Abstract
Two-line hybrid rice production requires environmental genetic male-sterile (EGMS) lines that become sterile in certain environmental conditions. In this study, we aimed to identify the genetic type(s), sterility thresholds, optimum planting date, and efficient seeding density of eight male-sterile lines, including four lines developed at the University of Arkansas, Rice Research and Extension Center (RREC) and four at the Dale Bumpers National Rice Research Center (DBNRRC) both located in Stuttgart, AR, USA. The lines were screened in growth chambers using different temperatures and day lengths to identify the type of EGMS line and sterility thresholds. A single location was used in testing the first year of the study and three locations used in the second year of the planting date study. All experiments were arranged in randomized blocks with three replications to identify the optimum planting date and seeding rate. Three planting dates were tested each year for the planting date experiment, and three seeding densities were used in the seed density study. The growth chamber study showed that all RREC lines were thermosensitive genic male-sterile lines, and the sterility threshold temperature for one of these lines was >32.2 °C while that of others was >29.4 °C. The type of sterility and threshold of DBNRRC lines were inconclusive due to their genetic heterogenicity and environmental response inconsistency. In Arkansas, April 25 was the optimum planting date for sterile conditions, and a 431 seed/m2 seeding rate was required to produce an adequate number of panicles for hybrid seed production while avoiding wasting expensive male-sterile seeds.
1. Introduction
Hybrid rice (Oryza sativa) production has expanded worldwide due to its 15% to 20% greater yield than pure-line cultivars. Hybrid rice is the F1 seed derived from crossing two genetically diverse inbred parents, resulting in greater performance due to heterosis [1,2]. Large-scale hybrid seed production is possible using sterile male lines developed to serve as the female parents. Two types of male sterility are utilized for F1 hybrid rice production, with the first one known as environmental genetic male sterility (EGMS), in which the sources of sterility are several genes and specific environmental conditions regulate their expression. The second type is cytoplasmic male sterility (CMS), which results from specific nuclear and mitochondrial interactions. EGMS is used in a two-line hybrid production system, whereas CMS is used in a three-line hybrid rice production system [2,3]. This study focuses on male-sterile lines used in the two-line production system.
In the two-line system, pollen sterility is induced by environmental conditions such as photoperiod, temperature, or both, affecting sterility genes’ expression in EGMS lines. EGMS lines are classified into five categories according to which sterility gene(s) they possess: thermosensitive genic male sterile (TGMS), reverse thermosensitive genic male sterile (rTGMS), photoperiod-sensitive genic male sterile (PGMS), reverse photoperiod-sensitive genic male sterile (rPGMS), and photo-thermosensitive genic male sterile (PTGMS). TGMS, PGMS, and PTGMS lines are predominantly used for hybrid rice production. The threshold for sterility in most TGMS lines is >30 °C for daytime (dt) and >24 °C for nighttime (nt). One of the first developed TGMS line, “5460S”, was released in 1990 [4]. The sterility threshold for rTGMS is <24 °C (dt) and <16 °C (nt). Most PGMS lines have a threshold of day-length >13.75 h. One of the first developed PGMS line, “Nongken 58S”, was released in 1990 [5]. PTGMS lines are more sensitive to temperature than photoperiod when daytime temperatures are <30 °C or nighttime temperatures are <24 °C. Thus, the photoperiod has no effect. However, when the temperature is within the range of 24 °C–30 °C, the photoperiod affects the sterility of the PTGMS lines, inducing sterility when the photoperiod is 14 h or longer in comparison with inducing fertility when the photoperiod is 13 h [6].
EGMS lines are susceptible to sudden temperature change during the R2 growth stage, when meiosis occurs, which causes pollen to become partially or fully fertile [3]. This poses a serious problem for hybrid seed production because the seed produced from the male-sterile line could be from self-pollination, affecting the purity of the hybrid seed. Therefore, identifying thresholds for male sterility is important in hybrid rice production. This study aims to identify the nature (type of gene(s) possessed) and environmental thresholds of EGMS male sterility in four Arkansas male-sterile lines and four USDA male-sterile lines.
Four EGMS lines of 236 s, 801 s, 805 s, and 811 s were developed at the University of Arkansas for hybrid rice production [7]. These lines were developed by crossing genetically distant parental lines from a subset or core collection [8]. Prior to the University of Arkansas research initiative, scientists at Dale Bumper National Rice Research Center, USDA-ARS at Stuttgart, AR conducted a study to develop new sources of male sterility conferred by a dominant sterility gene using gamma-irradiation in 1993 and 1994 [9]. As a result, four male-sterile mutant lines were developed from the following cultivars: “Kaybonnet” (GSOR 1), “Orion” (GSOR 2), “Cypress” (GSOR 3), and “LaGrue” (GSOR 4). The results from the study determined that GSORs 1, 2, and 4 possessed dominant male-sterility genes, and GSOR 3 possessed recessive male-sterility genes [9].
In hybrid rice production via a two-line system, it is important to determine the optimum planting date so that the male-sterile plant meets the environment required for sterility at its critical reproductive stage. The most critical time for inducing sterility and fertility occurs in the reproductive stages: R2 [3]. The weather could cause the ms plants to become fertile during hybrid rice seed production. Late-formed tillers (shoots that develop from the leaf axils at each unelongated node of the main shoots and other tillers [10]) that encounter low temperatures during the R2 growth stage could become partially fertile. The seeding rate can be increased to reduce late-formed tillers in male-sterile plants. This increases plant density allowing for fewer tiller formations. Testing different seeding rates will reveal the effectiveness of reducing late-formed tillers and maximizing effective tillering (tillers with exerted, sterile panicles).
Our objectives in this study are to identify the genetic type(s), sterility thresholds, optimum planting date, and efficient seeding density of eight male-sterile lines, including four lines developed at the University of Arkansas, Rice Research and Extension Center (RREC) and four at the Dale Bumpers National Rice Research Center (DBNRRC) both located in Stuttgart, Arkansas, USA. Rice breeders can use the results of this study for EGMS line development, geneticists for further genetic analysis of sterile gene(s), and agronomists and seed producers to produce hybrid seeds in Arkansas rice lands at optimum conditions.
2. Materials and Methods
2.1. Seed Materials
The seeds of the 811 s, 236 s, 801 s, and 805 s inbred lines were received through the seed source of the RREC hybrid breeding program. The homogeneity of each line was ensured through a molecular study and the evaluation of several phenotypic characteristics such as heading date (when 50% of panicles have partially exerted from plant sheath), plant height, and percentage of sterility that were recorded. Homozygous plants were identified, ratooned, and placed at a lower temperature (21 °C) and shorter day-length (<12 h) for seed production. Seeds from the GSOR 1, 2, 3, and 4 experimental lines were obtained from the germplasm collection at Dale Bumper’s National Rice Research Center, USDA-ARS Stuttgart, AR, USA [2].
2.2. Growth Chamber Study
To address objectives one and two of this study, a randomized complete block design (RCBD) with three replications and 16 treatments, including 4 different daylight of 12.5 h, 13 h, 13.5 h, and 14 h, and 4 sets of dt/nt temperatures of 23.9 °C/21.1 °C, 26.7 °C/23.9 °C, 29.4 °C/26.7 °C, and 32.2 °C/29.4 °C, was conducted in growth chamber condition to determine the type of EGMS sterility and identify the environmental threshold sterility on eight ms lines, 236 s, 811 s, 805 s, 811 s, GSOR1, GSOR2, GSOR 3, and GSOR4 [2]. This study followed a method described by Lee et al. [11]. In each replication, four plants from each sterile line were grown in a 3.8 L plastic pot with Baccto potting soil and kept in the greenhouse condition where the temperature was 31.1 °C high and 22.2 °C low with day-length at 13 h using high-pressure sodium light bulbs [2] (Table S1). The plant growth stages were monitored according to the development described by Moldenhauer et al. [12]. Watering and fertilizer applications were implemented according to Arkansas’s standard rice growth recommendations, including a flood established at the V4–V5 growth stage and 330 kg/ha of nitrogen at the time of flooding [12]. Prior to the R1 stage, plants from each line were transferred to each G.C. The R1 was determined by panicle differentiation of the main tiller in which internode length (the panicle is forming inside the plant and is being pushed upwards as it grows) reached ½ to ¾ inches (1.3 cm to 1.9 cm) [12]. The plant growth stages were monitored daily. In this study, four Conviron A1000 GCs located in RREC were used [2].
To identify the level of sterility in the tested plants, pollen was collected from anthers and suspended with a 1% iodine-potassium iodide solution (KI-I2) [11]. Three panicles from each plant were randomly selected approximately 90 days after emergence. Each panicle was sampled once it was 25% exerted from the main stem and the time for sampling was prior to flowering (before 9:00 AM) to ensure other pollen would not contaminate the samples. Results from 24 panicles were considered adequate to represent the response of each line to each environment. Of each selected panicle, six anthers were harvested from three spikelets (singly selected at the top, middle, and bottom of the panicle branch) for pollen stain testing. The three spikelets were pooled to represent each panicle sample [2]. The average percentage of sterile pollen was determined by counting the amount of sterile pollen in relation to the total pollen observed. Sterility levels were assessed based on pollen appearance observed with a 10× microscope magnification and classified according to Virmani et al. [6]. The results of each panicle were pooled to represent the average pollen sterility percentage for each plant. In this classification, plants possessing >99% sterile pollen were categorized as completely sterile (CS), <99%–>91% sterile pollen was categorized as sterile (S), <90%–>71% sterile pollen was categorized as partial sterile (PS), <70%–>31% sterile pollen was categorized as partially fertile, <30%–>21% was categorized as fertile, and <20%–>0% was categorized at fully fertile [6]. Since the results of this study will be used for the hybrid breeding program and producing hybrid seeds, plants with <90% sterility were categorized here as fertile because anything less than the sterile category (<90%) would result in too much self-pollinated (selfed) seed. There was a low threshold for allowable self-pollinated seed in the hybrid seed. A mixture of self-pollinated and hybrid seeds led to a lower production, somewhat proportional to the percentage of selfed seeds [2].
2.3. Field Studies
2.3.1. Determination of Optimal Planting Date for the Male-Sterile Lines
A two-year study was conducted to identify the optimum planting date for sterile lines under field conditions (objective three). For the first year, 2017, we conducted a single location study in RREC. Male-sterile lines 801 s, 811 s, and 236 s were tested in an RCBD with three replications and three planting dates of April 25 and May 02 and 11 (805 s was excluded due to limited seed availability). The male-sterile line 801 s was planted only on the third date. The plots were composed of seven rows 2.1 m long and spaced 0.2 m apart. Approximately 200 seeds of each male-sterile line were sown in each plot using a 7-row Almaco heavy-duty grain drill. Planting, flood establishment at V4–V5, and 330 kg/ha of nitrogen were implemented according to Arkansas’s standard rice growth recommendations. Air temperatures were recorded every hour to observe any temperatures above or below the sterility thresholds of each EMGS line. Thresholds were based on the results from the growth chamber study [2]. Temperatures were recorded by a weather station deployed by the University of Arkansas Cooperative Extension Service at the RREC within 400 m proximity [13]. Ten bordered plants from each plot were randomly selected to avoid contamination of nearby foreign pollen; panicles from the main stem, first tiller, and second tiller were collected, and pollen was collected from three spikelets (singly selected from the top, middle, and bottom of the panicle branches) for testing via pollen staining as previously mentioned in the lab within 1 h of sampling in the field. By the end of the season, 1890 panicles were collected. Each plot was evaluated on the average number of sterile panicles observed using the pollen staining procedure, which was assessed immediately after collecting.
For the second year, 2018, four male-sterile lines 236 s, 801 s, 805 s, and 811 s were tested in an RCBD with three replications and three locations in Arkansas: Rice Research and Extension RREC, Southeast Research and Extension Center Research Station (SREC hereafter) (33°47’51” N 91°16’53” W) in Rohwer, Arkansas, and Pine Tree Research Station (PTRS) (35°07’39” N 90°57’50” W) located near Colt, Arkansas. The three planting dates tested were May 16, 23, and 27 at the three locations. Since the GSOR lines expressed a high level of fertility in the growth chamber and the first year of the field study, these lines were excluded from the second field study. A total of 50 seeds were hand-planted for each plot (108 plots in total) with a dimension of 1.5 m × 1.5 m. There were five rows with spacing at 30.5 cm and spacing between seeds at 15 cm. As previously discussed, management was implemented according to Arkansas’s standard rice growth recommendations. A HOBO MX2302 external temperature/R.H. sensor data logger (Onset, location of company) was deployed in the plots at each location to provide more accurate temperatures during growth [14]. In addition, the HOBOs were used with an RS3-B solar radiation shield to ensure accurate ambient temperature readings [15]. Each HOBO was programmed to read every 5 min daily. Ten plants from each plot were randomly selected; three panicles from each plant, including one from each main stem, first tiller, and second tiller, were collected and tested via pollen staining. Each plot was evaluated on the average number of sterile panicles observed using the pollen staining. At the end of the season, 3240 panicles were collected.
2.3.2. Determination of Optimal Seed Density for the Male-Sterile Lines
A two-year study was performed to determine the optimum seeding rate to maximize the number of sterile panicles for hybrid seed production. For the first year, 2018, we conducted an RCBD with three replications and three seeding rates of 216, 431, and 861 seeds/m2 using male-sterile line 811 s at the RREC (Table S2A–C). The late planting date was 20 July to increase the chance of having conditions suitable for inducing fertility of the late tillers. Based on the data from the first year, the second-year field study, in 2020, was conducted as a RCBD with three replications and four seeding rates: 216, 324, 431, and 648 seeds/m2 at the RREC (Table S2D–E). The planting date for the second seeding rate experiment was July 13 to increase the possibility of the fertility of late tillers. The number of seeds per area was determined using a formula described by Runsick et al. [16]. The field management was implemented according to Arkansas’s standard rice growth recommendations. The plots were flooded when plants were at the V4–V5 stages. A total of 330 kg/ha of nitrogen was implemented. Each plot had seven rows with 20.3 cm row spacing. At the end of the first-year planting season, when the plants were matured and ready to be harvested, plants from rows 3 and 5 of each plot were harvested separately, and the tiller from each plant was counted. However, plants from three center rows of 3, 4, and 5 were selected for the tiller number evaluation in the second year. Tillers from the selected rows were counted and classified into three categories: tillers that produced panicles with seed (fertile panicles), panicles without seed (sterile panicles), and tillers with no panicles. In the fertile category, panicles with seeds indicated fertile pollen in spikelets on panicles developing during temperatures below the sterile threshold. Part of the test was to look at the potential of pollen fertility due to late tiller production during cooler temperatures. All tillers with panicles producing seed were grouped and counted. These panicles had a very low amount of seed (<1 g). However, they were still categorized as panicles with seed because any self-fertilized panicles would increase the impurity of hybrid rice seed production.
2.4. Statistical Analysis
The mean for each line was calculated from the average value of three replications in an Excel® file. A statistical analysis was performed using JMP Pro 14 software [17] for an ANOVA analysis using Fit Y by X and Fit Model-Full Factorial followed by Student’s t-test to evaluate the significance and interaction between different treatments.
3. Results
3.1. Identification of Type and Environmental Threshold for Sterility in the Male-Sterile Lines
To address objectives one and two, we conducted a growth chamber study at the RREC in 2017. Lines 801 s, 805 s, and 811 s expressed similar results among the environments (Table 1). Pollen fertility was maintained in environments 1, 2, 5, 6, 9, 10, 13, and 14, while pollen sterility was induced under environments 3, 4, 7, 8, 11, 12, 15, and 16. These results revealed that the sterility did not depend on the photoperiod, but the temperature changed the fertility/sterility rate. These results indicated that 801 s, 805 s, and 811 s were TGMS with a daytime temperature of >29.4 °C and a nighttime temperature of >26.7 °C.

Table 1.
Growth chamber results in 2017 (S—sterile) (F—fertile) of 16 environments.
Line 236 s expressed a higher temperature threshold for inducing pollen sterility. Pollen fertility was maintained in environments 1–3, 5–7, 9–11, and 13–15, whereas pollen sterility was induced under environments 4, 8, 12, and 16. These results revealed that the sterility did not depend on the photoperiod, but the fertility/sterility rate changed due to the temperature. As was the case for the previous lines, 236 s was revealed to be TGMS, but with a higher daytime temperature threshold of >32.2 °C and nighttime temperature >29.4 °C. The GSOR lines expressed a high percentage of pollen fertility in all photothermal environments. Overall, 89% of the GSOR plants were completely male fertile. Therefore, based on different thresholds for sterility/fertility, it can be assumed that there were two different TGMS in this study, one found in 811, 805 s, and 801 s, and the other was detected in the 236 s male sterile line.
3.2. Planting Date Study
We conducted a one-location and multilocation field study for 2 years to address the third objective. In 2017, the single location study revealed that TGMS line 236 s expressed partial sterility among different planting dates (Figure 1). The percentage of sterility from the first, second, and third planting dates was 87%, 53%, and 60%, respectively. In addition, nearly all of the critical sterility-inducing days (R1 to heading date) for planting dates 2 and 3 were below the threshold for sterility, thus causing partial fertility among the plants. The evaluation suggested that a critical growth stage for inducing pollen sterility was near R1, approximately 25 days before heading. According to Virmani et al. [3], the critical timing for inducing sterility varies from 15 to 25 days before heading or 5 to 15 days after panicle initiation (R0), which corresponds with the results. This finding was supported by a previous publication from Moldenhauer et al. [12], showing how the number of potential grains per panicle was greatly affected by the environment at the R1 stage. These findings indicated that the most critical timing for inducing sterility was R1 + 10 days after, near the R2 stage.
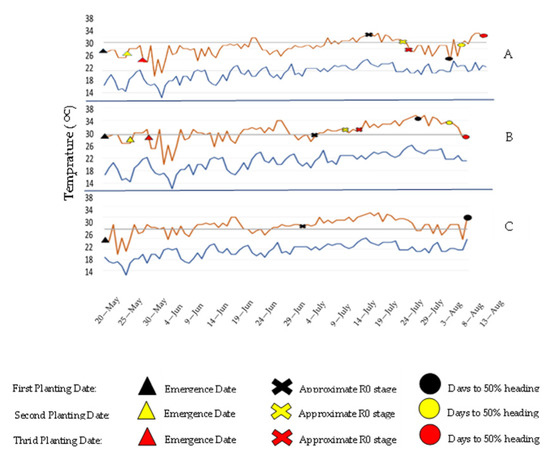
Figure 1.
High and low temperatures during TGMS lines 236s (A), 811s (B), and 801s (C) growth in 2017. Temperatures provided by weather stations for Arkansas; black, yellow, and red colors represent first, second, and third planting dates; X, triangle, and circle shapes represent different plant growth stages, including planting dates, R1 stage, and 50% date of heading, respectively.
Line 801 s expressed nearly complete sterility, with 93% of the 30 plants testing as completely sterile. All of the critical sterility-inducing days (R1 + 10 days) were above the temperature threshold, while ten days before heading, the temperatures were below the threshold (Figure 1). Thus, there was little to no effect on inducing sterility ten days before heading. The results suggested that the planting date used (May 10) was the optimum date to avoid a fertile environment.
Line 811 s expressed complete sterility under all three planting dates. All critical sterility-inducing days were above the threshold. The evaluation suggested that all daytime temperatures were above its critical threshold of 29.4 °C. Thus, all three planting dates were acceptable to meet sterile conditions. The nighttime temperature threshold might have been lower than the growth chamber study suggested (26.7 °C), or it had less effect on sterility than daytime temperatures. The GSOR lines were mostly fertile under the conditions of the 2017 trial. However, due to the low amount of seed and the high rate of fertile pollen results, the GSOR lines were removed for further evaluation (Figure 1).
The results showed that the 50% date of heading dates varied among 236 s, 801 s, and 811 s. The results showed that 801 s and 805 s reached 50% heading at 75 days, whereas 236 s took 90 days. It is important to note that these lines’ growth development was different and could reach critical sterility timing at different dates. Therefore, heading dates should be noted on ms lines to determine planting dates for reaching sterility thresholds.
The multilocation study in 2018 revealed that all four Arkansas TGMS lines, 236 s, 801 s, 805 s, and 811 s, expressed sterility at the three locations for all three planting dates. In addition, the mean daytime high temperature of 37.1 °C during the critical stages for inducing sterility for all three planting dates at the three locations was above the sterility threshold of the four TGMS lines (Figure 2).
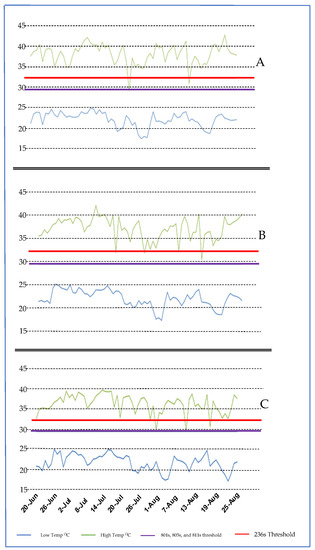
Figure 2.
Temperatures in three locations in 2018 of (A) PTRS, (B) RREC, and (C) SREC; red line, temperature threshold for inducing sterility in 236 s.
At PTRS, 95% of the days during critical stages were above both sterility thresholds for all EGMS lines (Figure 2). PTRS had the lowest daytime temperatures with a mean of 36.4 °C. Even though 3 days were below the thresholds, these temperatures for these days did not affect sterility. Thus, more time must be required below threshold temperatures to maintain fertility.
At the RREC, 94% of the days during critical stages were above both sterility thresholds, and 100% were above the 801 s, 805 s, and 811 s sterility thresholds (Figure 2). RREC had the second-lowest day temperatures with a mean maximum daily temperature of 37.1 °C. There were 4 days below the sterility thresholds. At SREC, 97% of the days during critical stages were above both sterility thresholds, and 98% were above 801 s, 805 s, and 811 s sterility thresholds (Figure 2). SREC had the least low day temperatures with a mean of 37.9 °C. There were only 2 days below the sterility thresholds.
The result revealed a significant difference (p > 2.8 × 10−3) in daytime temperatures among the locations. The mean daytime temperature in these three locations, from the highest to the lowest, was SREC>RREC>PTRS. Student’s t-test showed that the daytime temperatures in SREC were significantly higher than in PTRS. While there were no statistical differences in RREC and two other locations, according to the mean temperature, the RREC could be considered the intermediate environment of the three locations. These three locations cover much rice production acreage in Arkansas, indicating that maintaining male-sterile inbred rice is possible.
Low nighttime temperatures were recorded, but as in the case of the previous field study in the first year of study, it did not have a likely effect on inhibiting pollen sterility. In addition, low nighttime temperatures did not vary greatly across the three Arkansas test locations and did not affect sterility thresholds as strongly as daytime high temperatures.
3.3. Planting Density Test
In 2018, the first year of study, only 811 s were tested; however, in year 2 (2020) 811 s and 801 s were analyzed (Table S2F). The results from the first year of the study showed a significant difference between the seeding rate and the number of sterile panicles (p-value < 0.0250) and total panicles/tillers (p-value < 0.0139). In addition, Student’s t-test showed that 431 seeds/m2 significantly improved the number of sterile panicles compared to two other seeding rates. However, Student’s t-test seeding rate showed that 861 seeds/m2 significantly improved the total tiller with panicle compared to 216 seeds/m2. A further analysis showed no significant differences between these three seed rates, the number of tillers with no panicles, the number of fertile panicles, and the total number of tillers. Moreover, the data showed that the number of tillers with no panicles in 861 seeds/m2 was less than two other seed rates (Table 2).

Table 2.
Evaluating different levels of male-sterile seed rates on the traits associated with panicles and tillers from year 2018.
Male-sterile seeds are costly because of the cost and efforts to produce TGMS. Thus, the optimum seed rate was the lowest, producing a statistically adequate number of sterile panicles for hybrid seed production. These results recommended a seeding rate of 324 seeds/m2 because there was no significant gain in using the higher seeding rate for panicle production.
While conducting the second year of study, the temperature was lower than the threshold required for sterility; thus, the male-sterile plants showed partial fertility. In this part of the study, we compared the effect of seed rate and male-sterile genotypes on the number of tillers that produced panicles, the number of tillers without panicles, and the total tillers (Table 3).

Table 3.
Effect of seed rate and male-sterile genotypes on the number of tillers that produced panicles, number of tillers without panicles, and total tillers from year 2020.
The results showed that the seed rates and the male-sterile genotypes significantly affected the number of tillers with panicles (Table 3). Comparing the mean of four seed rates using Student t-test showed a higher seed rate produced more panicles due to a higher number of plants per meter square. The 60 seeds/m2 produced the highest number of tillers with panicles following 40 seeds/m2. No significant differences were found between 20 and 30 seeds/m2, but the mean of 30 seeds/m2 was higher than 20 seeds/m2. The results showed that the male-sterile 811 s significantly produced tillers with panicles compared to 801 s. Moreover, there was a significant seed rate by 801 s interaction on the number of tillers with panicles. Student t-test on seed rate × 801 s showed an increased seed rate resulted in an increased number of tillers with panicles produced.
A significant effect of male-sterile genotype was found on the number of tillers without panicles (Table 3). The average tillers without panicles in 811 s were 48.19 against 29.86 in 801 s. A further analysis revealed a significant seed rate by 811 s interaction on the number of tillers without panicles. Moreover, the Student t-test on seed rate × 811 s indicated that an increased seed rate resulted in an increased number of tillers without panicles.
The ANOVA results showed significant differences between the total number of tillers with seed rates. The Student’s t study revealed an increased number of tillers due to increased seed rates. Significant differences were found between the number of tillers of two male-sterile lines; the average tillers in 801 s were 224.25 against 188.08 from 811 s. Moreover, a significant interaction between seed rates and 801 male-sterile genotypes was observed. A significant interaction between seed rates and 801 s was observed such that the total of tiller was increased in 801 s due to the increased seed rate in this study.
Combining the data from both years of the number of total tillers for 811 s, there was no significant difference between seeding rates of 216 and 431 seeds/m2, but there was a highly significant difference (p-value < 0.0001) between the years (Table 4). No significant interaction existed between year and seed rates (Table 4). The results showed that year significantly (p-value ≤ 0.0331) impacted on the total number of tillers with panicles, while there was no significant effect of different seed rates and interaction year × seed rates on the number of tillers with panicles. The year’s highly significant effect (p-value < 0.0001) was found on tillers with no panicle. Moreover, significant differences were found in the interaction of year × seed rates on tillers without panicle. Thus, the result suggested that the environment affected the total amount of panicles produced.

Table 4.
Effect of seed rates and environment years 2018 (1) and 2020 (2) on the number of tillers that produced panicles, number of tillers without panicles, and total tillers.
4. Discussion
The primary objective was to determine the environmental threshold for inducing sterility of these newly developed EGMS lines so that they may be properly and successfully used in the production of Arkansas hybrid seed. This research showed that the Arkansas EGMS lines developed by the RREC Hybrid Rice Breeding Program [7] were TGMS with lines 801 s, 805 s, and 811 s expressing sterility at >29.4 °C and 236 s at a threshold of >32.2 °C based on the growth chambers studies devised [11]. The findings suggested that a critical growth stage for inducing pollen sterility was R1 + 10 days, approximately 25 days before heading. According to Virmani et al. [3], the critical timing for inducing sterility varies from 15 to 25 days before heading or 5 to 15 days after panicle initiation (R0), which corresponds with the results. It also relates with material from Moldenhauer et al. [12], which explains how at the R1 stage, the number of potential grains per panicle is greatly affected by the environment. Therefore, it is important to know about both hybrid seed production and seed production of the EGMS lines. Knowing the thresholds and critical timing for inducing sterility enables the breeders/producers to plant successfully in the right conditions considering which seed production is desired. Furthermore, the planting date is important for successful hybrid seed production because the EGMS lines must be grown in an environment above sterility thresholds at R1 +10 days. Hence, the proper planting date must be considered, and the optimum planting date of April 25 in Arkansas was determined, considering the results. Moreover, the results suggested that increasing the seed rate [16] increased the number of tillers; however, there were no significant differences between 431 and 648 seeds/m2. Male-sterile seed production is expensive. Thus, this study suggests that the seed rate of 431 seeds/m2 should be adequate for hybrid seed production. This study suggests considering several factors to improve hybrid seed production, including the location of hybrid seed production, planting date, the male-sterile threshold, and the number of panicles per plant.
5. Conclusions
This study aimed to provide information for rice breeders regarding strategies for hybrid rice parental line development and rice producers regarding field management for hybrid rice seed production. High-temperature thresholds induced the sterility of the Arkansas EGMS lines, thus indicating these lines were TGMS. Lines 801 s, 805 s, and 811 s expressed sterility at >29.4 °C and 236 s at a threshold of >32.2 °C. In order to achieve male sterility in Arkansas hybrid seed production, the TGMS lines must be planted by April 25 to meet high-temperature thresholds during the critical stage for inducing sterility. Another point is that a seed rate of 431 seeds/m2 is adequate for hybrid seed production because of the number of tillers available while avoiding overseeding the TGMS lines to help with hybrid seed production costs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12102519/s1, Table S1: Environmental Conditions for the Growth Chamber Study. Table S2: Field Data and Grwoth Chambers Environmental Condition. S2-A-Growth Chamber Study. S2-B-Field-Year 1-Tiller Count. S2-C-Field-summary year1-seed rate. S2-D-Field-Year 2-raw data. S2-E-Field-Summary Year 2. S2-F-Combined 811-data-for 2 year.
Author Contributions
Conceptualization, E.S., D.G.N. and P.C.; Data Curation, D.G.N.; Formal analysis, E.S. and D.G.N.; Funding acquisition, E.S.; Investigation, D.G.N.; Methodology, P.C., S.F. and E.S.; Investigation, D.G.N.; Project administration; E.S.; Resources, E.S.; software, E.S. and D.G.N.; Supervision, E.S.; Validation, P.C., E.S., S.F. and D.G.N.; Visualization, D.G.N., P.C. and E.S.; writing—original draft preparation, D.G.N.; Writing review and editing, S.F., E.S., D.G.N. and P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Arkansas Rice Research and Promotion Grant numbers: GR004940.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to express their gratitude to Arkansas rice producers via monies administered by the Arkansas Rice Research and Promotion Board and the University of Arkansas System, Division of Agriculture. The authors extend their appreciation to Dale Bumper’s National Rice Research Center, USDA-ARS at Stuttgart, AR, for the seed acquisition of GSOR 1, GSOR 2, GSOR 3, and GSOR 4. We acknowledge that part of this study was performed as Dustin North’s master thesis; however, the data and contej were not published in any peer-reviewed journal, and Dustin North’s research supervisor for MS study, Ehsan Shakiba, permit publishing this study [2].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kuyek, D.; Zamora, O.B.; Quijano, R. Hybrid Rice in Asia: An Unfolding Threat; GRAIN; KMP; MASIPAG; UBINIG: Los Baños, Philippines, 2000. [Google Scholar]
- North, D. Characterization and Application of Arkansas Male Sterile Lines for Hybrid Rice Production. Master’s Thesis, Iowa State University, Ames, IA, USA, 2019. Available online: https://dr.lib.iastate.edu/handle/20.500.12876/31251 (accessed on 1 November 2018).
- Virmani, S.S.; Sun, Z.X.; Mou, T.M.; Jauhar Ali, A.; Mao, C.X. Two-line Hybrid Rice Breeding Manual; Inernational Rice Research Institue (IRRI): Los Baños, Philippines, 2003. [Google Scholar]
- Yang, R.C.; Wang, N.Y.; Mang, K.; Chau, Q.; Yang, R.R.; Chen, S. Preliminary studies on application of indica photo-thermo genic male sterile line 5460S in hybrid rice breeding. Hybrid Rice 1990, 1, 32–34. [Google Scholar]
- Shi, M.S.; Deng, J.Y. The discovery, determination and utilization of Hubei photosensitive genic male sterile rice (Oryza sativa L. subsp. japonica). Acta Genet. Sin. 1986, 13, 107–112. [Google Scholar]
- Virmani, S.S.; Viraktamath, B.C.; Casal, C.L.; Toledo, R.S.; Lopes, M.T.; Manalo, J.O. Hybrid Rice Breeding Manual; Inernational Rice Research Institue (IRRI): Los Baños, Philippines, 1997. [Google Scholar]
- Yan, Z.; Yan, W.; Deren, C. Hybrid rice breeding. In B.R. Wells Rice Research Studies; 2010; pp. 61–63. [Google Scholar]
- Yan, W.G.; Rutger, J.N.; Bryant, R.J.; Bockelman, H.E.; Fjellstrom, R.G.; Chen, M.H.; Tai, T.H.; McClung, A.M. Development and evaluation of a core subset of the USDA rice (Oryza sativa L.) germplasm collection. Crop Sci. 2007, 47, 869–878. [Google Scholar] [CrossRef]
- Zhu, X.; Rutger, J. Inheritance of induced dominant and recessive genetic male sterile mutants in rice (Oryza sativa L.). SABRAO J. Breed. Genet. 1999, 31, 17–22. [Google Scholar]
- Hardke, J. Glossary of rice industry terms. In Rice Production Handbook: 208; University of Arkansas Division of Agriculture Extension Service: Little Rock, AR, USA, 2018. [Google Scholar]
- Lee, D.S.; Chen, L.J.; Suh, H.S. Genetic characterization and fine mapping of a novel thermo-sensitive genic male-sterile gene tms6 in rice (Oryza sativa L.). Theor. Appl. Genet. 2005, 111, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Moldenhauer, K.; Counce, P.; Hardke, J. Rice growth and development. In Arkansas Rice Production Handbook; University of Arkansas Division of Agriculture Cooperative Extension Service-MP192: Little Rock, AR, USA, 2018; pp. 9–20. [Google Scholar]
- Weather Stations for Arkansas; University of Arkansas Division of Agriculture Cooperative Extension Service: Little Rock, AR, USA, 2017; Available online: https://dd60.uaex.edu/ARAgWeather/default.asp (accessed on 1 November 2017).
- HOBO External Temperature/R.H. Sensor Data Logger; Onset Computer Corporation: Boume, MA, USA, 2018; Available online: https://www.onsetcomp.com/products/data-loggers/mx2302a (accessed on 1 November 2018).
- Solar Radiation Shield; Onset Computer Corporation: Boume, MA, USA, 2018; Available online: https://www.onsetcomp.com/products/mounting/rs3-b (accessed on 1 November 2018).
- Runsick, S.; Wilson, C.E. Rice Seeding Rate Recommendations for Arkansas; University of Arkansas Division of Agriculture Extension Service: Little Rock, AR, USA, 2009. [Google Scholar]
- JMP®. Title of the Software; Version 14; SAS Institute Inc.: Cary, NC, USA, 1989–2021. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).