Biofortification and Quality of Collard Greens as a Function of Iron Concentration in Nutrient Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Area Characterization and Location
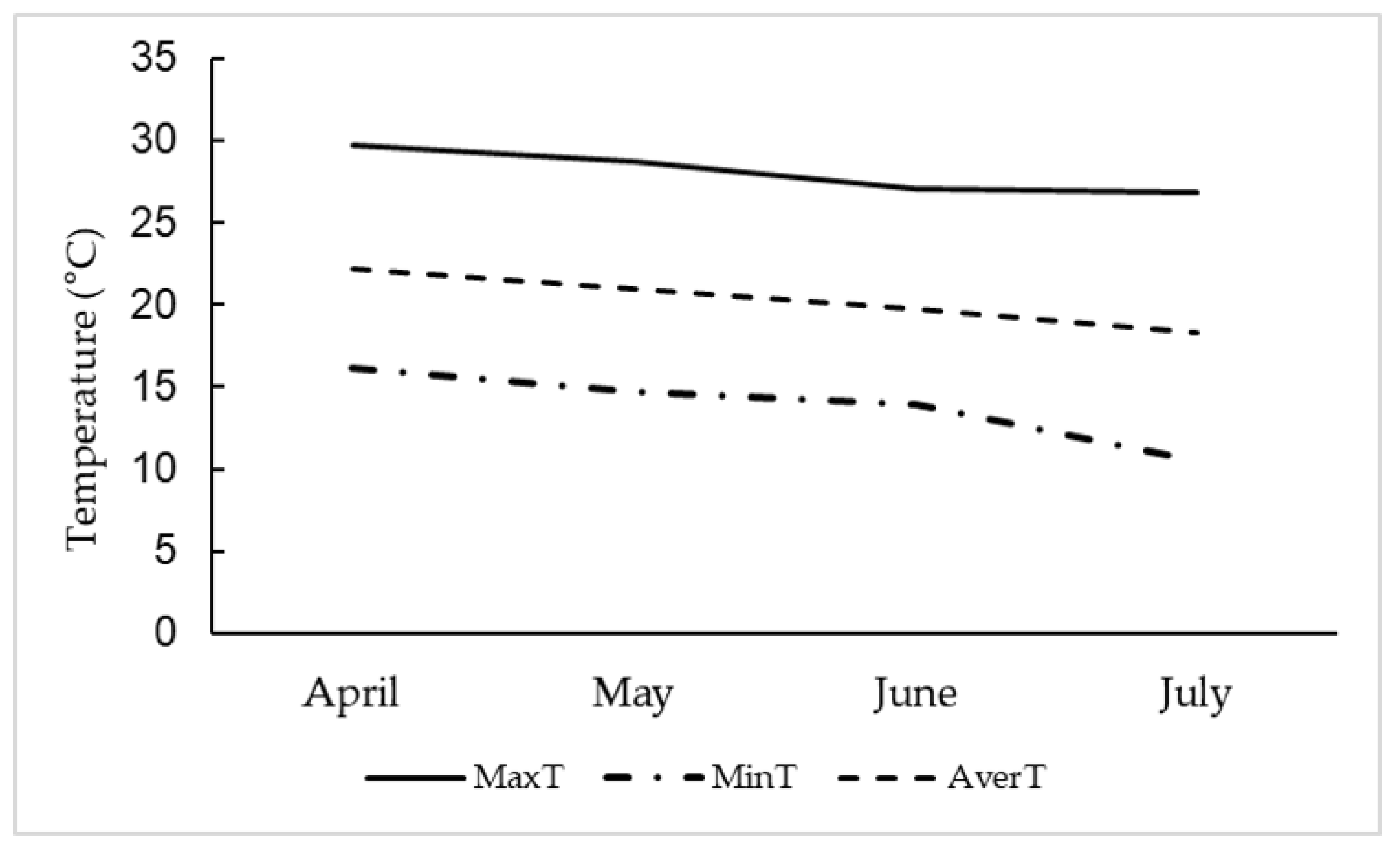
2.2. Experiment Installation and Conduction
2.3. Treatments and Experimental Design
2.4. Evaluated Characteristics
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on Iron and Its Importance for Human Health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Bilska-Wilkosz, A.; Iciek, M.; Górny, M.; Kowalczyk-Pachel, D. The Role of Hemoproteins: Hemoglobin, Myoglobin and Neuroglobin in Endogenous Thiosulfate Production Processes. Int. J. Mol. Sci. 2017, 18, 1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaz, N.; Guerinot, M. Lou All Together Now: Regulation of the Iron Deficiency Response. J. Exp. Bot. 2021, 72, 2045–2055. [Google Scholar] [CrossRef]
- Clemens, S. Zn and Fe Biofortification: The Right Chemical Environment for Human Bioavailability. Plant Sci. 2014, 225, 52–57. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture 2020; FAO: Roma, Italy, 2020. [Google Scholar]
- Poniedzialek, B.; Perkowska, K.; Rzymski, P. Food Fortification. What’s in It for the Malnourished World? In Vitamins and Minerals Biofortification of Edible Plants; Benkeblia, N., Ed.; Wiley Blackweel: Croydon, UK, 2020; pp. 27–44. ISBN 9781119511113. [Google Scholar]
- Liberal, Â.; Pinela, J.; Vívar-Quintana, A.M.; Ferreira, I.C.F.R.; Barros, L. Fighting Iron-Deficiency Anemia: Innovations in Food Fortificants and Biofortification Strategies. Foods 2020, 9, 1871. [Google Scholar] [CrossRef]
- Wishart, K. Increased Micronutrient Requirements during Physiologically Demanding Situations: Review of the Current Evidence. Vitam. Miner. 2017, 6, 166. [Google Scholar] [CrossRef]
- Chatterjee, R.; Chowdhury, R.S.; Dukpa, P.; Thirumdasu, R.K. Iron Fortification in Leafy Vegetables: Present Status and Future Possibilities. Innovare J. Agric. Sci. 2016, 4, 1–3. [Google Scholar]
- Connorton, J.M.; Balk, J. Iron Biofortification of Staple Crops: Lessons and Challenges in Plant Genetics. Plant Cell Physiol. 2019, 60, 1447–1456. [Google Scholar] [CrossRef] [Green Version]
- Carrasco-Gil, S.; Rios, J.J.; Álvarez-Fernández, A.; Abadía, A.; García-Mina, J.M.; Abadía, J. Effects of Individual and Combined Metal Foliar Fertilisers on Iron- and Manganese-Deficient Solanum Lycopersicum Plants. Plant Soil 2016, 402, 409–410. [Google Scholar] [CrossRef] [Green Version]
- Buturi, C.V.; Sabatino, L.; Mauro, R.P.; Navarro-León, E.; Blasco, B.; Leonardi, C.; Giuffrida, F. Iron Biofortification of Greenhouse Soilless Lettuce: An Effective Agronomic Tool to Improve the Dietary Mineral Intake. Agronomy 2022, 12, 1793. [Google Scholar] [CrossRef]
- Buturi, C.V.; Mauro, R.P.; Fogliano, V.; Leonardi, C.; Giuffrida, F. Mineral Biofortification of Vegetables as a Tool to Improve Human Diet. Foods 2021, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Stazi, S.R.; De Pascale, S.; Rouphael, Y. Iron Biofortification of Red and Green Pigmented Lettuce in Closed Soilless Cultivation Impacts Crop Performance and Modulates Mineral and Bioactive Composition. Agronomy 2019, 9, 290. [Google Scholar] [CrossRef]
- Di Gioia, F.; Petropoulos, S.A.; Ozores-Hampton, M.; Morgan, K.; Rosskopf, E.N. Zinc and Iron Agronomic Biofortification of Brassicaceae Microgreens. Agronomy 2019, 9, 677. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving Vegetable Quality in Controlled Environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C. Enhancing Quality of Fresh Vegetables Through Salinity Eustress and Biofortification Applications Facilitated by Soilless Cultivation. Front Plant Sci. 2018, 22, 1254. [Google Scholar] [CrossRef] [PubMed]
- Sambo, P.; Nicoletto, C.; Giro, A.; Pii, Y.; Valentinuzzi, F.; Mimmo, T.; Lugli, P.; Orzes, G.; Mazzetto, F.; Astolfi, S.; et al. Hydroponic Solutions for Soilless Production Systems: Issues and Opportunities in a Smart Agriculture Perspective. Front. Plant Sci. 2019, 10, 923. [Google Scholar] [CrossRef] [PubMed]
- Cecílio Filho, A.B.; Bianco, M.S.; Tardivo, C.F.; Pugina, G.C.M. Agronomic viability of New Zealand spinach and kale intercropping. An. Acad. Bras. Cien. 2017, 89, 2975–2986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furlani, P.R. Instrução Para o Cultivo de Hortaliças de Folha Pela Técnica de Hidroponia NFT; Instituto Agronômico: Campinas, Brazil, 1999. [Google Scholar]
- Miyazawa, M.; Muraoka, T.; Melo, W.J. de Análises Químicas de Tecido Vegetal. In Manual de Análises Químicas de Solos, Plantas e Fertilizantes; Embrapa: Brasilia, Brazil, 2009; pp. 107–184. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Strohecker, R.; Zaragoza, M.F.; Henning, H.M. Analisis de Vitaminas: Métodos Comprobados; Paz Montalvo: Madrid, Spain, 1967. [Google Scholar]
- Barbosa, J.C.; Maldonado Junior, W. AgroEstat—Sistema Para Análises Estatísticas de Ensaios Agronômicos; Version 1.1.0.711; Jaboticabal: São Paulo, Brazil, 2014. [Google Scholar]
- Trani, P.E.; Tivelli, S.W.; Blat, S.F.; Prela-Pantano, A.; Teixeira, É.P.; Araújo, H.S.; Feltran, J.C.; Passos, F.A.; Figueiredo, G.J.B.; Novo, M.D.C.d.S.S. Couve de Folha: Do Plantio à Pós-Colheita; IAC: Campinas, Brazil, 2015. [Google Scholar]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary Reference Intakes: Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef]
- Khan, N.; Khan, M.I.R.; Asgher, M.; Fatma, M.; Masood, A.; Syeed, S. Salinity Tolerance in Plants: Revisiting the Role of Sulfur Metabolites. J. Plant Biochem. Physiol. 2014, 02, 1000120. [Google Scholar] [CrossRef] [Green Version]
- Korus, A.; Słupski, J.; Gebczyński, P.; Banaś, A. Effect of Preliminary Processing and Method of Preservation on the Content of Glucosinolates in Kale (Brassica oleracea L. Var. Acephala) Leaves. LWT—Food Sci. Technol. 2014, 59, 1003–1008. [Google Scholar] [CrossRef]
- Becker, M.; Asch, F. Iron Toxicity in Rice—Conditions and Management Concepts. J. Plant Nutr. Soil Sci. 2005, 168, 558–573. [Google Scholar] [CrossRef]
- Tewari, R.K.; Kumar, P.; Sharma, P.N. Morphology and Physiology of Zinc-Stressed Mulberry Plants. J. Plant Nutr. Soil Sci. 2008, 171, 286–294. [Google Scholar] [CrossRef]
- Ghasemi-Fasaei, R.; Ronaghi, A. Interaction of Iron with Copper, Zinc, and Manganese in Wheat as Affected by Iron and Manganese in a Calcareous Soil. J. Plant Nutr. 2008, 31, 839–848. [Google Scholar] [CrossRef]
- Adiloğlu, S. Relation of Chelated Iron (Eddha-Fe) Applications with Iron Accumulation and Some Plant Nutrient Elements in Basil (Ocimum basilicum L.). Polish J. Environ. Stud. 2021, 30, 3471–3479. [Google Scholar] [CrossRef]
- Rout, G.R.; Sahoo, S. Role of Iron in Plant Growth and Metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Marschner, P.; Crowley, D.; Rengel, Z. Rhizosphere Interactions between Microorganisms and Plants Govern Iron and Phosphorus Acquisition along the Root Axis—Model and Research Methods. Soil Biol. Biochem. 2011, 43, 883–894. [Google Scholar] [CrossRef]
- Chatterjee, C.; Gopal, R.; Dube, B.K. Impact of Iron Stress on Biomass, Yield, Metabolism and Quality of Potato (Solanum tuberosum L.). Sci. Hortic. 2006, 108, 1–6. [Google Scholar] [CrossRef]
- Adamski, J.M.; Peters, J.A.; Danieloski, R.; Bacarin, M.A. Excess Iron-Induced Changes in the Photosynthetic Characteristics of Sweet Potato. J. Plant Physiol. 2011, 168, 2056–2062. [Google Scholar] [CrossRef]
- Monteiro, H.P.; Winterbourn, C.C. The Superoxide-Dependent Transfer of Iron from Ferritin to Transferrin and Lactoferrin. Biochem. J. 1988, 256, 923. [Google Scholar] [CrossRef] [Green Version]
- Andre, C.M.; Evers, D.; Ziebel, J.; Guignard, C.; Hausman, J.F.; Bonierbale, M.; Zum Felde, T.; Burgos, G. In Vitro Bioaccessibility and Bioavailability of Iron from Potatoes with Varying Vitamin C, Carotenoid, and Phenolic Concentrations. J. Agric. Food Chem. 2015, 63, 9012–9021. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kader, A.A. Preharvest and Postharvest Factors Influencing Vitamin C Content of Horticultural Crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]




| Fe Concentration in Nutrient Solution (mg L−1) 1 | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 6 | 8 | 10 | 4 | 6 | 8 | 10 | 4 | 6 | 8 | 10 | 4 | 6 | 8 | 10 | ||||
| 14 DAT | 28 DAT | 42 DAT | 56 DAT | ||||||||||||||||
| S | 0 | −1 | −1 | −2 | S | 0 | 0 | −1 | −2 | S | 0 | 0 | 0 | 0 | S | 0 | −1 | −1 | −2 |
| Cu | −3 | −4 | −4 | −4 | Cu | −2 | −4 | −4 | −4 | Cu | −1 | −2 | −3 | −4 | Cu | −3 | −4 | −4 | −4 |
| Fe | +5 | +1 | +10 | +5 | Fe | +2 | +5 | +10 | +15 | Fe | +10 | +20 | +15 | +10 | Fe | +5 | +10 | +20 | +20 |
| Zn | −2 | −3 | −3 | −3 | Zn | 0 | −1 | −1 | −1 | Zn | 0 | 0 | −1 | −1 | Zn | 0 | −1 | −1 | −2 |
| Chl 2 | +1 | +2 | +4 | +4 | Chl | 0 | 0 | 0 | 0 | Chl | +1 | +2 | +3 | +4 | Chl | +2 | +3 | +3 | +1 |
| Car 3 | +1 | +2 | +4 | +4 | Car | 0 | 0 | 0 | 0 | Car | 0 | +1 | +2 | +3 | Car | +1 | +2 | +2 | +2 |
| AA 4 | 0 | 0 | −1 | −3 | AA | 0 | 0 | −1 | −1 | AA | 0 | 0 | 0 | 0 | AA | 0 | −1 | −2 | −3 |
| Qual. 5 | +2 | +3 | +9 | +1 | Qual. | 0 | 0 | +4 | +7 | Qual. | +10 | +21 | +16 | +12 | Qual. | +5 | +8 | +17 | +12 |
| Collard greens leaf quality 6 | |||||||||||||||||||
| Fe (mg L−1) | 4 | 6 | 8 | 10 | |||||||||||||||
| Grade | 4.3 | 8.0 | 11.5 | 8.0 | |||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercês, J.K.R.d.; Medelo, M.J.Y.; Cecílio Filho, A.B. Biofortification and Quality of Collard Greens as a Function of Iron Concentration in Nutrient Solution. Agronomy 2022, 12, 2493. https://doi.org/10.3390/agronomy12102493
Mercês JKRd, Medelo MJY, Cecílio Filho AB. Biofortification and Quality of Collard Greens as a Function of Iron Concentration in Nutrient Solution. Agronomy. 2022; 12(10):2493. https://doi.org/10.3390/agronomy12102493
Chicago/Turabian StyleMercês, Julia Karoline Rodrigues das, Maria José Yañez Medelo, and Arthur Bernardes Cecílio Filho. 2022. "Biofortification and Quality of Collard Greens as a Function of Iron Concentration in Nutrient Solution" Agronomy 12, no. 10: 2493. https://doi.org/10.3390/agronomy12102493
APA StyleMercês, J. K. R. d., Medelo, M. J. Y., & Cecílio Filho, A. B. (2022). Biofortification and Quality of Collard Greens as a Function of Iron Concentration in Nutrient Solution. Agronomy, 12(10), 2493. https://doi.org/10.3390/agronomy12102493








