plateau. Master’s Thesis, Northwest A&F University, Xianyang, China, 2014. [Google Scholar]
- Franck, N.; Vaast, P.; Génard, M.; Dauzat, J. Soluble sugars mediate sink feedback down–regulation of leaf photosynthesis of coffea arabica in the field. Tree Physiol. 2006, 26, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.A.; Wagenmakers, P.S.; de Jager, A. Effects of light on flavonoid and chlorogenic acid levels in the skin of ‘jonagold’ apples. Sci. Hortic. 2001, 88, 289–298. [Google Scholar] [CrossRef]
- Sinoquet, H.; Sonohat, G.; Potel, A.-M.; Monney, P.; Lauri, P.-E. Using virtual plants to estimate light distribution at intra-canopy scale in apple trees: Method presentation and assessment. Acta Hortic. 2008, 803, 225–234. [Google Scholar] [CrossRef]
- Zhang, J.J.; Serra, S.; Leisso, R.S.; Musacchi, S. Effect of light microclimate on the quality of ‘d’ ‘anjou’ pears in mature open-centre tree architecture. Biosyst. Eng. 2016, 141, 1–11. [Google Scholar] [CrossRef]
- Liguori, G.; Farina, V.; Gullo, G.; Inglese, P. Tree and orchard variability of silver king nectarine (prunus persica (L.) batsch) fruit quality components. Hort. Sci. 2013, 40, 72–77. [Google Scholar] [CrossRef]
- Sun, W.T.; Niu, J.Q.; Dong, T.; Liu, X.L.; Yin, X.N.; Ma, M. Effect of thinning and reshaping on the canopy structure and leaf quality at late growth stage in dense apple orchard in loess plateau of eastern gansu, china. Chin. J. Appl. Ecol. 2018, 29, 3008–3016. [Google Scholar]
- Sansavini, S.; Corelli, L. Canopy efficiency of apple as affected by microclimatic factors and tree structure. Acta Hortic. 1991, 322, 69–77. [Google Scholar] [CrossRef]



| Tree Shapes | Y-Shaped | Trunk | Single-Arm | Double-Arm | Spindle |
|---|---|---|---|---|---|
| Height (cm) | - | 480 | - | - | 467.7 |
| Crown width (cm) | 3.6 bB ± 0.10 | 1.7 dD ± 0.23 | 2.8 cC ± 0.15 | 5.5 aA ± 0.17 | 3.3 bB ± 0.17 |
| Number of branches | 19.0 bB ± 0.99 | 20.7 bB ± 1.22 | 14.0 ± cC1.87 | 27.0 aA ± 1.80 | 18.3 bB ± 1.75 |
| Length of main branch (cm) | 141.5 cB ± 4.05 | 128.8 dB ± 7.30 | 163.6 bA ± 7.75 | 177.1 aA ± 1.95 | 179.3 aA ± 7.75 |
| Thickness (mm) | 25.4 cB ± 0.80 | 22.9 dB ± 1.05 | 32.7 aA ± 0.95 | 29.8 bA ± 1.35 | 30.8 abA ± 2.00 |
| Length of new shoots (cm) | 52.5 bB ± 0.85 | 45.6 cC ± 2.25 | 53.5 bB ± 2.35 | 52.2 bB ± 1.35 | 63.3 aA ± 1.10 |
| Branches per ha (Ten thousand × 15) | 4.4 aA ± 0.15 | 4.8 aA ± 0.20 | 3.7 bB ± 0.35 | 3.4 bB ± 0.25 | 4.4 bB ± 0.20 |
| Number of long fruit branches | 27.5 cC ± 1.05 | 25.3 cC ± 1.45 | 25.4 cC ± 1.45 | 56.7 aA ± 1.85 | 42.0 bB ± 1.05 |
| Number of middle fruit branches | 25.7 bB ± 1.10 | 16.7 dD ± 0.75 | 13.6 eE ± 1.05 | 35.3 aA ± 1.06 | 22.0 cC ± 0.93 |
| Number of short fruit branches | 393.5 bB ± 2.75 | 186.0 dD ± 8.75 | 345.6 bB ± 5.20 | 597.3 cC ± 8.80 | 397.0 bB ± 8.75 |
| Number of axillary flower buds | 42.0 dD ± 7.65 | 68.3 cC ± 7.35 | 77.6 cC ± 4.95 | 136.0 bB ± 5.35 | 165.0 aA ± 3.01 |
| Tree Shapes | Leaf Area Index(LAI) | Mean Leaf Inclination Angle (MFIA) | Direct Transmission Coefficient (TC) | Photosynthetically Active Radiation (PAR) |
|---|---|---|---|---|
| Y-Shaped | 4.07 aA ± 0.105 | 13.02 dCD ± 0.560 | 0.203 bB ± 0.005 | 44.29 cC ± 2.070 |
| Trunk | 4.19 aA ± 0.135 | 11.07 dD ± 0.395 | 0.165 cC ± 0.008 | 38.99 dC ± 2.545 |
| Single-arm | 3.68 bB ± 0.110 | 22.65 aA ± 2.005 | 0.255 aA ± 0.025 | 60.36 bB ± 2.055 |
| Double-arm | 3.55 bB ± 0.155 | 18.22 bB ± 1.175 | 0.262 aA ± 0.012 | 68.67 aA ± 2.450 |
| Spindle | 3.74 bB ± 0.110 | 15.75 cBC ± 1.495 | 0.240 aA ± 0.011 | 57.59 bB ± 2.030 |
| Tree Shapes | LCP /(μmol·m−2·s−1) | LSP /(μmol·m−2·s−1) | Pnmax /(μmol·m−2·s−1) | AQY | Rd /(μmol·m−2·s−1) |
|---|---|---|---|---|---|
| Y-Shaped | 35.92 bB ± 2.040 | 1669.37 dC ± 20.33 | 20.30 cC ± 0.225 | 0.0292 abA ± 0.003 | 1.0250 bcA ± 0.015 |
| Trunk | 31.19 cC ± 1.205 | 1528.24 eD ± 28.07 | 19.89 cC ± 0.830 | 0.0333 aA ± 0.003 | 1.0398 abcA ± 0.008 |
| Single-arm | 36.69 bB ± 2.230 | 1825.70 bB ± 24.31 | 23.69 bB ± 0.905 | 0.0285 abA ± 0.005 | 1.0460 abA ± 0.013 |
| Double-arm | 42.12 aA ± 1.815 | 1942.37 aA ± 41.22 | 26.55 aA ± 1.190 | 0.0249 bA ± 0.004 | 1.0490 aA ± 0.013 |
| Spindle | 36.71 bB ± 2.065 | 1711.58 cC ± 19.80 | 20.62 cC ± 1.380 | 0.0277 abA ± 0.003 | 1.0170 cA ± 0.009 |
| Tree Shapes | CCP /(μmol·mol−1) | CSP /(μmol·mol−1) | Pnmax /(μmol·mol−1) | CE /(μmol·mol−1) |
|---|---|---|---|---|
| Y-Shaped | 56.55 abAB ± 1.995 | 1790.89 cB ± 32.53 | 40.01 cBC ± 0.915 | 0.051 a ± 0.003 |
| Trunk | 55.68 cbAB ± 1.015 | 1690.89 dC ± 25.52 | 37.33 dC ± 1.125 | 0.05 a ± 0.002 |
| Single-arm | 53.26 bcB ± 2.135 | 1910.89 bA ± 35.49 | 43.35 bB ± 1.055 | 0.05 a ± 0.004 |
| Double-arm | 52.66 Bc ± 1.520 | 1969.10 aA ± 34.33 | 54.99 aA ± 1.465 | 0.055 a ± 0.003 |
| Spindle | 59.70 aA ± 1.785 | 1892.60 bA ± 29.43 | 40.89 cB ± 1.675 | 0.056 a ± 0.004 |
| Tree Shapes | Amount of Fruit | Picking Time | Number | Single Fruit Weight (g) | Picking Percentage (%) |
|---|---|---|---|---|---|
| Y-Shaped | 263.33 | 9 August | 6.0 | 280.00 | 2.28 |
| 18 August | 39.6 | 258.38 | 15.06 | ||
| 28 August | 208.0 | 208.56 | 78.99 | ||
| Trunk | 128.67 | 9 August | 13.0 | 296.15 | 10.10 |
| 18 August | 12.3 | 247.36 | 9.58 | ||
| 28 August | 85.7 | 152.68 | 66.58 | ||
| Single-arm | 138.33 | 9 August | 40.7 | 321.61 | 29.40 |
| 18 August | 49.0 | 287.76 | 35.42 | ||
| 28 August | 38.3 | 251.24 | 27.71 | ||
| Double-arm | 364.00 | 9 August | 50.7 | 299.39 | 13.92 |
| 18 August | 97.3 | 295.39 | 26.74 | ||
| 28 August | 199.0 | 261.21 | 54.67 | ||
| Spindle | 163.00 | 9 August | 21.0 | 281.98 | 12.90 |
| 18 August | 17.7 | 257.50 | 10.84 | ||
| 28 August | 110.3 | 209.19 | 67.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Sun, W.; Li, H.; Wang, W.; Cao, G.; Wang, F. The Effects of the Tree Structure of Zaosu Pear on the Transport and Distribution of Photosynthetic Assimilates and Fruit Quality under Desert-Area Conditions. Agronomy 2022, 12, 2440. https://doi.org/10.3390/agronomy12102440
Zhao M, Sun W, Li H, Wang W, Cao G, Wang F. The Effects of the Tree Structure of Zaosu Pear on the Transport and Distribution of Photosynthetic Assimilates and Fruit Quality under Desert-Area Conditions. Agronomy. 2022; 12(10):2440. https://doi.org/10.3390/agronomy12102440
Chicago/Turabian StyleZhao, Mingxin, Wentai Sun, Hongxu Li, Wei Wang, Gang Cao, and Falin Wang. 2022. "The Effects of the Tree Structure of Zaosu Pear on the Transport and Distribution of Photosynthetic Assimilates and Fruit Quality under Desert-Area Conditions" Agronomy 12, no. 10: 2440. https://doi.org/10.3390/agronomy12102440
APA StyleZhao, M., Sun, W., Li, H., Wang, W., Cao, G., & Wang, F. (2022). The Effects of the Tree Structure of Zaosu Pear on the Transport and Distribution of Photosynthetic Assimilates and Fruit Quality under Desert-Area Conditions. Agronomy, 12(10), 2440. https://doi.org/10.3390/agronomy12102440




