Revealing the Genetic Architecture of Yield-Related and Quality Traits in Indian Mustard [Brassica juncea (L.) Czern. and Coss.] Using Meta-QTL Analysis
Abstract
1. Introduction
2. Materials and Methods
QTL Data Collection for Meta-QTL Analysis
3. Construction of the Consensus Map
3.1. QTL Projection
3.2. Prediction of MQTLs Associated with Yield-Related and Quality Traits and Their Validation with Genome-Wide Association Studies
3.3. Detection of Candidate Genes and Their Expression Analysis
4. Results
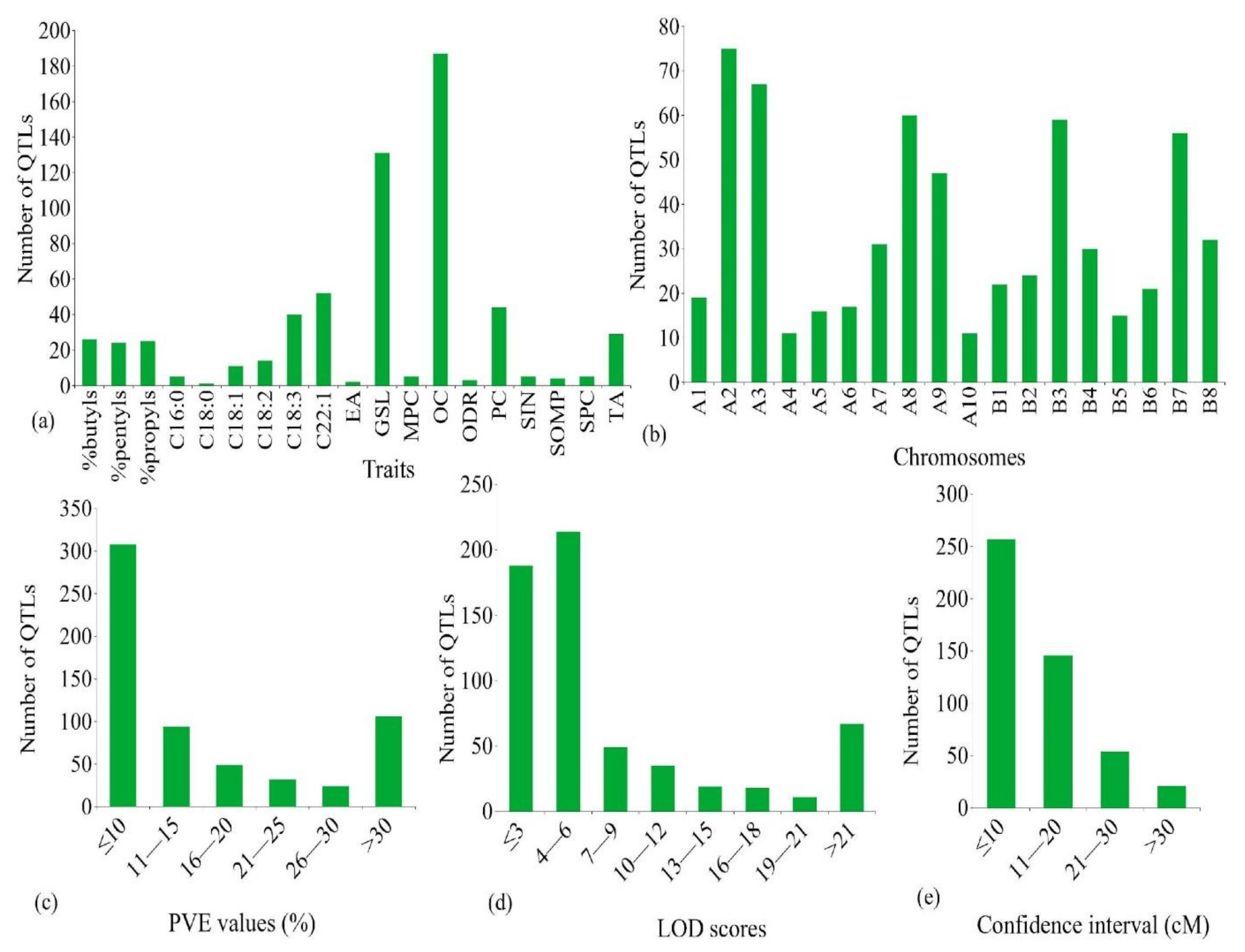
4.1. Distribution of QTLs on the B. juncea Genome and Their Salient Features
4.2. Features of the Consensus Map
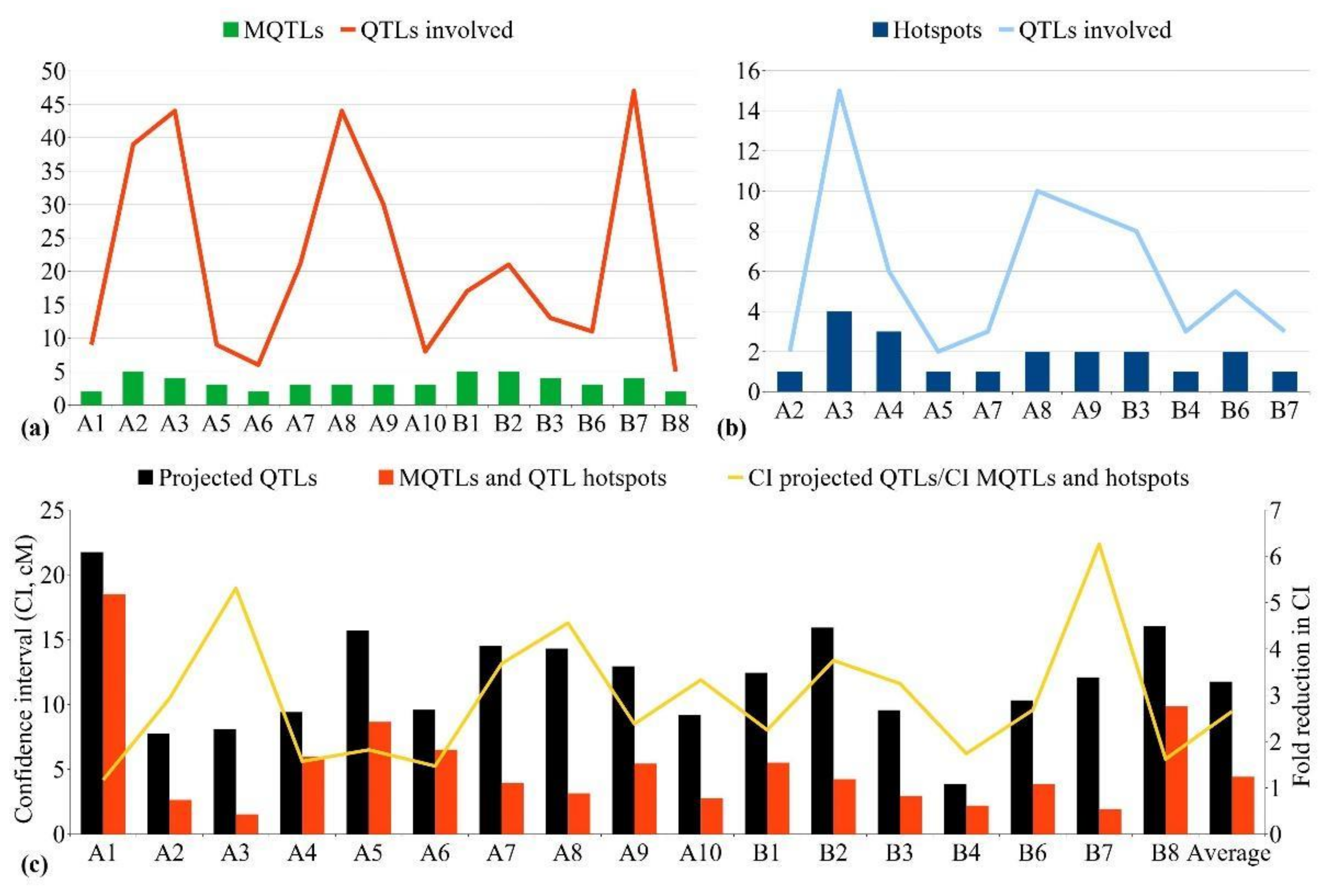
4.3. MQTLs for Yield-Related Traits and Their Distribution in the Genome
4.4. MQTLs for Quality Traits and Their Distribution in the Genome
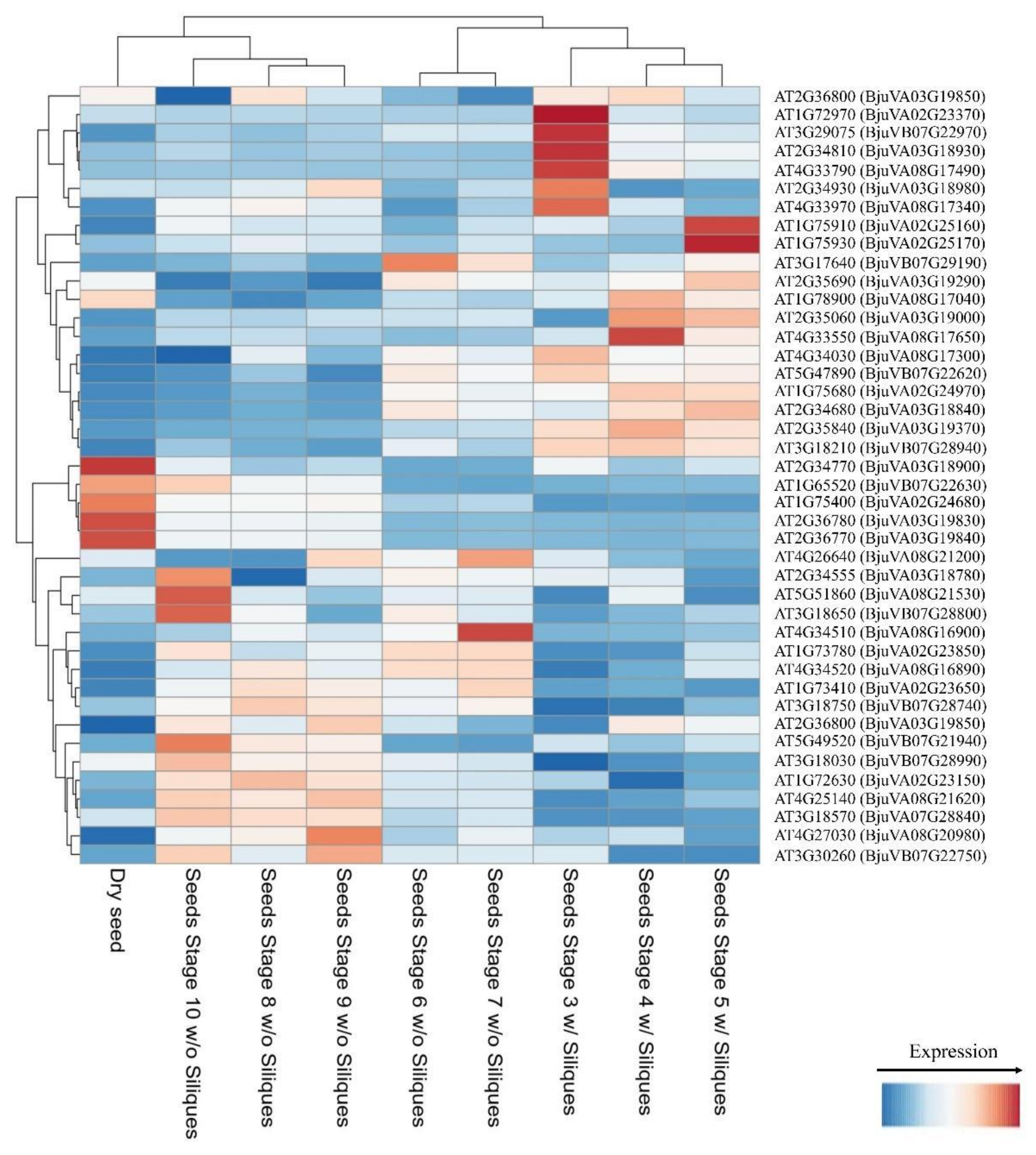
4.5. Validation of MQTLs with GWAS-Based Significant SNPs and Identification of Candidate Genes and Their Expressions at Different Developmental Stages
5. Discussion
5.1. MQTLs for Yield-Related and Quality Traits
5.2. Co-Localization of MQTLs: Inferring a Genetic Relationship between Yield-Related and Quality Traits
5.3. Candidate Genes for Yield-Related and Quality Traits
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagaharu, U. Genome analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Jpn. J. Bot. 1935, 7, 389–452. [Google Scholar]
- Mahmood, T.; Rahman, M.H.; Stringam, G.R.; Yeh, F.; Good, A. Molecular markers for yield components in Brassica juncea—Do these assist in breeding for high seed yield. Euphytica 2005, 144, 157–167. [Google Scholar] [CrossRef]
- Vangheesdaele, G.; Fournier, N. Chemical composition of Brassica juncea seeds used to prepare the mustard of Dijon. Rev. Fr. Corps Gras. 1980, 27, 15–22. [Google Scholar]
- Chauhan, J.S.; Singh, K.H.; Singh, V.V.; Kumar, S. Hundred years of rapeseed-mustard breeding in India: Accomplishments and future strategies. Indian J. Agric. Sci. 2011, 81, 1093–1109. [Google Scholar]
- McVetty, P.B.E.; Scarth, R. Breeding for improved oil quality in Brassica oilseed species. J. Crop Prod. 2002, 5, 345–369. [Google Scholar] [CrossRef]
- Tatum, V.; Chow, C.K. Effects of processing and storage on fatty acids in edible oils. In Fatty Acids in Foods and Their Health Implications; CRC: Boca Raton, FL, USA, 1992; pp. 411–425. [Google Scholar]
- Mahmood, T.; Rahman, M.H.; Stringam, G.R.; Yeh, F.; Good, A.G. Identification of quantitative trait loci (QTL) for oil and protein contents and their relationships with other seed quality traits in Brassica juncea. Theor. Appl. Genet. 2006, 113, 1211–1220. [Google Scholar] [CrossRef]
- Ramchiary, N.; Bisht, N.C.; Gupta, V.; Mukhopadhyay, A.; Arumugam, N.; Sodhi, Y.S.; Pental, D.; Pradhan, A.K. QTL analysis reveals context-dependent loci for seed glucosinolate trait in the oilseed Brassica juncea: Importance of recurrent selection backcross scheme for the identification of “true” QTL. Theor. Appl. Genet. 2007, 116, 77–85. [Google Scholar] [CrossRef]
- Arcade, A.; Labourdette, A.; Falque, M.; Mangin, B.; Chardon, F.; Charcosset, A.; Joets, J. BioMercator: Integrating genetic maps and QTL towards discovery of candidate genes. Bioinformatics 2004, 20, 2324–2326. [Google Scholar] [CrossRef] [PubMed]
- Goffinet, B.; Gerber, S. Quantitative trait loci: A meta-analysis. Genetics 2000, 155, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Mackay, T.F.C. The Genetic Architecture of Quantitative Traits. Annu. Rev. Genet. 2001, 35, 303–339. [Google Scholar] [CrossRef]
- Yadava, S.K.; Arumugam, N.; Mukhopadhyay, A.; Sodhi, Y.S.; Gupta, V.; Pental, D.; Pradhan, A.K. QTL mapping of yield-associated traits in Brassica juncea: Meta-analysis and epistatic interactions using two different crosses between east European and Indian gene pool lines. Theor. Appl. Genet. 2012, 125, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Rout, K.; Yadav, B.G.; Yadava, S.K.; Mukhopadhyay, A.; Gupta, V.; Pental, D.; Pradhan, A.K. QTL landscape for oil content in brassica juncea: Analysis in multiple bi-parental populations in high and “0” erucic background. Front. Plant Sci. 2018, 871, 1448. [Google Scholar] [CrossRef] [PubMed]
- Khahani, B.; Tavakol, E.; Shariati, V.; Fornara, F. Genome wide screening and comparative genome analysis for Meta-QTLs, ortho-MQTLs and candidate genes controlling yield and yield-related traits in rice. BMC Genom. 2020, 21, 294. [Google Scholar] [CrossRef]
- Sandhu, N.; Pruthi, G.; Raigar, O.P.; Singh, M.P.; Phagna, K.; Kumar, A.; Sethi, M.; Singh, J.; Ade, P.A.; Saini, D.K. Meta-QTL analysis in rice and cross-genome talk of the genomic regions controlling nitrogen use efficiency in cereal crops revealing phylogenetic relationship. Front. Genet. 2021, 12, 807210. [Google Scholar] [CrossRef]
- Said, J.I.; Song, M.; Wang, H.; Lin, Z.; Zhang, X.; Fang, D.D.; Zhang, J. A comparative meta-analysis of QTL between intraspecific Gossypium hirsutum and interspecific G. hirsutum × G. barbadense populations. Mol. Genet. Genom. 2015, 290, 1003–1025. [Google Scholar] [CrossRef]
- Rong, J.; Feltus, F.A.; Waghmare, V.N.; Pierce, G.J.; Chee, P.W.; Draye, X.; Saranga, Y.; Wright, R.J.; Wilkins, T.A.; May, O.L.; et al. Meta-analysis of polyploid cotton QTL shows unequal contributions of subgenomes to a complex network of genes and gene clusters implicated in lint fiber development. Genetics 2007, 176, 2577–2588. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Deng, D.; Ding, H.; Bian, Y.; Yin, Z.; Wu, Y.; Zhou, B.; Zhao, Y. A comprehensive meta-analysis of plant morphology, yield, stay-green, and virus disease resistance QTL in maize (Zea mays L.). Planta 2016, 243, 459–471. [Google Scholar] [CrossRef]
- Guo, J.; Chen, L.; Li, Y.; Shi, Y.; Song, Y.; Zhang, D.; Li, Y.; Wang, T.; Yang, D.; Li, C. Meta-QTL analysis and identification of candidate genes related to root traits in maize. Euphytica 2018, 214, 223. [Google Scholar] [CrossRef]
- Zhang, X.; Shabala, S.; Koutoulis, A.; Shabala, L.; Zhou, M. Meta-analysis of major QTL for abiotic stress tolerance in barley and implications for barley breeding. Planta 2017, 245, 283–295. [Google Scholar] [CrossRef]
- Danan, S.; Veyrieras, J.B.; Lefebvre, V. Construction of a potato consensus map and QTL meta-analysis offer new insights into the genetic architecture of late blight resistance and plant maturity traits. BMC Plant Biol. 2011, 11, 16. [Google Scholar] [CrossRef]
- Guo, B.; Sleper, D.A.; Lu, P.; Shannon, J.G.; Nguyen, H.T.; Arelli, P.R. QTLs associated with resistance to soybean cyst nematode in soybean: Meta-analysis of QTL locations. Crop Sci. 2006, 46, 595–602. [Google Scholar] [CrossRef]
- Sun, Y.N.; Pan, J.B.; Shi, X.L.; Du, X.Y.; Wu, Q.; Qi, Z.M.; Jiang, H.W.; Xin, D.W.; Liu, C.Y.; Hu, G.H.; et al. Multi-environment mapping and meta-analysis of 100-seed weight in soybean. Mol. Biol. Rep. 2012, 39, 9435–9443. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; King, C.A.; Chen, P.; Ray, J.D.; Cregan, P.B.; Carter, T.E.; Li, Z.; Abdel-Haleem, H.; Matson, K.W.; Schapaugh, W.; et al. Meta-analysis to refine map position and reduce confidence intervals for delayed-canopy-wilting QTLs in soybean. Mol. Breed. 2016, 36, 91. [Google Scholar] [CrossRef]
- Zhao-Ming, Q.; Ya-Nan, S.; Qiong, W.; Chun-Yan, L.; Guo-Hua, H.; Qing-Shan, C. A meta-analysis of seed protein concentration QTL in soybean. Can. J. Plant Sci. 2011, 91, 221–230. [Google Scholar] [CrossRef]
- Qi, Z.-M.; Wu, Q.; Han, X.; Sun, Y.-N.; Du, X.-Y.; Liu, C.-Y.; Jiang, H.-W.; Hu, G.-H.; Chen, Q.-S. Soybean oil content QTL mapping and integrating with meta-analysis method for mining genes. Euphytica 2011, 179, 499–514. [Google Scholar] [CrossRef]
- Gudi, S.; Saini, D.K.; Singh, G.; Halladakeri, P. Unravelling consensus genomic regions associated with quality traits in wheat (Triticum aestivum L.) using meta-analysis of quantitative trait loci. Planta 2021, 255, 115. [Google Scholar] [CrossRef]
- Avni, R.; Oren, L.; Shabtay, G.; Assili, S.; Pozniak, C.; Hale, I.; Ben-David, R.; Peleg, Z.; Distelfeld, A. Genome based meta-QTL analysis of grain weight in tetraploid wheat identifies rare alleles of GRF4 associated with larger grains. Genes 2018, 9, 636. [Google Scholar] [CrossRef]
- Saini, D.K.; Srivastava, P.; Pal, N.; Gupta, P.K. Meta-QTLs, ortho-meta-QTLs and candidate genes for grain yield and associated traits in wheat (Triticum aestivum L.). Theor. Appl. Genet. 2022, 135, 1049–1081. [Google Scholar] [CrossRef]
- Saini, D.K.; Chopra, Y.; Pal, N.; Chahal, A.; Srivastava, P.; Gupta, P.K. Root system architecture in bread wheat (Triticum aestivum L.) Meta-QTLs, ortho-MQTLs and candidate genes for nitrogen use efficiency and root system architecture in bread wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 2021, 27, 2245–2267. [Google Scholar] [CrossRef]
- Soriano, J.M.; Royo, C. Dissecting the genetic architecture of leaf rust resistance in wheat by QTL meta-analysis. Phytopathology 2015, 105, 1585–1593. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, V.P.; Saini, D.K.; Sharma, H.; Saripalli, G.; Kumar, S.; Balyan, H.S.; Gupta, P.K. Meta-QTLs, ortho-MQTLs, and candidate genes for thermotolerance in wheat (Triticum aestivum L.). Mol. Breed. 2021, 41, 69. [Google Scholar] [CrossRef]
- Pal, N.; Saini, D.K.; Kumar, S. Meta-QTLs, ortho-MQTLs and candidate genes for the traits contributing to salinity stress tolerance in common wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants. 2021, 27, 2767–2786. [Google Scholar] [CrossRef]
- Saini, D.K.; Chahal, A.; Pal, N.; Srivastava, P.; Gupta, P.K. Meta-analysis reveals consensus genomic regions associated with multiple disease resistance in wheat (Triticum aestivum L.). Mol Breed 2022, 42, 11. [Google Scholar] [CrossRef]
- Pal, N.; Jan, I.; Saini, D.K.; Kumar, K.; Kumar, A.; Sharma, P.K.; Kumar, S.; Balyan, H.S.; Gupta, P.K. Meta-QTLs for multiple disease resistance involving three rusts in common wheat (Triticum aestivum L.). Theor. Appl Genet. 2022, 135, 2385–2405. [Google Scholar] [CrossRef]
- Singh, R.; Saripalli, G.; Gautam, T.; Kumar, A.; Jan, I.; Batra, R.; Kumar, J.; Kumar, R.; Balyan, H.S.; Sharma, S.; et al. Meta-QTLs, ortho-MetaQTLs and candidate genes for grain Fe and Zn contents in wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 2022, 28, 37–650. [Google Scholar] [CrossRef]
- Dhaka, N.; Rout, K.; Yadava, S.K.; Sodhi, Y.S.; Gupta, V.; Pental, D.; Pradhan, A.K. Genetic dissection of seed weight by QTL analysis and detection of allelic variation in Indian and east European gene pool lines of Brassica juncea. Theor. Appl. Genet. 2017, 130, 293–307. [Google Scholar] [CrossRef]
- Rout, K.; Sharma, M.; Gupta, V.; Mukhopadhyay, A.; Sodhi, Y.S.; Pental, D.; Pradhan, A.K. Deciphering allelic variations for seed glucosinolate traits in oilseed mustard (Brassica juncea) using two bi-parental mapping populations. Theor. Appl. Genet. 2015, 128, 657–666. [Google Scholar] [CrossRef]
- Ramchiary, N.; Padmaja, K.L.; Sharma, S.; Gupta, V.; Sodhi, Y.S.; Mukhopadhyay, A.; Arumugam, N.; Pental, D.; Pradhan, A.K. Mapping of yield influencing QTL in Brassica juncea: Implications for breeding of a major oilseed crop of dryland areas. Theor. Appl. Genet. 2007, 115, 807–817. [Google Scholar] [CrossRef]
- Aakanksha, A.; Yadava, S.K.; Yadav, B.G.; Gupta, V.; Mukhopadhyay, A.; Pental, D.; Pradhan, A.K. Genetic analysis of heterosis for yield influencing traits in Brassica juncea using a doubled haploid population and its backcross progenies. Front. Plant Sci. 2021, 12, 721631. [Google Scholar] [CrossRef]
- Aggarwal, R.A.K.; Sharma, R.; Kumar, R.; Mohapatra, T.; Sharma, R.P. Molecular mapping of loci affecting the contents of three major fatty acids in Indian mustard (Brassica juncea L). J. Plant Biochem. Biotechnol. 2003, 12, 131–137. [Google Scholar] [CrossRef]
- Cheung, W.Y.; Landry, B.S.; Raney, P.; Rakow, G.F.W. Molecular mapping of seed quality traits in Brassica juncea L. Czern. and Coss. Acta Hortic. 1998, 459, 139–148. [Google Scholar] [CrossRef]
- Gupta, V.; Mukhopadhyay, A.; Arumugam, N.; Sodhi, Y.S.; Pental, D.; Pradhan, A.K. Molecular tagging of erucic acid trait in oilseed mustard (Brassica juncea) by QTL mapping and single nucleotide polymorphisms in FAE1 gene. Theor. Appl. Genet. 2004, 108, 743–749. [Google Scholar] [CrossRef]
- Jagannath, A.; Sodhi, Y.S.; Gupta, V.; Mukhopadhyay, A.; Arumugam, N.; Singh, I.; Rohatgi, S.; Burma, P.K.; Pradhan, A.K.; Pental, D. Eliminating expression of erucic acid-encoding loci allows the identification of “hidden” QTL contributing to oil quality fractions and oil content in Brassica juncea (Indian mustard). Theor. Appl. Genet. 2011, 122, 1091–1103. [Google Scholar] [CrossRef]
- Khattak, A.N.; Wang, T.; Yu, K.; Yang, R.; Wan, W.; Ye, B.; Tian, E. Exploring the basis of 2-propenyl and 3-butenyl glucosinolate synthesis by QTL mapping and RNA-sequencing in Brassica juncea. PLoS ONE 2019, 14, e0220597. [Google Scholar] [CrossRef]
- Lionneton, E.; Aubert, G.; Ochatt, S.; Merah, O. Genetic analysis of agronomic and quality traits in mustard (Brassica juncea). Theor. Appl. Genet. 2004, 109, 792–799. [Google Scholar] [CrossRef]
- Mahmood, T.; Ekuere, U.; Yeh, F.; Good, A.G.; Stringam, G.R. Molecular mapping of seed aliphatic glucosinolates in Brassica juncea. Genome 2003, 46, 753–760. [Google Scholar] [CrossRef]
- Mahmood, T.; Ekuere, U.; Yeh, F.; Good, A.G.; Stringam, G.R. RFLP linkage analysis and mapping genes controlling the fatty acid profile of Brassicajuncea using reciprocal DH populations. Theor. Appl. Genet. 2003, 107, 283–290. [Google Scholar] [CrossRef]
- Lionneton, E.; Ravera, S.; Sanchez, L.; Aubert, G.; Delourme, R.; Ochatt, S. Development of an AFLP-based linkage map and localization of QTLs for seed fatty acid content in condiment mustard (Brassica juncea). Genome 2002, 45, 1203–1215. [Google Scholar] [CrossRef]
- Sharma, R.; Aggarwal, R.A.K.; Kumar, R.; Mohapatra, T.; Sharma, R.P. Construction of an RAPD linkage map and localization of QTLs for oleic acid level using recombinant inbreds in mustard (Brassica juncea). Genome 2002, 45, 467–472. [Google Scholar] [CrossRef]
- Singh, S.; Mohapatra, T.; Singh, R.; Hussain, Z. Mapping of QTLs for oil content and fatty acid composition in Indian mustard [Brassica juncea (L.) Czern. and Coss.]. J. Plant Biochem. Biotechnol. 2013, 22, 80–89. [Google Scholar] [CrossRef]
- Pradhan, A.K.; Gupta, V.; Mukhopadhyay, A.; Arumugam, N.; Sodhi, Y.S.; Pental, D. A high-density linkage map in Brassica juncea (Indian mustard) using AFLP and RFLP markers. Theor. Appl. Genet. 2003, 106, 607–614. [Google Scholar] [CrossRef]
- Paritosh, K.; Yadava, S.K.; Singh, P.; Bhayana, L.; Gupta, V.; Bisht, N.C.; Zhang, J.; Kudrna, D.A.; Wing, R.A.; Bhasker, V.; et al. A chromosome-scale assembly of allotetraploid Brassica juncea (AABB) elucidates comparative architecture of the A and B genomes. Plant Biotechnol. J. 2021, 19, 602–614. [Google Scholar] [CrossRef] [PubMed]
- Endelman, J.B.; Plomion, C. LPmerge: An R package for merging genetic maps by linear programming. Bioinformatics 2014, 30, 1623–1624. [Google Scholar] [CrossRef]
- Visscher, P.M.; Goddard, M.E. Prediction of the Confidence Interval of Quantitative Trait Loci Location. Behav. Genet. 2004, 34, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, Y.; Sosnowski, O.; Charcosset, A.; Joets, J. BioMercator 4: A complete framework to integrate QTL, meta-QTL, and genome annotation. In European Conference on Computational Biology; INRA, UMR Génétique Quantitative et Evolution—Le Moulon: Gif-sur-Yvette, France, 2014. [Google Scholar]
- Sosnowski, O.; Charcosset, A.; Joets, J. BioMercator V3: An upgrade of genetic map compilation and quantitative trait loci meta-analysis algorithms. Bioinformatics 2012, 28, 2082–2083. [Google Scholar] [CrossRef] [PubMed]
- Veyrieras, J.B.; Goffinet, B.; Charcosset, A. MetaQTL: A package of new computational methods for the meta-analysis of QTL mapping experiments. BMC Bioinform. 2007, 8, 49. [Google Scholar] [CrossRef]
- Schmid, M.; Davison, T.S.; Henz, S.R.; Pape, U.J.; Demar, M.; Vingron, M.; Weigel, D.; Lohmann, J.U. A gene expression map of Arabidopsis thaliana development. Nat. Genet. 2005, 37, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Metsalu, T.; Vilo, J.; Science, C.; Liivi, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Jiaqin, S.; Ruiyuan, L.; Dan, Q.; Congcong, J.; Yan, L.; Morgan, C.; Bancroft, I.; Jianyi, Z.; Jinling, M. Unraveling the complex trait of crop yield with quantitative trait loci mapping in Brassica napus. Genetics 2009, 182, 851–861. [Google Scholar] [CrossRef]
- Zhang, Y.; Thomas, C.L.; Xiang, J.; Long, Y.; Wang, X.; Zou, J.; Luo, Z.; Ding, G.; Cai, H.; Graham, N.S.; et al. QTL meta-analysis of root traits in Brassica napus under contrasting phosphorus supply in two growth systems. Sci. Rep. 2016, 6, 33113. [Google Scholar] [CrossRef]
- Löffler, M.; Schön, C.C.; Miedaner, T. Revealing the genetic architecture of FHB resistance in hexaploid wheat (Triticum aestivum L.) by QTL meta-analysis. Mol. Breed. 2009, 23, 473–488. [Google Scholar] [CrossRef]
- Saini, D.K.; Chopra, Y.; Singh, J.; Sandhu, K.S.; Kumar, A.; Bazzer, S.; Srivastava, P. Comprehensive evaluation of mapping complex traits in wheat using genome-wide association studies. Mol. Breed 2022, 42, 11. [Google Scholar] [CrossRef]
- Gupta, M.; Choudhary, M.; Singh, A.; Sheoran, S.; Singla, D.; Rakshit, S. Meta-QTL analysis for mining of candidate genes and constitutive gene network development for fungal disease resistance in maize (Zea mays L.). Crop J. 2022. [Google Scholar] [CrossRef]
- Daryani, P.; Darzi Ramandi, H.; Dezhsetan, S.; Mirdar Mansuri, R.; Hosseini Salekdeh, G.; Shobbar, Z.S. Pinpointing genomic regions associated with root system architecture in rice through an integrative meta-analysis approach. Theor. Appl. Genet. 2022, 135, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Akhatar, J.; Banga, S.S. Genome-wide association mapping for grain yield components and root traits in Brassica juncea (L.) Czern & Coss. Mol. Breed. 2015, 35, 1–14. [Google Scholar] [CrossRef]
- Akhatar, J.; Singh, M.P.; Sharma, A.; Kaur, H.; Kaur, N.; Sharma, S.; Bharti, B.; Sardana, V.K.; Banga, S.S. Association mapping of seed quality traits under varying conditions of nitrogen application in Brassica juncea L. Czern & Coss. Front. Genet. 2020, 11, 744. [Google Scholar] [CrossRef]
- Akhatar, J.; Goyal, A.; Kaur, N.; Atri, C.; Mittal, M.; Singh, M.P.; Kaur, R.; Rialch, I.; Banga, S.S. Genome wide association analyses to understand genetic basis of flowering and plant height under three levels of nitrogen application in Brassica juncea (L.) Czern & Coss. Sci. Rep. 2021, 11, 4278. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.K.; Pal, L.; Kaur, J.; Bhatia, D. Genome wide association studies for yield and its component traits under terminal heat stress in Indian mustard (Brassica juncea L.). Euphytica, 2019; 215, 188. [Google Scholar] [CrossRef]
- Tandayu, E.; Borpatragohain, P.; Mauleon, R.; Kretzschmar, T. Genome-wide association reveals trait loci for seed glucosinolate accumulation in Indian mustard (Brassica juncea L.). Plants 2022, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, J.; Li, Z.; Li, X.; He, Z.; Zhang, L.; Sha, T.; Lyu, X.; Chen, S.; Gu, Y.; et al. Genomic signatures of vegetable and oilseed allopolyploid Brassica juncea and genetic loci controlling the accumulation of glucosinolates. Plant Biotechnol. J. 2021, 19, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.K.; Kang, M.S.; Akash, M.W.; Singh, P. Selection indices for improving selection efficiency in Indian mustard. J. Crop Improv. 2019, 33, 25–41. [Google Scholar] [CrossRef]
- Zhao, J. QTLs for Oil Content and their Relationships to other Agronomic Traits in an European x Chinese Oilseed Rape Population. Ph.D. Dissertation, Universität Göttingen, Göttingen, Germany, 2002; p. 131. [Google Scholar]
- Medema, M.H.; Kottmann, R.; Yilmaz, P.; Cummings, M.; Biggins, J.B.; Blin, K.; De Bruijn, I.; Chooi, Y.H.; Claesen, J.; Coates, R.C.; et al. Minimum Information about a Biosynthetic Gene cluster. Nat. Chem. Biol. 2015, 11, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Hou, X.; Xing, G.; Liu, J.; Duan, A.; Xu, Z.; Li, M.; Zhuang, J.; Xiong, A. Critical Reviews in Biotechnology Advances in AP2/ERF super-family transcription factors in plant. Crit. Rev. Biotechnol. 2020, 40, 750–776. [Google Scholar] [CrossRef]
- Tapia-López, R.; García-Ponce, B.; Dubrovsky, J.G.; Garay-Arroyo, A.; Pérez-Ruíz, R.V.; Kim, S.H.; Acevedo, F.; Pelaz, S.; Alvarez-Buylla, E.R. An AGAMOUS-related MADS-box gene, XAL1 (AGL12), regulates root meristem cell proliferation and flowering transition in Arabidopsis. Plant Physiol. 2008, 146, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Withers, J.; Yao, J.; Mecey, C.; Howe, G.A.; Melotto, M.; Yang, S. Transcription factor-dependent nuclear localization of a transcriptional repressor in jasmonate hormone signaling. Proc. Natl. Acad. Sci. USA 2012, 109, 20148–20153. [Google Scholar] [CrossRef]
- Sonoda, Y.; Yao, S.; Sako, K.; Sato, T.; Kato, W.; Ohto, M.; Ichikawa, T. SHA1, a novel RING finger protein, functions in shoot apical meristem maintenance in Arabidopsis. Plant J. 2007, 3, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hua, L.; Dong, S.; Chen, H.; Zhu, X.; Jiang, J.; Zhang, F.; Li, Y.; Fang, X.; Chen, F. OsMAPK6, a mitogen-activated protein kinase, influences rice grain size and biomass production. Plant J. 2015, 84, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bai, B.; Lee, M.; Alfiko, Y.; Suwanto, A.; Yue, G.H. Cloning and characterization of EgGDSL, a gene associated with oil content in oil palm. Sci. Rep. 2018, 8, 226. [Google Scholar] [CrossRef]
- Karunarathna, N.L.; Wang, H.; Harloff, H.; Jiang, L.; Jung, C. Elevating seed oil content in a polyploid crop by induced mutations in SEED FATTY ACID REDUCER genes. Plant Biotechnol. J. 2020, 18, 2251–2266. [Google Scholar] [CrossRef] [PubMed]
- Geilen, K.; Heilmann, M.; Hillmer, S.; Böhmer, M. WRKY43 regulates polyunsaturated fatty acid content and seed germination under unfavourable growth conditions. Sci. Rep. 2017, 7, 14235. [Google Scholar] [CrossRef] [PubMed]
- Siloto, R.M.P.; Findlay, K.; Lopez-Villalobos, A.; Yeung, E.C.; Nykiforuk, C.L.; Moloney, M.M. The accumulation of oleosins determines the size of seed oilbodies in Arabidopsis. Plant Cell 2006, 18, 1961–1974. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.S.; Kim, J.S. Understanding of MYB transcription factors involved in glucosinolate biosynthesis in Brassicaceae. Molecules 2017, 22, 1549. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.A.; Choudhury, A.R.; Kancharla, P.K. The FAD2 Gene in Plants: Occurrence, Regulation, and Role. Front. Plant Sci. 2017, 8, 1789. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Li, Y.; Liu, Y.; He, L.; Liu, A.; Wen, J.; Mysore, K.S. The 3-ketoacyl-CoA synthase WFL is involved in lateral organ development and cuticular wax synthesis in Medicago truncatula. Plant Mol. Biol. 2020, 105, 193–204. [Google Scholar] [CrossRef] [PubMed]




| Cross (Population Type-Size) | Traits | Types of Markers (Number) | Mapping Method | QTLs | Reference |
|---|---|---|---|---|---|
| Yield-related traits | |||||
| Varuna/Herra (DH-123), TM4/DONSKAJA-IV (DH-100), DONSKAJA-IV/EH-2 (DH-184), EH-2/PUSA JAIKISAN (DH-182) | TSW | IP and SSR (860) | CIM | 65 | [37] |
| Varuna/Herra (DH-123) | PH, BN, MSL, SMS, SPP, SL, TW, SD, and DTF | AFLP, RFLP and SSR (1448) | CIM | 65 | [39] |
| RLM-514/canola quality inbred line (DH-112) | SL, and SN | RFLP (300) | IM (MQM) | 11 | [2] |
| Varuna/Herra (DH-123), TM4/DONSKAJA-IV (DH-100) | PH, PB, SB, MSL, SMS, SPP, SL, SPS, TSW, SD, and DTF | AFLP, RFLP, IP, SSRs, SCAR, CAPS and gene markers (2662) | CIM | 178 | [12] |
| Varuna/EH-2 (DH-164), VEH/Varuna (BC-164), and VEH/EH-2 (BC-164) | PH, DTF, PB, SB, MSL, SPY, SL, SD, SPP, and TSW | Mainly SNP markers | CIM | 596 | [40] |
| Quality traits | |||||
| Varuna/BEC-144 (RILs-94) | C22:1, and C18:3 | RAPD (130) | IM | 5 | [41] |
| J9-4317/J90-2733 (DH-119) | C22, OC, C18:3, and GSL | RFLP (343) | IM | 12 | [42] |
| Varuna/Herra (DH-123) | C22:1 | SNPs | IM | 2 | [43] |
| Varuna/Herra (DH-ZE-123, DH-SE-110) | C18:3, C18:1, and OC | AFLP, and RFLP (1029) | CIM | 17 (ZE) 17 (SE) | [44] |
| G266Å/G302 (RILs-167) | GSL | ILP (304) | IM | 4 | [45] |
| BJ-90/BJ-70 (DH-131) | OC, C18:1, C18:2, C18:3, C22:1, SIN, GNA, and GSL | AFLP, and RAPD (273) | CIM | 26 | [46] |
| RLM-514/poor canola quality inbred line (DH-112) | GSL | RFLP (316) | IM (MQM) | 71 | [47] |
| RLM-514/poor canola quality inbred line (DH-112) | C22:1, C18:1, and C18:3 | RFLP (300) | IM (MQM) | 11 | [48] |
| RLM-514/poor canola quality inbred line (DH-112) | OC, MPC, SPC, and SOMP | RFLP (316) | IM (MQM) | 20 | [7] |
| BJ 99/BJ70 (DH-131) | OC, C16:0, C18:0, C18:1, C18:2, C18:3, and C22:1 | AFLP, and RAPD (273) | CIM | 17 | [49] |
| Heera/Varuna (F1DH-123, BC4DH-167) | % Propyls, % Butyls, % Pentyls and GSL | AFLP, RFLP and SSR (1448) | CIM | 26 | [8] |
| Donskaja-IV/EH-2 (DH-184) Varuna/Heera (DH-123) | % Propyls, % Butyls, % Pentyls, TA, and GSL | IPs, SSRs, SNPs and Indels (653); AFLPs, RFLPs, IPs, SSRs, gene markers and SNPs (1908) | CIM | 118 | [38] |
| J8/EH-2 (DH-166), Donskaja-IV/EH-2 (DH-163), Pusa Jaikisan/EH-2 (DH-182), TM-4/Donskaja-IV (DH-100), Varuna/Heera (110–123) | C 22:1, and OC | IP, SSRs, SNPs, RFLP and AFLP (388–470; 650–653; 841; 881; 420–2408) | CIM | 128 | [13] |
| Varuna/BEC144 (RILs-94) | C18:1 | RAPD (130) | IM | 2 | [50] |
| Varuna/BEC-144 (RILs-88) | OC, C22:1, EA, C18:3, and C16:0 | AFLP (91) | CIM | 14 | [51] |
| MQTLs | Associated Traits a | B. juncea Genes | Arabidopsis Orthologs | Function Descriptions |
|---|---|---|---|---|
| Yield-related traits | ||||
| YRTs_MQTLA7.2 | SPS, SD, PB, DTF, PH, MSL, SB, SPY, SPP, SL, and TW | BjuVA07G37280 | AT1G70700 | Tify domain |
| YRTs_MQTLA7.2 | BjuVA07G37450 | AT1G70920 | Homeobox domain | |
| YRTs_MQTLA7.2 | BjuVA07G37850 | AT1G71450 | AP2/ERF domain | |
| YRTs_MQTLA7.2 | BjuVA07G37930 | AT1G71692 | Transcription factor, MADS-box | |
| YRTs_MQTLA7.2 | BjuVA07G38410 | AT1G72180 | Protein kinase domain | |
| YRTs_MQTLA7.2 | BjuVA07G38660 | AT1G72360 | AP2/ERF domain | |
| YRTs_MQTLA7.3 | SB, SD, SPY, SPS, MSL, TSW, PB, and PH | BjuVA07G38850 | AT1G72630 | Protein EARLY FLOWERING 4 domain |
| YRTs_MQTLA7.3 | BjuVA07G38870 | AT1G72650 | SANT/Myb domain | |
| YRTs_MQTLA7.3 | BjuVA07G39210 | AT1G73190 | Major intrinsic protein | |
| YRTs_MQTLA9.2 | SPP, TSW, SD, SB, SL, and MSL | BjuVA09G08320 | AT5G63780 | Zinc finger, RING-CH-type |
| YRTs_MQTLA9.2 | BjuVA09G08330 | AT5G63840 | Glycoside hydrolase family 31 | |
| YRTs_MQTLA10.1 | PH, TW, PB, MSL, SMS, SL, TSW, SPP, SPS, DTF, SB, and SD | BjuVA10G02820 | AT1G04440 | Protein kinase domain |
| YRTs_MQTLB3.1 | SPS, SL, PB, MSL, and SB | BjuVB03G34480 | AT1G02930 | Glutathione S-transferase, N-terminal |
| YRTs_MQTLB3.1 | BjuVB03G34880 | AT1G73670 | Protein kinase domain | |
| YRTs_MQTLB3.1 | BjuVB03G35600 | AT1G72650 | SANT/Myb domain | |
| Quality traits | ||||
| Quality_MQTLA2.4 | OC, %pentyls, %butyls, and %propyls | BjuVA02G23150 | AT1G72630 | Protein EARLY FLOWERING 4 domain |
| Quality_MQTLA2.4 | BjuVA02G23370 | AT1G72970 | Glucose-methanol-choline oxidoreductase, N-terminal | |
| Quality_MQTLA2.4 | BjuVA02G23650 | AT1G73410 | SANT/Myb domain | |
| Quality_MQTLA2.4 | BjuVA02G23710 | AT1G73550 | Bifunctional inhibitor/plant lipid transfer protein/seed storage helical domain | |
| Quality_MQTLA2.4 | BjuVA02G23850 | AT1G73780 | Bifunctional inhibitor/plant lipid transfer protein/seed storage helical domain | |
| Quality_MQTLA2.5 | C18:3, OC, C22:1, C18:1, C16:0, C18:2, and C18:0 | BjuVA02G24750 | AT1G47620 | Cytochrome P450 |
| Quality_MQTLA2.5 | BjuVA02G24680 | AT1G75400 | Zinc finger, RING-type | |
| Quality_MQTLA2.5 | BjuVA02G24970 | AT1G75680 | Glycoside hydrolase family 9 | |
| Quality_MQTLA2.5 | BjuVA02G25160 | AT1G75910 | GDSL lipase/esterase | |
| Quality_MQTLA2.5 | BjuVA02G25170 | AT1G75930 | GDSL lipase/esterase | |
| Quality_MQTLA3.1 | %butyls, C16:0, OC, %propyls, %pentyls, and GSL | BjuVA03G18780 | AT2G34555 | Oxoglutarate/iron-dependent dioxygenase |
| Quality_MQTLA3.1 | BjuVA03G18840 | AT2G34680 | Leucine-rich repeat | |
| Quality_MQTLA3.1 | BjuVA03G18900 | AT2G34770 | Fatty acid hydroxylase | |
| Quality_MQTLA3.1 | BjuVA03G18930 | AT2G34810 | FAD-linked oxidase, N-terminal | |
| Quality_MQTLA3.1 | BjuVA03G18980 | AT2G34930 | Leucine-rich repeat | |
| Quality_MQTLA3.2 | TA, %pentyls, %butyls, GSL, OC, C18:1, and ODR | BjuVA03G19000 | AT2G35060 | Potassium transporter |
| Quality_MQTLA3.2 | BjuVA03G19290 | AT2G35690 | Acyl-CoA oxidase, C-terminal | |
| Quality_MQTLA3.2 | BjuVA03G19370 | AT2G35840 | HAD-superfamily hydrolase, subfamily IIB | |
| Quality_MQTLA3.2 | BjuVA03G19840 | AT2G36770 | UDP-glucuronosyl/UDP-glucosyltransferase | |
| Quality_MQTLA3.2 | BjuVA03G19830 | AT2G36780 | UDP-glucuronosyl/UDP-glucosyltransferase | |
| Quality_MQTLA3.2 | BjuVA03G19850 | AT2G36800 | UDP-glucuronosyl/UDP-glucosyltransferase | |
| Quality_MQTLA8.1 | C22:1, OC, and C18:3 | BjuVA08G21620 | AT4G25140 | Oleosin |
| Quality_MQTLA8.1 | BjuVA08G21200 | AT4G26640 | WRKY domain | |
| Quality_MQTLA8.1 | BjuVA08G21050 | AT4G26965 | NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 12 | |
| Quality_MQTLA8.1 | BjuVA08G20980 | AT4G27030 | B domain of TMEM189, localization domain | |
| Quality_MQTLA8.1 | BjuVA08G21530 | AT5G51860 | Transcription factor, MADS-box | |
| Quality_MQTLA8.2 | OC, C22:1, GSL, PC, TA, and C18:3 | BjuVA08G17650 | AT4G33550 | Bifunctional inhibitor/plant lipid transfer protein/seed storage helical domain |
| Quality_MQTLA8.2 | BjuVA08G17660 | AT4G33550 | Bifunctional inhibitor/plant lipid transfer protein/seed storage helical domain | |
| Quality_MQTLA8.2 | BjuVA08G17490 | AT4G33790 | Fatty acyl-coenzyme A reductase, NAD-binding domain | |
| Quality_MQTLA8.2 | BjuVA08G17340 | AT4G33970 | Leucine-rich repeat domain superfamily | |
| Quality_MQTLA8.2 | BjuVA08G17300 | AT4G34030 | Acetyl-coenzyme A carboxyltransferase, N-terminal | |
| Quality_MQTLA8.2 | BjuVA08G16900 | AT4G34510 | Very-long-chain 3-ketoacyl-CoA synthase | |
| Quality_MQTLA8.2 | BjuVA08G16890 | AT4G34520 | Very-long-chain 3-ketoacyl-CoA synthase | |
| Quality_MQTLA8.2 | BjuVA08G17040 | AT1G78900 | ATPase, F1/V1/A1 complex, alpha/beta subunit, nucleotide-binding domain | |
| Quality_MQTLB7.3 | PC, C18:3, C22:1, and OC | BjuVB07G22630 | AT1G65520 | Enoyl-CoA hydratase/isomerase |
| Quality_MQTLB7.3 | BjuVB07G22620 | AT5G47890 | Ribosomal protein/NADH dehydrogenase domain | |
| Quality_MQTLB7.3 | BjuVB07G21940 | AT5G49520 | WRKY domain | |
| Quality_MQTLB7.3 | BjuVB07G22970 | AT3G29075 | PLD-regulated protein1-like | |
| Quality_MQTLB7.3 | BjuVB07G22750 | AT3G30260 | Transcription factor, MADS-box | |
| Quality_MQTLB7.4 | C18:3, OC, %pentyls, %butyls, C22:1, and PC | BjuVB07G29190 | AT3G17640 | Leucine-rich repeat |
| Quality_MQTLB7.4 | BjuVB07G28990 | AT3G18030 | Flavoprotein | |
| Quality_MQTLB7.4 | BjuVB07G28940 | AT3G18210 | Oxoglutarate/iron-dependent dioxygenase | |
| Quality_MQTLB7.4 | BjuVB07G28840 | AT3G18570 | Oleosin | |
| Quality_MQTLB7.4 | BjuVB07G28810 | AT3G18620 | Palmitoyltransferase, DHHC domain | |
| Quality_MQTLB7.4 | BjuVB07G28800 | AT3G18650 | Transcription factor, MADS-box | |
| Quality_MQTLB7.4 | BjuVB07G28740 | AT3G18750 | Protein kinase domain | |
| MQTL Name | Position | CI (95%) | Flanking Markers | LOD Score (PVE) | a Traits (N QTLs) | N QTLs (N Studies) |
|---|---|---|---|---|---|---|
| YRTs_MQTLA7.2 | 22.06 | 1.32 | E3M4_7/Bn-A07-p21096706 | 9.4 (17.24) | SPS (5), SD (7), PB (8), DTF (10), PH (10), MSL (7), SB (2), SPY, SPP, SL, TW | 53 (3) |
| YRTs_MQTLA7.3 | 29.45 | 0.98 | At1g70800/e31m50h244 | 5.58 (11.68) | SB (3), SD, SPY (4), SPS (4), MSL (2), TSW (9), PB, PH | 25 (2) |
| YRTs_MQTLA9.2 | 97.41 | 1.62 | Usuper-Scaffold_19_1295439/Bn-A09-p2343357 | 5.59 (10.40) | SPP (2), TSW, SD, SB (3), SL (2), MSL | 10 (2) |
| YRTs_MQTLB3.1 | 0.76 | 1.09 | USuper-Scaffold_30_7841960/Bj-B8-p821821 | 6.82 (13.25) | SPS, SL, PB (9), MSL (3), SB | 15 (3) |
| MQTL Name | Position (cM) | CI (95%) | Flanking Markers | LOD Score (PVE) | a Traits (N QTLs) | N QTLs (N Studies) |
|---|---|---|---|---|---|---|
| Quality_MQTLA2.4 | 61.45 | 0.47 | USuper-Scaffold_48_5983547/E1M7_10 | 39.79 (28.25) | OC, %pentyls (2), %butyls (4), %propyls (3) | 10 (3) |
| Quality_MQTLA2.5 | 68.94 | 0.49 | UGM0053/Bn-A02-p13999424 | 13.09 (32.36) | C18:3 (2), OC, C22:1 (2), C18:1 (2), C16:0, C18:2(2), C18:0 | 11 (3) |
| Quality_MQTLA3.1 | 76.45 | 0.69 | USuper-Scaffold_33_8156896/USuper-Scaffold_33_8903409 | 15.84 (13.64) | %butyls (4), C16:0, OC, %propyls (3), %pentyls (3), GSL, | 13 (4) |
| Quality_MQTLA3.2 | 79.16 | 0.67 | USuper-Scaffold_33_8156896/USuper-Scaffold_33_8903409 | 12.86 (23.61) | TA (3), %pentyls, (3) %butyls (3), GSL (3), OC, C18:1, ODR | 15 (3) |
| Quality_MQTLA8.1 | 19.29 | 0.89 | Bn-A08-p10031059/AG-AT_145 | 17.98 (28.58) | C22:1 (6), OC (19), C18:3 (4) | 29 (6) |
| Quality_MQTLA8.2 | 30.79 | 1.76 | At4g28200(56PP)/Bn-A08-p14737862 | 21.05 (26.72) | OC (5), C22:1 (3), GSL, PC, TA, C18:3 | 12 (5) |
| Quality_MQTLB7.3 | 29.92 | 1.21 | Bj-B3-p1835733/Bj-B3-p1683461 | 25.14 (33.08) | PC (5) C18:3, C22:1(4), OC (4) | 14 (4) |
| Quality_MQTLB7.4 | 39.2 | 1.17 | Bj-B3-p1091801/Bc-B3-p1053078 | 21.81 (18.77) | C18:3, OC (5), %pentyls, %butyls, C22:1 (6), PC | 15 (4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Saini, D.K.; Kumar, M.; Priyanka, V.; Akhatar, J.; Kaushik, D.; Sharma, A.; Dhanda, P.S.; Kaushik, P. Revealing the Genetic Architecture of Yield-Related and Quality Traits in Indian Mustard [Brassica juncea (L.) Czern. and Coss.] Using Meta-QTL Analysis. Agronomy 2022, 12, 2442. https://doi.org/10.3390/agronomy12102442
Kumar R, Saini DK, Kumar M, Priyanka V, Akhatar J, Kaushik D, Sharma A, Dhanda PS, Kaushik P. Revealing the Genetic Architecture of Yield-Related and Quality Traits in Indian Mustard [Brassica juncea (L.) Czern. and Coss.] Using Meta-QTL Analysis. Agronomy. 2022; 12(10):2442. https://doi.org/10.3390/agronomy12102442
Chicago/Turabian StyleKumar, Rahul, Dinesh Kumar Saini, Mukesh Kumar, Veerala Priyanka, Javed Akhatar, Deepak Kaushik, Amit Sharma, Parmdeep Singh Dhanda, and Prashant Kaushik. 2022. "Revealing the Genetic Architecture of Yield-Related and Quality Traits in Indian Mustard [Brassica juncea (L.) Czern. and Coss.] Using Meta-QTL Analysis" Agronomy 12, no. 10: 2442. https://doi.org/10.3390/agronomy12102442
APA StyleKumar, R., Saini, D. K., Kumar, M., Priyanka, V., Akhatar, J., Kaushik, D., Sharma, A., Dhanda, P. S., & Kaushik, P. (2022). Revealing the Genetic Architecture of Yield-Related and Quality Traits in Indian Mustard [Brassica juncea (L.) Czern. and Coss.] Using Meta-QTL Analysis. Agronomy, 12(10), 2442. https://doi.org/10.3390/agronomy12102442






