Toxic Effects of Tetracycline and Its Removal by the Freshwater Microalga Chlorella pyrenoidosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Pre-Culture of Microalgae
2.3. Toxicity Assay
2.3.1. Experimental Design
2.3.2. Determination of Microalgal Growth
2.3.3. Determination of Photosynthetic Pigments
2.3.4. Determination of Chlorophyll Fluorescence
2.3.5. Assays of Oxidative Stress
2.4. Removal Kinetics and Mechanisms of TC by Microalgae
2.5. Instrumental Analysis of TC
2.6. Statistical Analysis
3. Results and Discussion
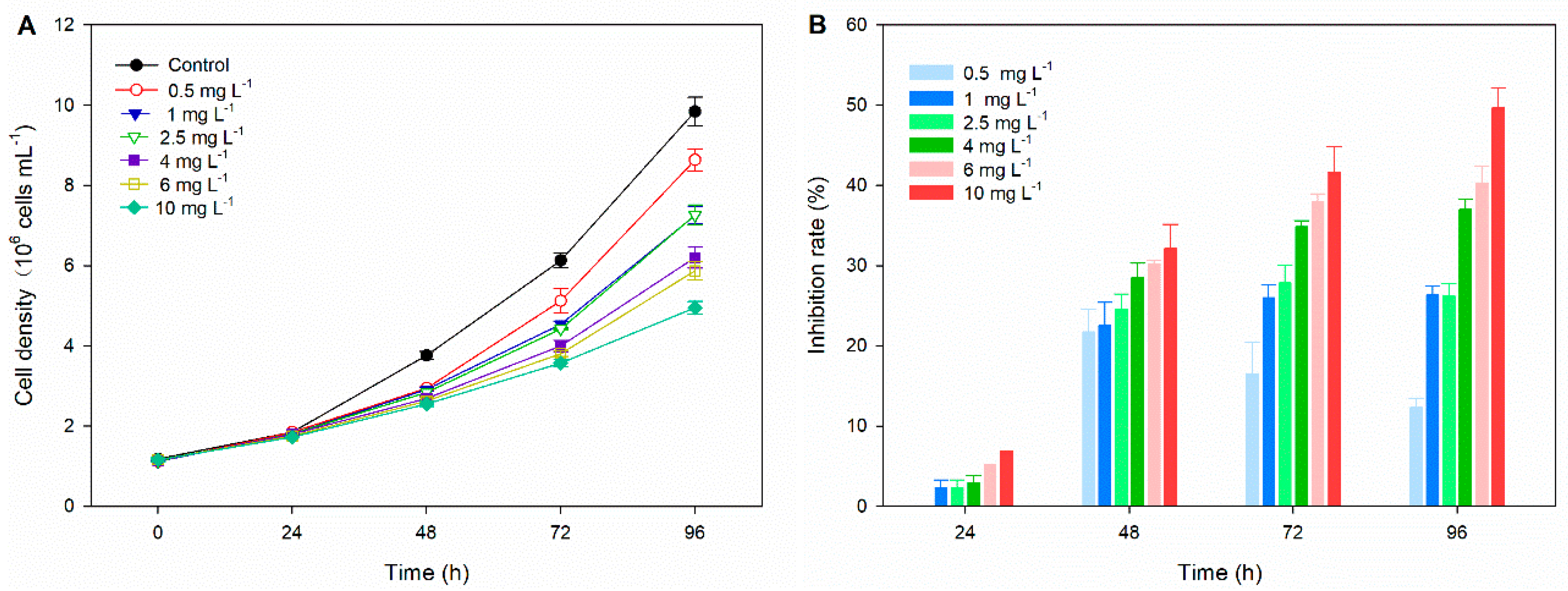
3.1. Microalgal Growth
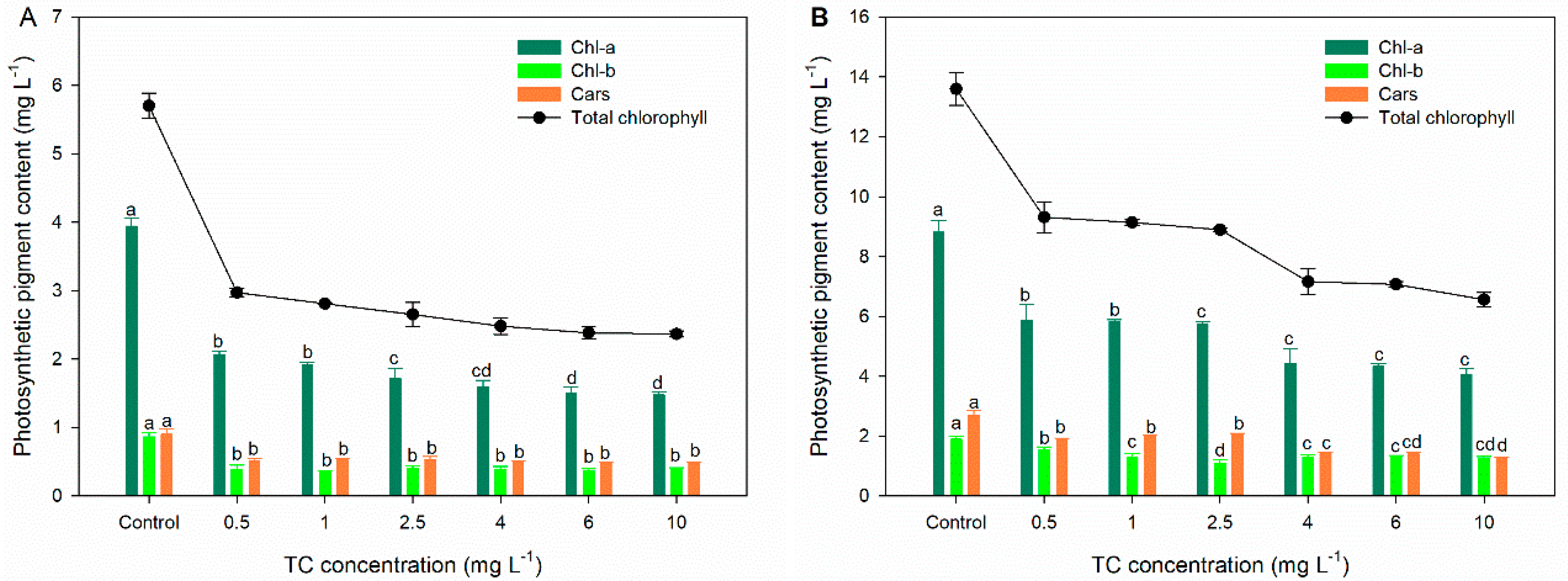
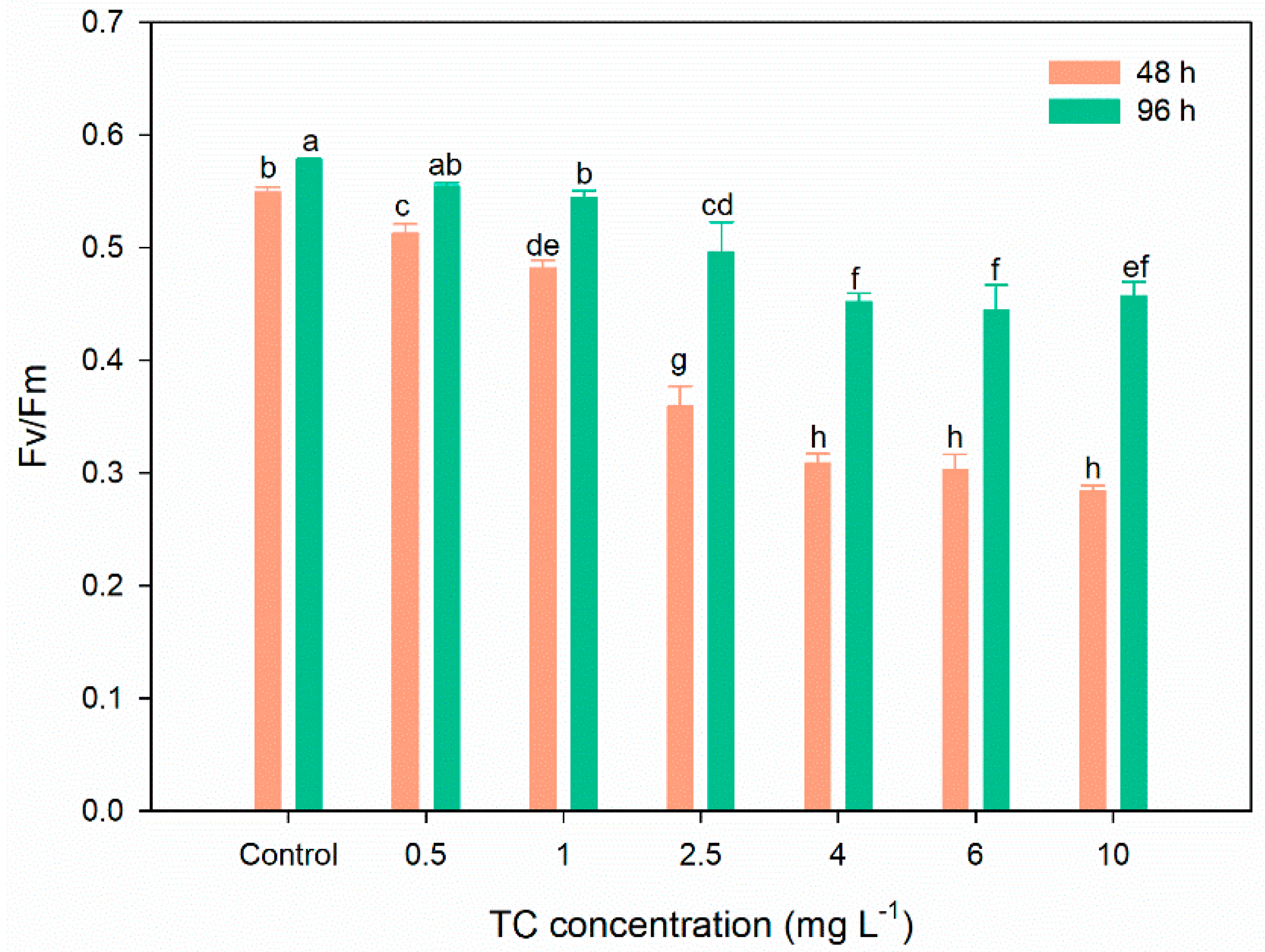
3.2. Photosynthetic Performance
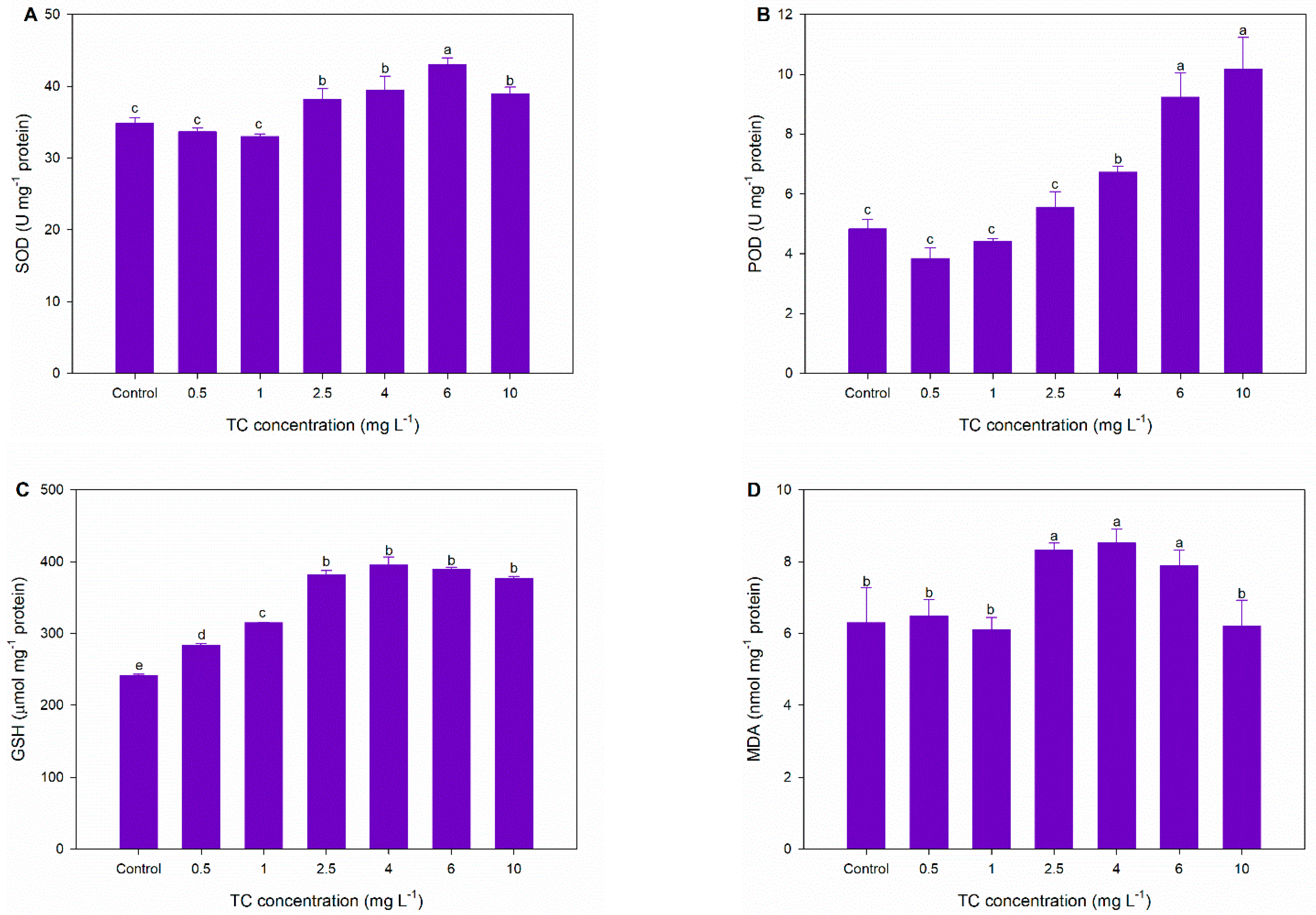
3.3. Antioxidant Responses
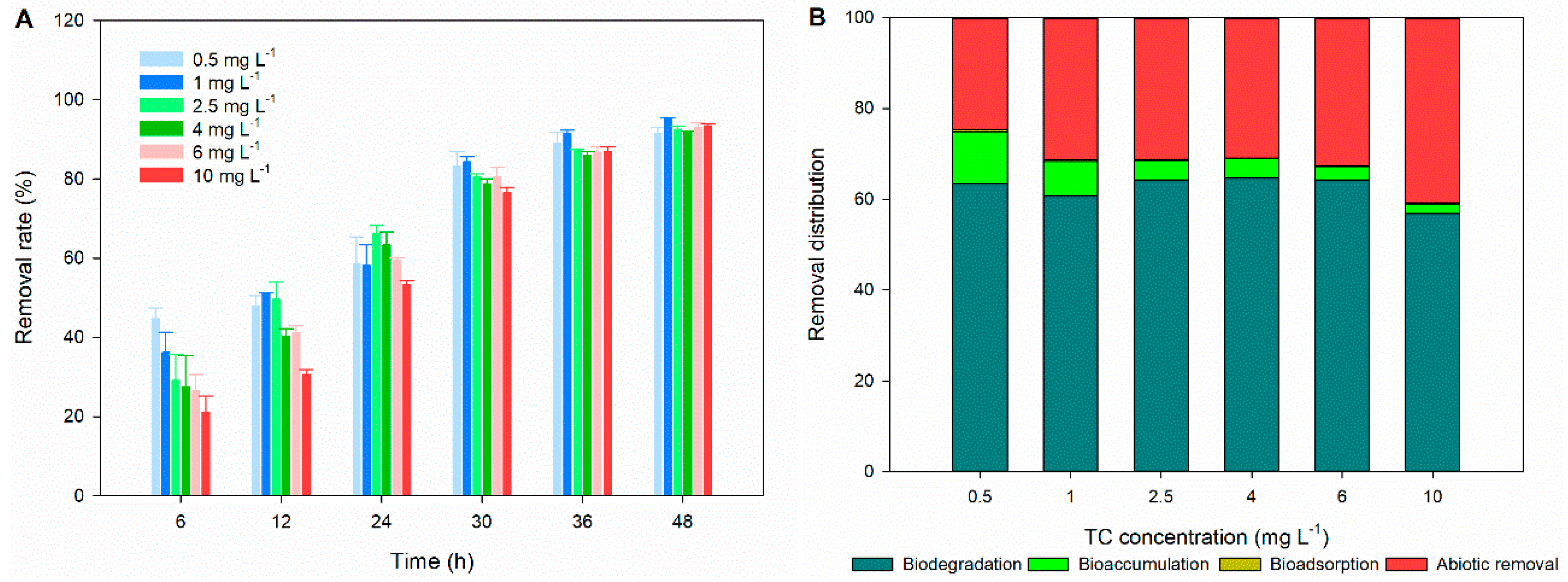
3.4. Removal Efficiency and Mechanism of TC
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the aquatic environment–a review–part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, P.; Xie, Z.; Wang, Z.; Hu, B. Effect of iron plaque on antibiotic uptake and metabolism in water spinach (Ipomoea aquatic Forsk.) grown in hydroponic culture. J. Hazard. Mater. 2021, 417, 125981. [Google Scholar] [CrossRef] [PubMed]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Lundström, S.V.; Östman, M.; Bengtsson-Palme, J.; Rutgersson, C.; Thoudal, M.; Sircar, T.; Blanck, H.; Eriksson, K.M.; Tysklind, M.; Flach, C.-F.; et al. Minimal selective concentrations of tetracycline in complex aquatic bacterial biofilms. Sci. Total Environ. 2016, 553, 587–595. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.; Liao, C.; Jiang, G. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci. Total Environ. 2021, 753, 141975. [Google Scholar] [CrossRef]
- Tang, J.; Shi, T.; Wu, X.; Cao, H.; Li, X.; Hua, R.; Tang, F.; Yue, Y. The occurrence and distribution of antibiotics in Lake Chaohu, China: Seasonal variation, potential source and risk assessment. Chemosphere 2015, 122, 154–161. [Google Scholar] [CrossRef]
- Xie, Z.; Tang, J.; Wu, X.; Li, X.; Hua, R. Bioconcentration, metabolism and the effects of tetracycline on multiple biomarkers in Chironomus riparius larvae. Sci. Total Environ. 2019, 649, 1590–1598. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, J.; Xin, Q. Effects of tetracycline on developmental toxicity and molecular responses in zebrafish (Danio rerio) embryos. Ecotoxicology 2015, 24, 707–719. [Google Scholar] [CrossRef]
- Kim, H.Y.; Asselman, J.; Jeong, T.Y.; Yu, S.; De Schamphelaere, K.A.C.; Kim, S.D. Multigenerational effects of the antibiotic tetracycline on transcriptional responses of Daphnia magna and its relationship to higher levels of biological organizations. Environ. Sci. Technol. 2017, 51, 12898–12907. [Google Scholar] [CrossRef]
- Zainab, S.M.; Junaid, M.; Xu, N.; Malik, R.N. Antibiotics and antibiotic resistant genes (ARGs) in groundwater: A global review on dissemination, sources, interactions, environmental and human health risks. Water Res. 2020, 187, 116455. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Hu, Y.; Cheng, J.; Chen, Y. Research progress on distribution, migration, transformation of antibiotics and antibiotic resistance genes (ARGs) in aquatic environment. Crit. Rev. Biotechnol. 2018, 38, 1195–1208. [Google Scholar] [CrossRef]
- Shao, S.; Wu, X. Microbial degradation of tetracycline in the aquatic environment: A review. Crit. Rev. Biotechnol. 2020, 40, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Leichtweis, J.; Vieira, Y.; Welter, N.; Silvestri, S.; Dotto, G.L.; Carissimi, E. A review of the occurrence, disposal, determination, toxicity and remediation technologies of the tetracycline antibiotic. Process. Safe. Environ. 2022, 160, 25–40. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Xu, J.; Xie, Z.; Tang, J.; Li, X.; Fan, S. Simultaneous carbonization, activation, and magnetization for producing tea waste biochar and its application in tetracycline removal from the aquatic environment. J. Environ. Chem. Eng. 2021, 9, 105324. [Google Scholar] [CrossRef]
- Mei, Y.; Xu, J.; Zhang, Y.; Li, B.; Xu, H. Effect of Fe–N modification on the properties of biochars and their adsorption behavior on tetracycline removal from aqueous solution. Bioresour. Technol. 2021, 325, 124732. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, L.; Li, J.; Rong, S.; Jiang, J.; Liu, S. Photocatalytic Degradation of tetracycline by a novel (CMC)/MIL-101 (Fe)/β-CDP composite hydrogel. Front. Chem. 2021, 8, 593730. [Google Scholar] [CrossRef]
- Wang, J.S.; Yi, X.H.; Xu, X.; Ji, H.; Alanazi, A.M.; Wang, C.C.; Zhao, C.; Kaneti, Y.V.; Wang, P.; Liu, W. Eliminating tetracycline antibiotics matrix via photoactivated sulfate radical-based advanced oxidation process over the immobilized MIL-88A: Batch and continuous experiments. Chem. Eng. J. 2022, 431, 133213. [Google Scholar] [CrossRef]
- Pan, L.; Cao, Y.; Zang, J.; Huang, Q.; Wang, L.; Zhang, Y.; Fan, S.; Tang, J.; Xie, Z. Preparation of iron-loaded granular activated carbon catalyst and its application in tetracycline antibiotic removal from aqueous solution. Int. J. Environ. Res. Public Health 2019, 16, 2270. [Google Scholar] [CrossRef] [Green Version]
- Jingrui, X.; Asraful Alam, M.; Jing, W.; Wenchao, W.; Norhana Balia Yusof, Z.; Daroch, M.; Zhang, D.; Lifen, L.; Russel, M. Enhanced removal of tetracycline from synthetic wastewater using an optimal ratio of co-culture of Desmodesmus sp. and Klebsiella pneumoniae. Bioresour. Technol. 2022, 351, 127056. [Google Scholar] [CrossRef]
- Xiong, Q.; Hu, L.X.; Liu, Y.S.; Zhao, J.L.; He, L.Y.; Ying, G. Microalgae-based technology for antibiotics removal: From mechanisms to application of innovational hybrid systems. Environ. Int. 2021, 155, 106594. [Google Scholar] [CrossRef] [PubMed]
- Russel, M.; Meixue, Q.; Alam, M.A.; Lifen, L.; Daroch, M.; Blaszczak-Boxe, C.; Kumar Gupta, G. Investigating the potentiality of Scenedesmus obliquus and Acinetobacter pittii partnership system and their effects on nutrients removal from synthetic domestic wastewater. Bioresour. Technol. 2020, 299, 122571. [Google Scholar] [CrossRef] [PubMed]
- Hena, S.; Gutierrez, L.; Croué, J.-P. Removal of pharmaceutical and personal care products (PPCPs) from wastewater using microalgae: A review. J. Hazard. Mater. 2021, 403, 124041. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Wei, L.; Xiong, Q.; Xu, S.; Li, W.; Lv, S.; Lu, Q.; Wan, L.; Wen, Z.; Zhou, W. Use of microalgae based technology for the removal of antibiotics from wastewater: A review. Chemosphere 2020, 238, 124680. [Google Scholar] [CrossRef] [PubMed]
- De Godos, I.; Muñoz, R.; Guieysse, B. Tetracycline removal during wastewater treatment in high-rate algal ponds. J. Hazard. Mater. 2012, 229–230, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Norvill, Z.N.; Toledo-Cervantes, A.; Blanco, S.; Shilton, A.; Guieysse, B.; Muñoz, R. Photodegradation and sorption govern tetracycline removal during wastewater treatment in algal ponds. Bioresour. Technol. 2017, 232, 35–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michelon, W.; Matthiensen, A.; Viancelli, A.; Fongaro, G.; Gressler, V.; Soares, H.M. Removal of veterinary antibiotics in swine wastewater using microalgae-based process. Environ. Res. 2022, 207, 112192. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Lyu, T.; Zhan, L.; Matamoros, V.; Angelidaki, I.; Cooper, M.; Pan, G. Mitigating antibiotic pollution using cyanobacteria: Removal efficiency, pathways and metabolism. Water Res. 2021, 190, 116735. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Xiao, Y.; Pan, H.; Mei, Y. Toxic effects of tetracycline and its degradation products on freshwater green algae. Ecotoxicol. Environ. Saf. 2019, 174, 43–47. [Google Scholar] [CrossRef]
- Yang, W.; Tang, Z.; Zhou, F.; Zhang, W.; Song, L. Toxicity studies of tetracycline on Microcystis aeruginosa and Selenastrum capricornutum. Environ. Toxicol. Pharmacol. 2013, 35, 320–324. [Google Scholar] [CrossRef]
- Mao, Y.; Yu, Y.; Ma, Z.; Li, H.; Yu, W.; Cao, L.; He, Q. Azithromycin induces dual effects on microalgae: Roles of photosynthetic damage and oxidative stress. Ecotoxicol. Environ. Saf. 2021, 222, 112496. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, J.; Tao, M.T.; Xu, C.M.; Chen, M. Quantitative characterization of toxicity interaction within antibiotic-heavy metal mixtures on Chlorella pyrenoidosa by a novel area-concentration ratio method. Sci. Total Environ. 2021, 762, 144180. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, K.; Li, W.; Zhang, M.; Li, P.; Han, J. Removal mechanisms of erythromycin by microalgae Chlorella pyrenoidosa and toxicity assessment during the treatment process. Sci. Total Environ. 2022, 848, 157777. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Liu, Y.S.; Hu, L.X.; Shi, Z.Q.; Cai, W.W.; He, L.Y.; Ying, G.G. Co-metabolism of sulfamethoxazole by a freshwater microalga Chlorella pyrenoidosa. Water Res. 2020, 175, 115656. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Zhang, X.; Zhou, T.; Lv, G.; Zhao, Q. Exploring kinetics, removal mechanism and possible transformation products of tigecycline by Chlorella pyrenoidosa. Sci. Total Environ. 2022, 817, 152988. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.T.; Hou, J.H.; Su, C.I.; Chen, C.L. Effects of chloramphenicol, florfenicol, and thiamphenicol on growth of algae Chlorella pyrenoidosa, Isochrysis galbana, and Tetraselmis chui. Ecotoxicol. Environ. Saf. 2009, 72, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Zhu, Y.; Wang, Y.; Zhao, Q.; Huang, H. Effects of three antibiotics on growth and antioxidant response of Chlorella pyrenoidosa and Anabaena cylindrica. Ecotoxicol. Environ. Saf. 2021, 211, 111954. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.; Jin, M.; Wang, H.; Xu, Q.; Xu, L.; Wang, C.; Du, S.; Liu, H. Effect of differently methyl-substituted ionic liquids on Scenedesmus obliquus growth, photosynthesis, respiration, and ultrastructure. Environ. Pollut. 2019, 250, 155–165. [Google Scholar] [CrossRef]
- Lee, S.H.; Xiong, J.Q.; Ru, S.; Patil, S.M.; Kurade, M.B.; Govindwar, S.P.; Oh, S.E.; Jeon, B.H. Toxicity of benzophenone-3 and its biodegradation in a freshwater microalga Scenedesmus obliquus. J. Hazard. Mater. 2020, 389, 122149. [Google Scholar] [CrossRef]
- Zang, J.; Wu, T.; Song, H.; Zhou, N.; Fan, S.; Xie, Z.; Tang, J. Removal of tetracycline by hydrous ferric oxide: Adsorption kinetics, isotherms, and mechanism. Int. J. Environ. Res. Public Health 2019, 16, 4580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, L.; Wu, Y.; Ding, H.; Zhang, W. Toxicity, biodegradation, and metabolic fate of organophosphorus pesticide trichlorfon on the freshwater algae Chlamydomonas reinhardtii. J. Agric. Food Chem. 2020, 68, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Lin, K.; Yang, B.; Yang, M.; Li, J. Toxic effects and metabolic fate of carbamazepine in diatom Navicula sp. as influenced by humic acid and nitrogen species. J. Hazard. Mater. 2019, 378, 120763. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, W.; Li, J.; Yuan, M.; Zhang, J.; Xu, F.; Xu, H.; Zheng, X.; Wang, L. Ecotoxicological effects of sulfonamides and fluoroquinolones and their removal by a green alga (Chlorella vulgaris) and a cyanobacterium (Chrysosporum ovalisporum). Environ. Pollut. 2020, 263, 114554. [Google Scholar] [CrossRef] [PubMed]
- Cupellini, L.; Calvani, D.; Jacquemin, D.; Mennucci, B. Charge transfer from the carotenoid can quench chlorophyll excitation in antenna complexes of plants. Nat. Commun. 2020, 11, 662. [Google Scholar] [CrossRef] [Green Version]
- Wan, L.; Wu, Y.; Zhang, B.; Yang, W.; Ding, H.; Zhang, W. Effects of moxifloxacin and gatifloxacin stress on growth, photosynthesis, antioxidant responses, and microcystin release in Microcystis aeruginosa. J. Hazard. Mater. 2021, 409, 124518. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, X.; Gan, Y.; Cheng, H.; Fan, S.; Li, X.; Tang, J. Ecotoxicological effects of the antidepressant fluoxetine and its removal by the typical freshwater microalgae Chlorella pyrenoidosa. Ecotoxicol. Environ. Saf. 2022, 244, 114045. [Google Scholar] [CrossRef]
- Yang, M.; Fan, Z.; Xie, Y.; Fang, L.; Wang, X.; Yuan, Y.; Li, R. Transcriptome analysis of the effect of bisphenol A exposure on the growth, photosynthetic activity and risk of microcystin-LR release by Microcystis aeruginosa. J. Hazard. Mater. 2020, 397, 122746. [Google Scholar] [CrossRef]
- Wang, H.; Jin, M.; Mao, W.; Chen, C.; Fu, L.; Li, Z.; Du, S.; Liu, H. Photosynthetic toxicity of non-steroidal anti-inflammatory drugs (NSAIDs) on green algae Scenedesmus obliquus. Sci. Total Environ. 2020, 707, 136176. [Google Scholar] [CrossRef]
- Li, S.; Yu, Y.; Gao, X.; Yin, Z.; Bao, J.; Li, Z.; Chu, R.; Hu, D.; Zhang, J.; Zhu, L. Evaluation of growth and biochemical responses of freshwater microalgae Chlorella vulgaris due to exposure and uptake of sulfonamides and copper. Bioresour. Technol. 2021, 342, 126064. [Google Scholar] [CrossRef]
- Xie, Z.; Luan, H.; Zhang, Y.; Wang, M.; Cao, D.; Yang, J.; Tang, J.; Fan, S.; Wu, X.; Hua, R. Interactive effects of diclofenac and copper on bioconcentration and multiple biomarkers in crucian carp (Carassius auratus). Chemosphere 2020, 242, 125141. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Gan, Y.; Tang, J.; Fan, S.; Wu, X.; Li, X.; Cheng, H.; Tang, J. Combined effects of environmentally relevant concentrations of diclofenac and cadmium on Chironomus riparius larvae. Ecotoxicol. Environ. Saf. 2020, 202, 110906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wan, J.; Li, Z.; Wu, Z.; Dang, C.; Fu, J. Enhanced removal efficiency of sulfamethoxazole by acclimated microalgae: Tolerant mechanism, and transformation products and pathways. Bioresour. Technol. 2022, 347, 126461. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Wei, Y.; Sun, J.; Wang, J.; Zhao, Z.; Liu, Y.; Li, X.; Cao, J. Degradation of cefradine in alga-containing water environment: A mechanism and kinetic study. Environ. Sci. Pollut. Res. 2019, 26, 9184–9192. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dou, X.; Wu, J.; Meng, F. Attenuation pathways of erythromycin and biochemical responses related to algal growth and lipid synthesis in a microalga-effluent system. Environ. Res. 2021, 195, 110873. [Google Scholar] [CrossRef] [PubMed]
- Pala-Ozkok, I.; Ubay-Cokgor, E.; Jonas, D.; Orhon, D. Kinetic and microbial response of activated sludge community to acute and chronic exposure to tetracycline. J. Hazard. Mater. 2019, 367, 418–426. [Google Scholar] [CrossRef]
- Kiki, C.; Rashid, A.; Wang, Y.; Li, Y.; Zeng, Q.; Yu, C.P.; Sun, Q. Dissipation of antibiotics by microalgae: Kinetics, identification of transformation products and pathways. J. Hazard. Mater. 2020, 387, 121985. [Google Scholar] [CrossRef]
| TC Concentration (mg L−1) | Pseudo-Zero Order Model | Pseudo-First Order Model | Pseudo-Second Order Model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| k a (h−1) | T1/2 b (h) | R2 c | k (h−1) | T1/2 (h) | R2 | k (h−1) | T1/2 (h) | R2 | |
| 0.5 | 0.012 | 21.4 | 0.937 | 0.054 | 12.8 | 0.977 | 0.38 | 5.21 | 0.921 |
| 1 | 0.024 | 20.8 | 0.951 | 0.061 | 11.3 | 0.978 | 0.29 | 3.41 | 0.847 |
| 2.5 | 0.059 | 21.3 | 0.957 | 0.054 | 12.8 | 0.996 | 0.078 | 5.14 | 0.901 |
| 4 | 0.092 | 21.8 | 0.969 | 0.051 | 13.5 | 0.994 | 0.044 | 5.67 | 0.894 |
| 6 | 0.14 | 21.7 | 0.972 | 0.053 | 13.0 | 0.988 | 0.034 | 4.98 | 0.861 |
| 10 | 0.22 | 22.5 | 0.975 | 0.052 | 13.4 | 0.979 | 0.020 | 4.95 | 0.817 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Yang, J.; Zhao, S.; Wang, X.; Xie, Z. Toxic Effects of Tetracycline and Its Removal by the Freshwater Microalga Chlorella pyrenoidosa. Agronomy 2022, 12, 2497. https://doi.org/10.3390/agronomy12102497
Tang J, Yang J, Zhao S, Wang X, Xie Z. Toxic Effects of Tetracycline and Its Removal by the Freshwater Microalga Chlorella pyrenoidosa. Agronomy. 2022; 12(10):2497. https://doi.org/10.3390/agronomy12102497
Chicago/Turabian StyleTang, Jun, Jun Yang, Sirui Zhao, Xiaoyu Wang, and Zhengxin Xie. 2022. "Toxic Effects of Tetracycline and Its Removal by the Freshwater Microalga Chlorella pyrenoidosa" Agronomy 12, no. 10: 2497. https://doi.org/10.3390/agronomy12102497




