Morphological, Cytological, and Molecular Comparison between Diploid and Induced Autotetraploids of Callisia fragrans (Lindl.) Woodson
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material Acquisition, Surface Sterilization, and Culture Establishment
2.2. Polyploidy Induction
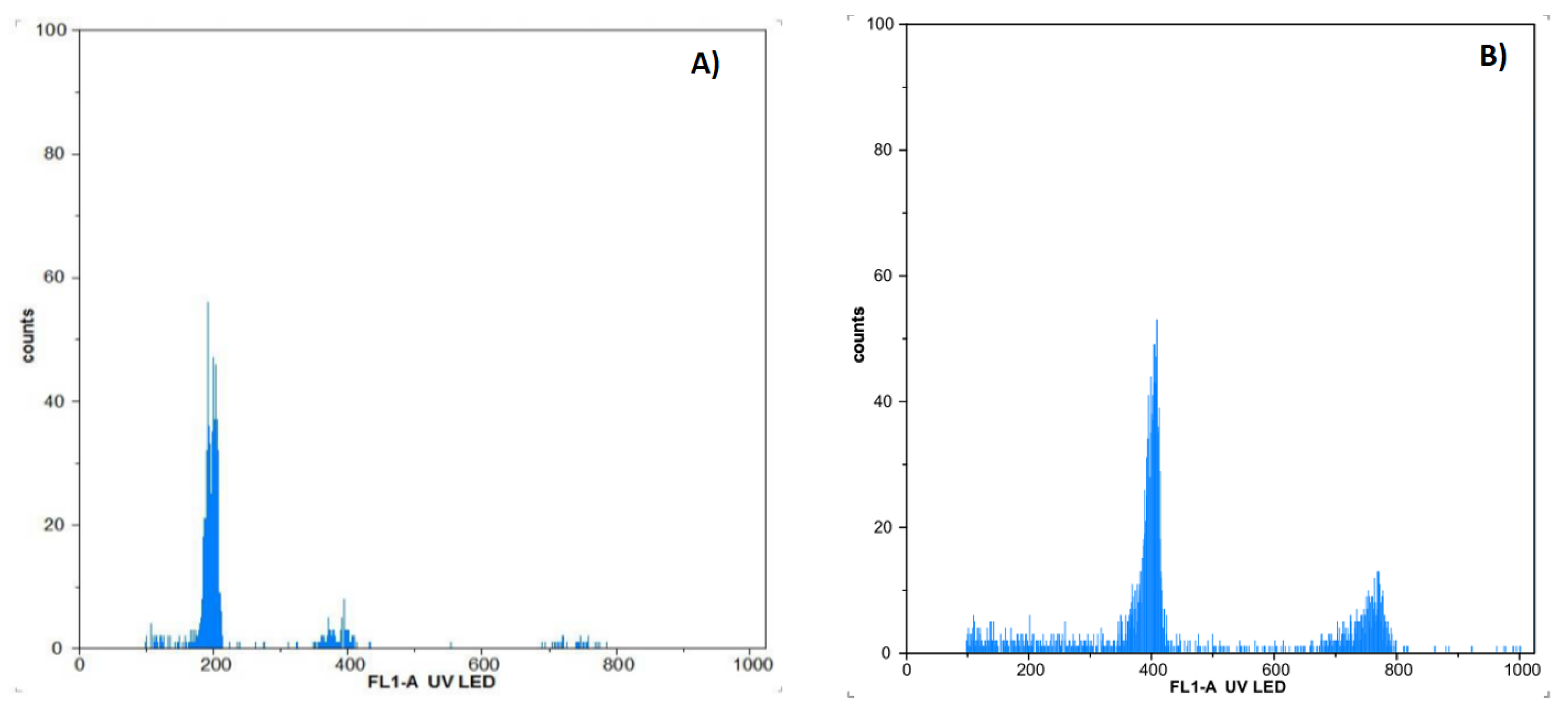
2.3. Flow Cytometry
2.4. Plant Transfer into Ex Vitro Conditions
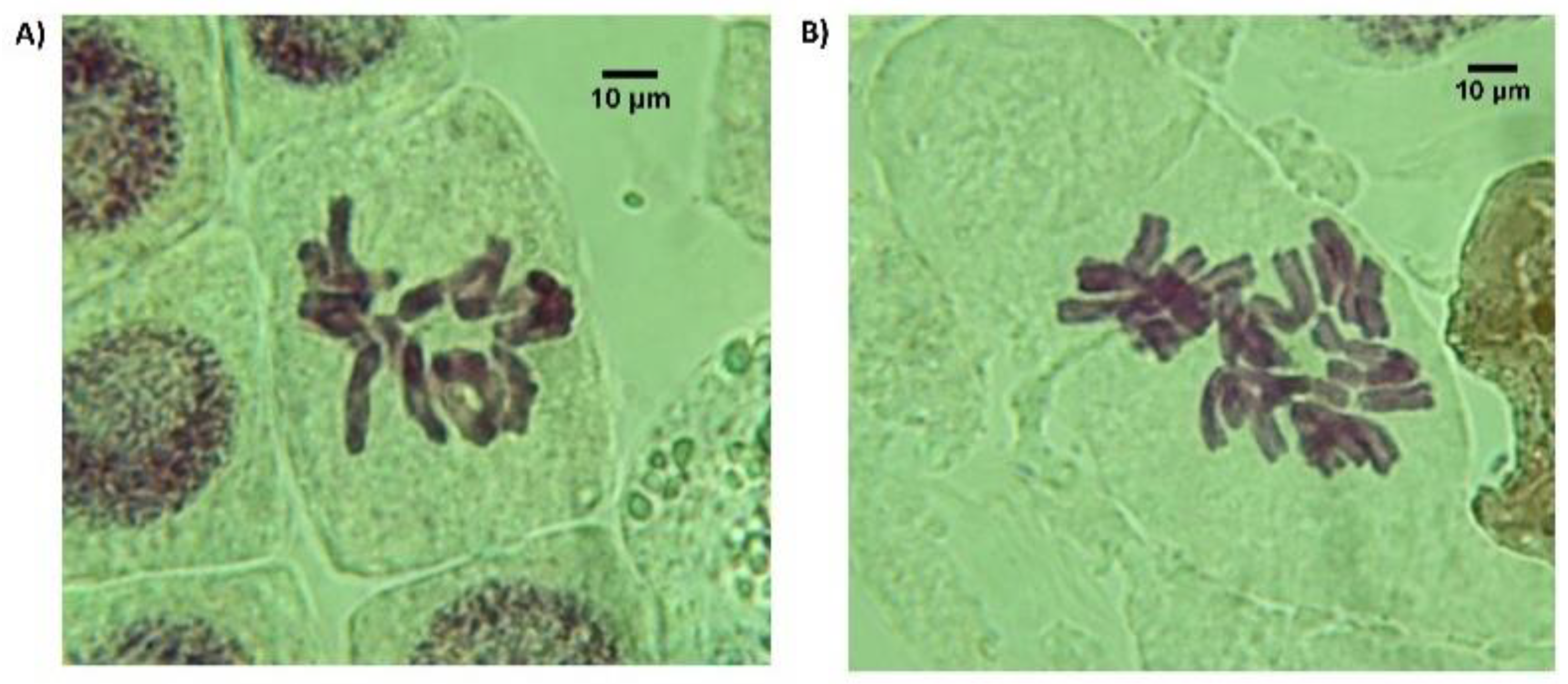
2.5. Chromosome Counting
2.6. DNA Extraction
2.7. iPBS Analysis
2.8. CDDP Analysis
2.9. DNA Fingerprinting
2.10. Mineralization and ICP-OES Elemental Analysis of Micro- and Macronutrients
2.11. Statistical Analysis
3. Results
3.1. Chromosome Doubling
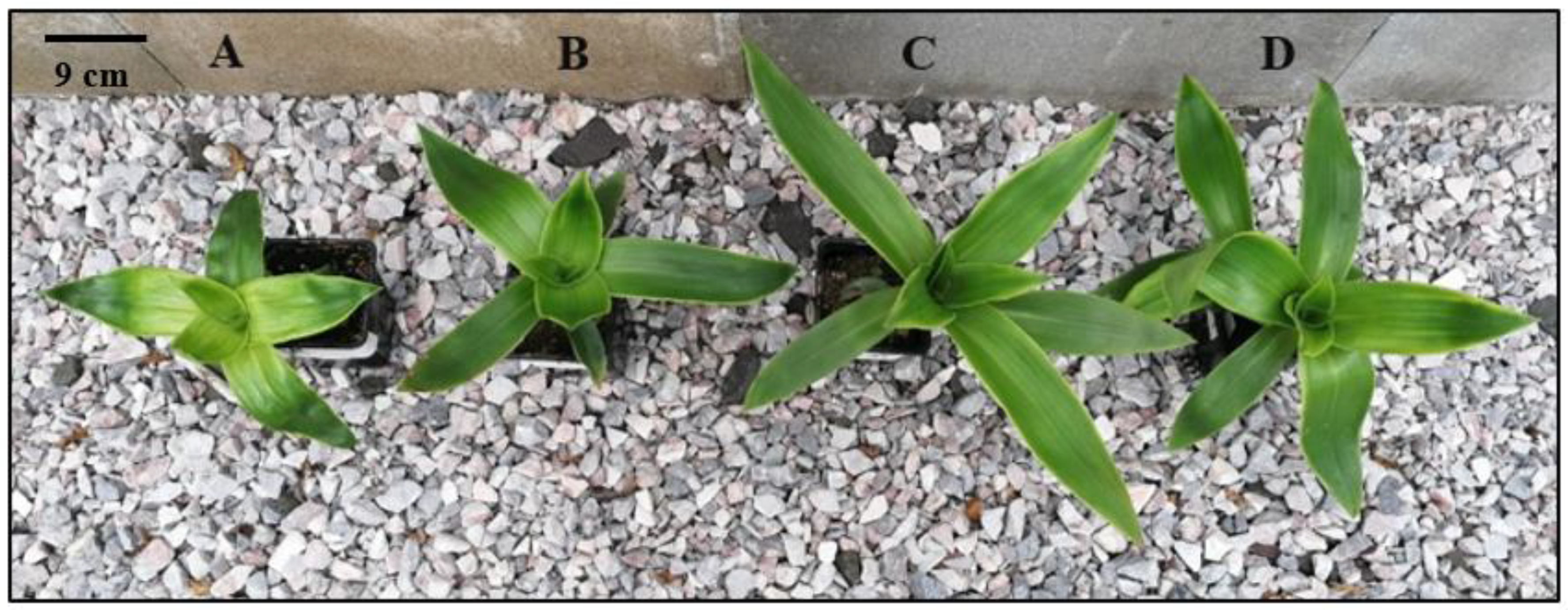
3.2. Morphological Differences between Diploid and the Autotetraploid Genotypes
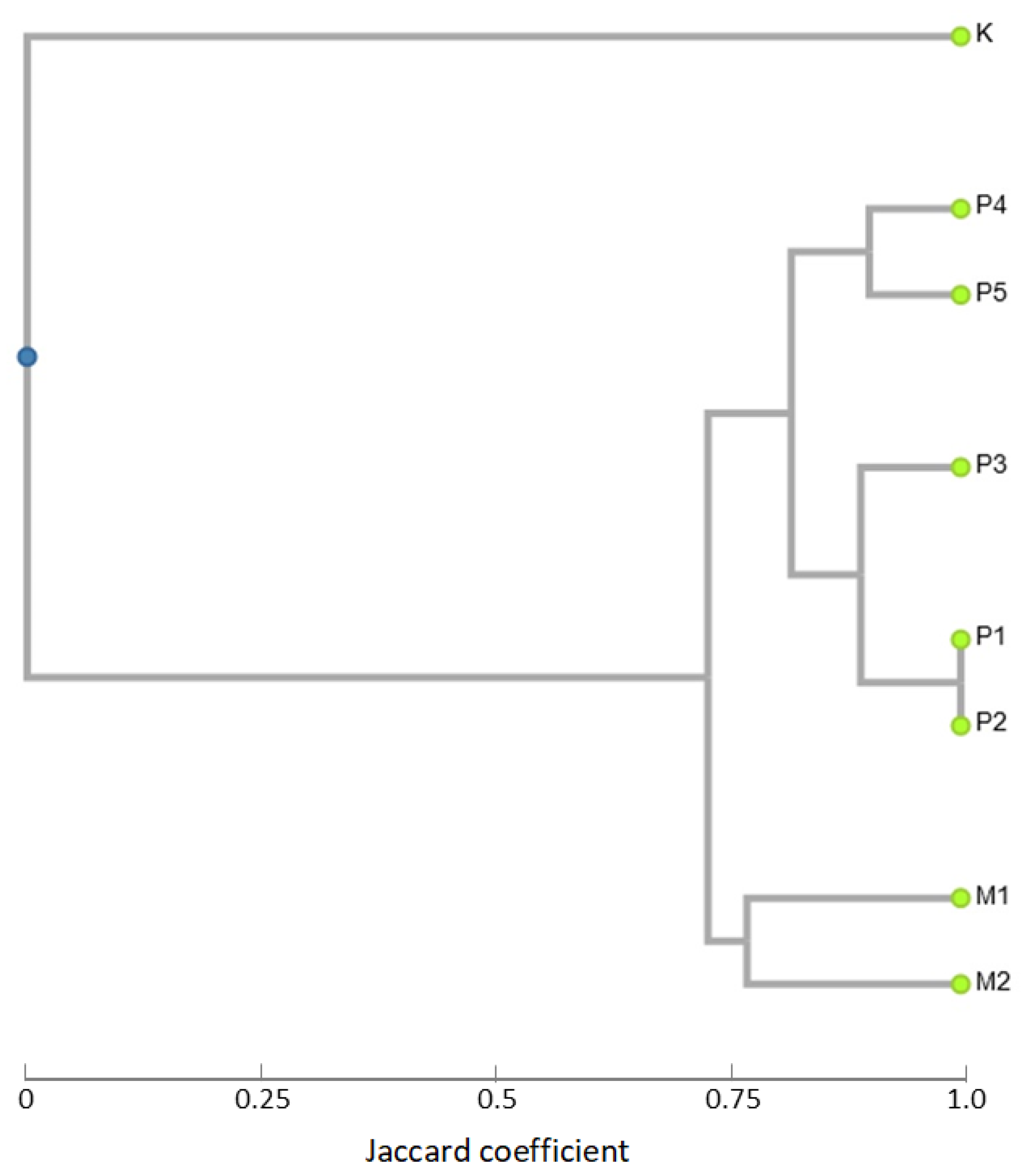
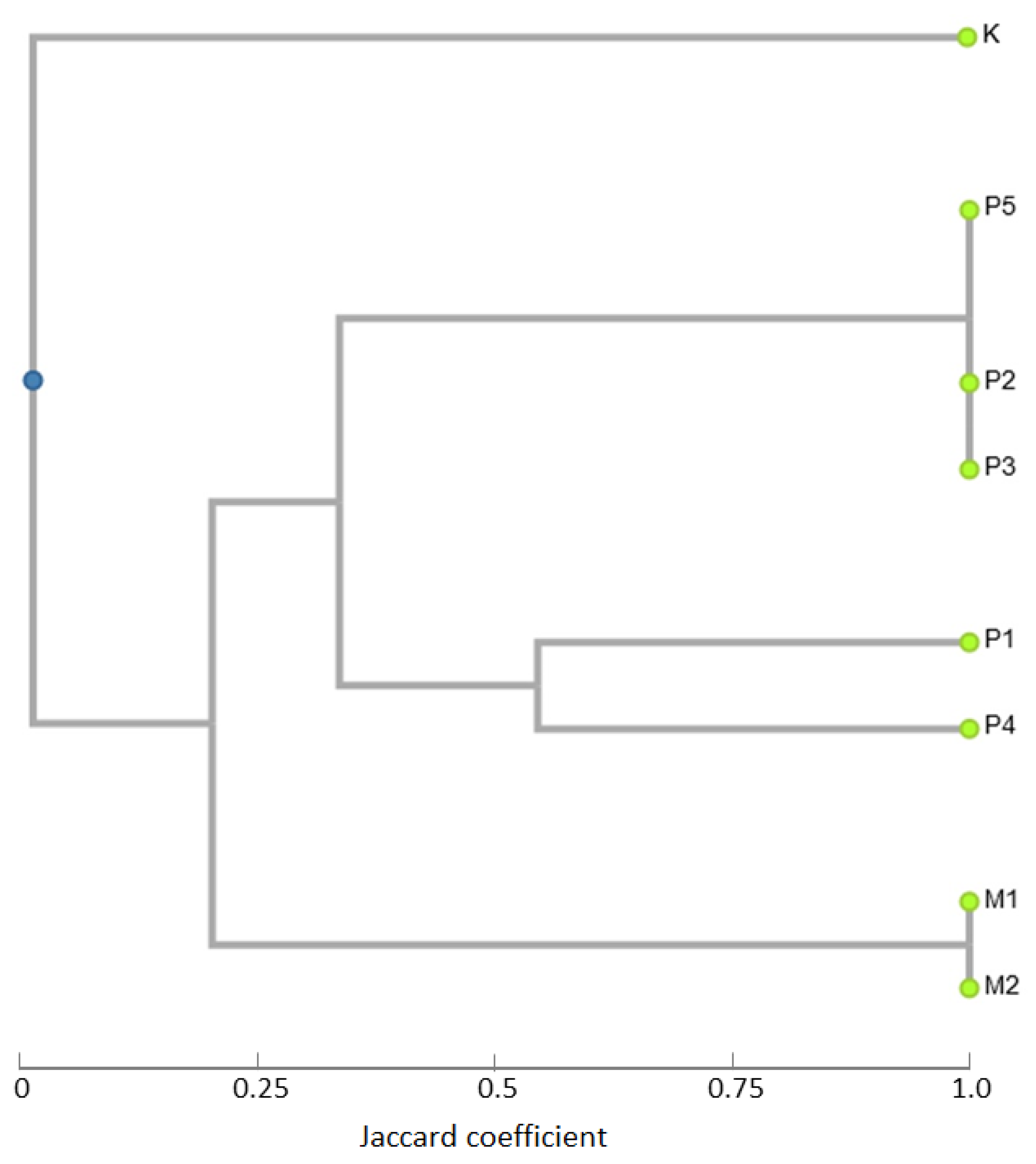
3.3. iPBS Analysis (Inter-Primer Binding Site Polymorphism)
3.4. Conserved DNA-Derived Polymorphism (CDDP) Analysis
3.5. Nutrient Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chernenko, T.V.; Ul’chenko, N.T.; Glushenkova, A.I.; Redzhepov, D. Chemical Investigation of Callisia Fragrans. Chem. Nat. Compd. 2007, 43, 253–255. [Google Scholar] [CrossRef]
- Saensouk, S.; Saensouk, P. A Karyological Study of Six Commelinaceae Species from Thailand. Cytologia 2020, 85, 57–62. [Google Scholar] [CrossRef]
- Malakyan, M.H. Radioprotective activity of Callisia fragrans grown in soilless (hydroponics) and soil culture conditions. Electron. J. Nat. Sci. 2015, 25, 20–23. [Google Scholar] [CrossRef] [Green Version]
- Pranskuniene, Z.; Ratkeviciute, K.; Simaitiene, Z.; Pranskunas, A.; Bernatoniene, J. Ethnobotanical Study of Cultivated Plants in Kaišiadorys District, Lithuania: Possible Trends for New Herbal Based Medicines. Evid.-Based Complement. Altern. Med. 2019, 2019, 3940397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Sohafy, S.M.; Nassra, R.A.; D’Urso, G.; Piacente, S.; Sallam, S.M. Chemical Profiling and Biological Screening with Potential Anti-Inflammatory Activity of Callisia fragrans Grown in Egypt. Nat. Prod. Res. 2021, 35, 5521–5524. [Google Scholar] [CrossRef] [PubMed]
- Karapetyan, A.S.; Mairapetyan, S.K. Quantitative Determination of Ascorbic Acid in Callisia fragrans under Open-Air Hydroponic Conditions. Bulg. Chem. Commun. 2020, 52, 30–32. [Google Scholar]
- Phan, L.T.M.; Nguyen, K.T.P.; Vuong, H.T.; Tran, D.D.; Nguyen, T.X.P.; Hoang, M.N.; Mai, T.P.; Nguyen, H.H. Supercritical Fluid Extraction of Polyphenols from Vietnamese Callisia fragrans Leaves and Antioxidant Activity of the Extract. J. Chem. 2020, 2020, 9548401. [Google Scholar] [CrossRef]
- Eng, W.-H.; Ho, W.-S. Polyploidization Using Colchicine in Horticultural Plants: A Review. Sci. Hortic. 2019, 246, 604–617. [Google Scholar] [CrossRef]
- Dehghan, E.; Reed, D.W.; Covello, P.S.; Hasanpour, Z.; Palazon, J.; Oksman-Caldentey, K.-M.; Ahmadi, F.S. Genetically Engineered Hairy Root Cultures of Hyoscyamus senecionis and H. muticus: Ploidy as a Promising Parameter in the Metabolic Engineering of Tropane Alkaloids. Plant Cell Rep. 2017, 36, 1615–1626. [Google Scholar] [CrossRef] [Green Version]
- Niazian, M.; Nalousi, A.M. Artificial Polyploidy Induction for Improvement of Ornamental and Medicinal Plants. Plant Cell Tissue Organ Cult. 2020, 142, 447–469. [Google Scholar] [CrossRef]
- Esmaeili, G.; Van Laere, K.; Muylle, H.; Leus, L. Artificial Chromosome Doubling in Allotetraploid Calendula officinalis. Front. Plant Sci. 2020, 11, 622. [Google Scholar] [CrossRef] [PubMed]
- Talebi, S.F.; Saharkhiz, M.J.; Kermani, M.J.; Sharafi, Y.; Raouf Fard, F. Effect of Different Antimitotic Agents on Polyploid Induction of Anise Hyssop (Agastache foeniculum L.). Caryologia 2017, 70, 184–193. [Google Scholar] [CrossRef]
- Dhooghe, E.; Van Laere, K.; Eeckhaut, T.; Leus, L.; Van Huylenbroeck, J. Mitotic Chromosome Doubling of Plant Tissues In Vitro. Plant Cell Tissue Organ Cult. 2011, 104, 359–373. [Google Scholar] [CrossRef]
- Homaidan Shmeit, Y.; Fernandez, E.; Novy, P.; Kloucek, P.; Orosz, M.; Kokoska, L. Autopolyploidy Effect on Morphological Variation and Essential Oil Content in Thymus vulgaris L. Sci. Hortic. 2020, 263, 109095. [Google Scholar] [CrossRef]
- Parsons, J.L.; Martin, S.L.; James, T.; Golenia, G.; Boudko, E.A.; Hepworth, S.R. Polyploidization for the Genetic Improvement of Cannabis sativa. Front. Plant Sci. 2019, 10, 476. [Google Scholar] [CrossRef] [PubMed]
- Sabzehzari, M.; Hoveidamanesh, S.; Modarresi, M.; Mohammadi, V. Morphological, Anatomical, Physiological, and Cytological Studies in Diploid and Tetraploid Plants of Plantago Psyllium. Plant Cell Tissue Organ Cult. 2019, 139, 131–137. [Google Scholar] [CrossRef]
- Tsuro, M.; Kondo, N.; Noda, M.; Ota, K.; Nakao, Y.; Asada, S. In Vitro Induction of Autotetraploid of Roman Chamomile (Chamaemelum nobile L.) by Colchicine Treatment and Essential Oil Productivity of Its Capitulum. In Vitro Cell. Dev. Biol.-Plant 2016, 52, 479–483. [Google Scholar] [CrossRef]
- Mei, B.; Xie, H.; Xing, H.; Kong, D.; Pan, X.; Li, Y.; Wu, H. Changes of Phenolic Acids and Antioxidant Activities in Diploid and Tetraploid Echinacea purpurea at Different Growth Stages. Rev. Bras. Farmacogn. 2020, 30, 510–518. [Google Scholar] [CrossRef]
- Hassanzadeh, F.; Zakaria, R.A.; Azad, N.H. Polyploidy Induction in Salvia officinalis L. and Its Effects on Some Morphological and Physiological Characteristics. Cytologia 2020, 85, 157–162. [Google Scholar] [CrossRef]
- Santos, G.C.; Cardoso, F.P.; Martins, A.D.; Rodrigues, F.A.; Pasqual, M.; Cossani, P.; Magno Queiroz Luz, J.; Rezende, R.A.L.S.; Soares, J.D.R. Polyploidy Induction in Physalis alkekengi. Biosci. J. 2020, 36, 827–835. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, F.; Fu, J.; Xiao, Y.; Wu, J. In Vitro Polyploid Induction Using Colchicine for Zingiber officinale Roscoe Cv. ‘Fengtou’ Ginger. Plant Cell Tissue Organ Cult. 2020, 142, 87–94. [Google Scholar] [CrossRef]
- Shaikh, N.; Verma, R.C.; Dass, P.; Khah, M.A. Meiotic Investigation in Reciprocal Translocation Heterozygotes of Commelina benghalensis L. (Commelinaceae) Induced by Gamma Irradiations. Cytologia 2020, 85, 213–217. [Google Scholar] [CrossRef]
- Shaikh, N.; Verma, R.C.; Khah, M.A.; Filimban, F.Z.; Albaz, A.A.; Alam, Q. Morphological and Chromosomal Characterization of Diploid and Colchicine Induced Autotetraploid Plants of Commelina benghalensis L. (Commelinaceae). Cytologia 2021, 86, 167–173. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Zahumenická, P.; Fernández, E.; Šedivá, J.; Žiarovská, J.; Ros-Santaella, J.L.; Martínez-Fernández, D.; Russo, D.; Milella, L. Morphological, Physiological and Genomic Comparisons between Diploids and Induced Tetraploids in Anemone sylvestris L. Plant Cell Tissue Organ Cult. 2018, 132, 317–327. [Google Scholar] [CrossRef]
- Tomaskova, D. Karyological Methods of Ploidy Level Determination in Sugar Beet Varieties. Genet. Slechteni 1996, 31, 161–170. [Google Scholar]
- Kalendar, R.; Antonius, K.; Smýkal, P.; Schulman, A.H. IPBS: A Universal Method for DNA Fingerprinting and Retrotransposon Isolation. Theor. Appl. Genet. 2010, 121, 1419–1430. [Google Scholar] [CrossRef] [PubMed]
- Collard, B.C.Y.; Mackill, D.J. Conserved DNA-Derived Polymorphism (CDDP): A Simple and Novel Method for Generating DNA Markers in Plants. Plant Mol. Biol. Rep. 2009, 27, 558–562. [Google Scholar] [CrossRef]
- Yun, R.Q.A.; Dinarti, D.; Husni, A.; Kosmiatin, M. Callus Regeneration and Polyploidy Induction of Allium cepa L Var. Bima Brebes Using Oryzalin. IOP Conf. Ser. Earth Environ. Sci. 2021, 948, 012043. [Google Scholar] [CrossRef]
- Ridwan, R.; Witjaksono, W. Induction of Autotetraploid Moringa Plant (Moringa oleifera) Using Oryzalin. Biodiversitas 2020, 21, 4086–4093. [Google Scholar] [CrossRef]
- Salma, U.; Kundu, S.; Mandal, N. Artificial Polyploidy in Medicinal Plants: Advancement in the Last Two Decades and Impending Prospects. J. Crop Sci. Biotechnol. 2017, 20, 9–19. [Google Scholar] [CrossRef]
- Kim, H.-E.; Han, J.-E.; Lee, H.; Kim, J.-H.; Kim, H.-H.; Lee, K.-Y.; Shin, J.-H.; Kim, H.-K.; Park, S.-Y. Tetraploidization Increases the Contents of Functional Metabolites in Cnidium officinale. Agronomy 2021, 11, 1561. [Google Scholar] [CrossRef]
- Yue, M.; Chen, C.; Liao, J.; Sun, J.; Shen, W. Oryzalin-induced chromosome doubling of Scutellaria barbata. Zhong Yao Cai 2011, 34, 4–8. [Google Scholar] [PubMed]
- Sabooni, N.; Gharaghani, A.; Jowkar, A.; Eshghi, S. Successful Polyploidy Induction and Detection in Blackberry Species by Using an In Vitro Protocol. Sci. Hortic. 2022, 295, 110850. [Google Scholar] [CrossRef]
- Eeckhaut, T.G.R.; Werbrouck, S.P.O.; Leus, L.W.H.; Van Bockstaele, E.J.; Debergh, P.C. Chemically Induced Polyploidization in Spathiphyllum wallisii Regel through Somatic Embryogenesis. Plant Cell Tissue Organ Cult. 2004, 78, 241–246. [Google Scholar] [CrossRef]
- Viehmannová, I.; Cusimamani, E.F.; Bechyne, M.; Vyvadilová, M.; Greplová, M. In Vitro Induction of Polyploidy in Yacon (Smallanthus sonchifolius). Plant Cell Tissue Organ Cult. 2009, 97, 21–25. [Google Scholar] [CrossRef]
- Greplova, M.; Polzerova, H.; Domkářová, J. Intra- and Inter-Specific Crosses of Solanum Materials after Mitotic Polyploidization In Vitro. Plant Breed. 2009, 128, 651–657. [Google Scholar] [CrossRef]
- Kaensaksiri, T.; Soontornchainaksaeng, P.; Soonthornchareonnon, N.; Prathanturarug, S. In Vitro Induction of Polyploidy in Centella asiatica (L.) Urban. Plant Cell Tissue Organ Cult. 2011, 107, 187–194. [Google Scholar] [CrossRef]
- Gao, S.L.; Zhu, D.N.; Cai, Z.H.; Xu, D.R. Autotetraploid Plants from Colchicine-Treated Bud Culture of Salvia miltiorrhiza Bge. Plant Cell Tissue Organ Cult. 1996, 47, 73–77. [Google Scholar] [CrossRef]
- Gao, S.L.; Chen, B.J.; Zhu, D.N. In Vitro Production and Identification of Autotetraploids of Scutellaria baicalensis. Plant Cell Tissue Organ Cult. 2002, 70, 289–293. [Google Scholar] [CrossRef]
- Wei, K.; Xu, J.; Li, L.; Cai, J.; Miao, J.; Li, M. In Vitro Induction and Generation of Tetraploid Plants of Sophora tonkinensis Gapnep. Pharmacogn. Mag. 2018, 14, 149. [Google Scholar] [CrossRef]
- Considine, M.J.; Wan, Y.; D’Antuono, M.F.; Zhou, Q.; Han, M.; Gao, H.; Wang, M. Molecular Genetic Features of Polyploidization and Aneuploidization Reveal Unique Patterns for Genome Duplication in Diploid Malus. PLoS ONE 2012, 7, e29449. [Google Scholar] [CrossRef] [PubMed]
- Persson Hovmalm, A.H.; Jeppsson, N.; Bartish, V.I.; Nybom, H. RAPD Analysis of Diploid and Tetraploid Populations of Aronia Points to Different Reproductive Strategies within the Genus: RAPD Analysis of Populations of Aronia. Hereditas 2005, 141, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Belogrudova, I.; Grauda, D.; Jakobsone, G.; Rashal, I. Usability of Retrotransposone-Based Molecular Marker System to Assess Genetic Diversity of Liparis loeselii. Acta Biol. Univ. Daugavp. 2012, 12, 4. [Google Scholar]
- Gailite, A.; Rungis, D. An Initial Investigation of the Taxonomic Status of Saussurea esthonica Baer Ex Rupr. Utilising DNA Markers and Sequencing. Plant Syst. Evol. 2012, 298, 913–919. [Google Scholar] [CrossRef]
- Baránek, M.; Meszáros, M.; Sochorová, J.; Čechová, J.; Raddová, J. Utility of Retrotransposon-Derived Marker Systems for Differentiation of Presumed Clones of the Apricot Cultivar Velkopavlovická. Sci. Hortic. 2012, 143, 1–6. [Google Scholar] [CrossRef]
- Petrovičová, L.; Balážová, Ž.; Vivodí k, M.; Gálová, Z. Detection Genetic Variability of Secale cereale L. by Scot Markers. Potravinarstvo 2017, 11, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Zang, D. Analysis of Genetic Relationships in Rosa rugosa Using Conserved DNA-Derived Polymorphism Markers. Biotechnol. Biotechnol. Equip. 2018, 32, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Vivodí k, M.; Gálová, Z.; Balážová, Ž. Genetic Divergence in Tunisian Castor Bean Genotypes Based on Trap Markers. Potravinarstvo 2020, 14, 510–518. [Google Scholar] [CrossRef]
- Ravi, R.S.D.; Siril, E.A.; Nair, B.R. The Efficiency of Cytochrome P450 Gene-Based Markers in Accessing Genetic Variability of Drumstick (Moringa oleifera Lam.) Accessions. Mol. Biol. Rep. 2020, 47, 2929–2939. [Google Scholar] [CrossRef]
- Stefunova, V.; Bezo, M.; Senkova, M. Genetic Analysis of Three Amaranth Species Using ISSR Markers. Emir. J. Food Agric. 2014, 26, 35. [Google Scholar] [CrossRef]
- Balážová, Å.; Gálová, Z.; Vivodík, M. Application of rye SSR markers for detection of genetic diversity in triticale. J. Microbiol. Biotechnol. Food Sci. 2016, 5, 623–626. [Google Scholar] [CrossRef] [Green Version]
- Kuťka Hlozáková, T.; Gálová, Z.; Gregová, E.; Vivodí k, M.; Balážová, Ž.; Miháliková, D. RAPD Analysis of the Genetic Polymorphism in European Wheat Genotypes. Potravinarstvo 2016, 10, 1–6. [Google Scholar] [CrossRef]
- Iarovsk, J.; Labajov, K. Using of Inter Microsatellite Polymorphism to Evaluate Gamma-Irradiated Amaranth Mutants. Emir. J. Food Agric. 2013, 25, 673. [Google Scholar] [CrossRef]
- Kalendar, R.; Schulman, A.H. Transposon-Based Tagging: IRAP, REMAP, and IPBS. In Molecular Plant Taxonomy; Methods in Molecular Biology; Besse, P., Ed.; Humana Press: Totowa, NJ, USA, 2014; Volume 1115, pp. 233–255. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, J.K.; Chandel, P.; Gupta, S.; Gopal, J.; Singh, B.P.; Bhardwaj, V. Analysis of Genetic Stability of In Vitro Propagated Potato Microtubers Using DNA Markers. Physiol. Mol. Biol. Plants 2013, 19, 587–595. [Google Scholar] [CrossRef] [PubMed]

| Concentration (µM) | Duration (weeks) | No. of Plants | No. of Plants after Treatment | Survival Rate (%) | Mixoploid Plants | Tetraploid Plants | Efficiency Rate (%) |
|---|---|---|---|---|---|---|---|
| 1 | 4 | 30 | 34 | 100 | 0 | 0 | 0 |
| 8 | 30 | 66 | 100 | 0 | 0 | 0 | |
| 5 | 4 | 30 | 47 | 100 | 1 | 0 | 2.13 |
| 8 | 30 | 63 | 100 | 0 | 6 | 9.52 | |
| 10 | 4 | 30 | 38 | 100 | 0 | 0 | 0 |
| 8 | 30 | 58 | 100 | 1 | 0 | 1.72 | |
| Total | 180 | 306 | 100 | 2 | 6 |
| Genotype | Average Number of Internodes | Average Number of Stolons | Average Number of Leaves | Average Height (cm) | Average Length of Stolons (cm) | Average Numberof Internodes on Stolon | Average Leaf Length (cm) |
|---|---|---|---|---|---|---|---|
| Control | 16.3 ± 1.7 a | 5.5 ± 1.35 a | 23.7 ± 2.26 a | 64 ± 6.58 a | 76.1 ± 10.04 a | 10.33 ± 0.92 a | 24.47 ± 1.48 c |
| P1 | 14.8 ± 2.15 a | 4.4 ± 1.17 a | 22 ± 1.76 a | 67.5 ± 10.61 a | 54.95 ± 10.8 b | 9.51 ± 0.86 a | 30.87 ± 0.42 a |
| P2 | 14.3 ± 1.16 a | 4.3 ± 1.25 a | 20.9 ± 2.08 a | 62.8 ± 6.09 a | 57.012 ±16.87 b | 6.92 ± 2.3 c | 28.69 ± 0.88 b |
| P3 | 14.7 ± 0.68 a | 5.6 ± 0.52 a | 17.9 ± 0.87 b | 61.9 ± 3.,48 a | 64.133 ±5.64 b | 9.33 ± 0.54 a | 31.86 ± 1.57 a |
| P4 | 11.9 ± 1.37 b | 4.5 ± 0.53 a | 17 ± 0.94 b | 62 ± 10.33 a | 61.48 ± 9.92 b | 8.19 ± 0.94 b | 31.34 ± 0.82 a |
| P5 | 17.3 ± 1.34 a | 4.5 ± 0.85 a | 21.3 ± 1.49 a | 64.5 ± 5.93 a | 48.835 ± 5.69 c | 8.3 ± 2.76 a | 22.35 ± 0.94 c |
| M1 | 12.3 ± 1.9 b | 5.2 ± 0.42 a | 18.8 ± 3.36 b | 46 ± 7.76 b | 56.86 ± 15.48 b | 8.62 ± 1.35 a | 32.7 ± 1.14 a |
| M2 | 12.2 ± 1.81 b | 6 ± 1.25 a | 18.2 ± 2.49 b | 46 ± 7.75 b | 57.38 ± 13.73 b | 8.82 ± 1.21 a | 32.73 ± 1.61 a |
| Genotype | Average Diameter (cm) | Petal Length (mm) | Petal Width (mm) |
|---|---|---|---|
| K | 2.08 ± 0.18 b | 3.54 ± 0.41 b | 1.94 ± 0.37 b |
| P1 | 2.08 ± 0.16 c | 6.045 ± 1.01 a | 2.33 ± 0.88 b |
| P3 | 3.11 ± 0.10 a | 5.96 ± 0.58 a | 2.68 ± 0.55 a |
| P4 | 2.22 ± 0.16 b | 5.97 ± 0.99 a | 2.42 ± 0.54 b |
| P5 | 1.92 ± 0.09 c | 4.94 ± 0.55 b | 2.41 ± 0.77 b |
| Technique | Primer/Primer Combination | Polymorphism (%) | Min Length of Generated Fragments | Max Length of Generated Fragments | Average Dice Coefficient of Genetic Similarity |
|---|---|---|---|---|---|
| iPBS | 1846 | 100 | 69 | 1190 | 0.52 |
| 2270 | 100 | 68 | 789 | 0.61 | |
| 1882 | 98 | 60 | 820 | 0.95 | |
| 1854 | 99 | 64 | 988 | 0.65 | |
| 1867 | 99 | 71 | 1850 | 0.89 | |
| 1868 | 100 | 90 | 1230 | 0.88 | |
| CDDP | WRKY-F + R1 | 96 | 55 | 560 | 0.52 |
| WRKY-F + R2a | 95 | 82 | 780 | 0.45 | |
| WRKY-F + R2b | 99 | 70 | 930 | 0.36 | |
| WRKY-F + R3 | 99 | 50 | 520 | 0.49 | |
| WRKY-F + R3b | 93 | 63 | 900 | 0.58 |
| Nutrient | Control (K) | P1 | P2 | P3 | P4 | P5 | M1 | M2 |
|---|---|---|---|---|---|---|---|---|
| K | 15,772.00 ± 174.00 g | 31,040.35 ± 92.75 d | 37,369.05 ± 68.15 b | 30,066.50 ± 39.50 e | 39,041.80 ± 9.40 a | 39,047.45 ± 15.05 a | 33,313.35 ± 5.95 c | 26,340.25 ± 10.05 f |
| Ca | 23,253.40 ± 105.50 b | 25,777.90 ± 89.40 a | 8862.80 ± 4.70 g | 13,685.25 ± 75.05 e | 13,122.80 ± 10.30 f | 17,231.55 ± 5.65 c | 17,334.80 ± 11.60 c | 14,798.30 ± 10.90 d |
| Mg | 12,866.10 ± 120.50 b | 14,295.50 ± 15.00 a | 4868.00 ± 10.00 h | 8039.85 ± 13.35 f | 6882.75 ± 5.55 g | 10,316.95 ± 6.25 c | 9353.75 ± 3.05 d | 8517.55 ± 4.15 e |
| Na | 195.95 ± 1.55 h | 1368.15 ± 2.95 de | 1758.25 ± 12.85 a | 1305.35 ± 7.85 f | 1706.50 ± 3.80 b | 1404.50 ± 4.70 cd | 1351.85 ± 2.55 e | 922.20 ± 1.50 g |
| Fe | 82.80 ± 0.88 d | 73.88 ± 0.41 e | 90.95 ± 0.15 c | 57.97 ± 0.00 f | 73.97 ± 0.37 e | 82.03 ± 0.05 d | 92.98 ± 0.35 bc | 93.74 ± 0.00 ab |
| Mn | 102.40 ± 0.70 e | 107.15 ± 0.25 e | 142.90 ± 0.30 b | 90.25 ± 0.55 f | 118.90 ± 0.30 d | 79.05 ± 0.25 g | 188.75 ± 2.55 a | 135.22 ± 0.22 c |
| Zn | 48.96 ± 0.45 c | 43.20 ± 0.25 d | 53.42 ± 0.46 b | 36.97 ± 0.10 f | 56.13 ± 0.20 a | 44.01 ± 0.15 d | 50.91 ± 0.25 c | 39.86 ± 0.41 e |
| Cu | 26.76 ± 0.22 d | 34.73 ± 0.35 c | 54.16 ± 0.00 a | 26.05 ± 0.25 d | 33.12 ± 0.77 c | 25.07 ± 0.08 d | 43.32 ± 0.14 b | 41.93 ± 0.10 b |
| Cr | 0.15 ± 0.00 f | 0.17 ± 0.00 cdef | 0.16 ± 0.00 ef | 0.12 ± 0.01 g | 0.18 ± 0.00 bcde | 0.08 ± 0.00 h | 0.17 ± 0.01 def | 0.21 ± 0.01 a |
| Mo | 0.40 ± 0.01 f | 0.52 ± 0.01 de | 0.46 ± 0.01 ef | 1.02 ± 0.01 b | 0.15 ± 0.00 g | NDh | 0.70 ± 0.02 c | 1.39 ± 0.02 a |
| Co | NDe | 0.29 ± 0.00 b | NDe | 0.20 ± 0.00 c | NDe | 0.12 ± 0.01 d | 0.35 ± 0.01 a | NDe |
| Ag | NDa | 0.07 ± 0.00 a | NDa | NDa | NDa | 0.07 ± 0.00 a | 0.11 ± 0.00 a | 0.02 ± 0.00 a |
| Sr | 49.07 ± 0.10 d | 43.65 ± 0.40 f | 54.03 ± 0.15 b | 38.05 ± 0.10 g | 56.07 ± 0.14 a | 46.08 ± 0.14 ce | 50.95 ± 0.29 c | 38.56 ± 0.40 g |
| Sb | NDf | 1.98 ± 0.05 a | 1.90 ± 0.01 a | 1.72 ± 0.01 b | 1.44 ± 0.01 c | 0.91 ± 0.01 d | 0.47 ± 0.00 e | NDf |
| Li | 12.04 ± 0.15 a | 8.07 ± 0.05 c | 2.44 ± 0.03 g | 7.68 ± 0.04 c | 4.04 ± 0.01 f | 4.85 ± 0.02 e | 6.22 ± 0.09 d | 9.76 ± 0.08 b |
| Ba | 8.77 ± 0.35 a | 7.25 ± 0.10 b | 3.10 ± 0.03 d | 5.31 ± 0.02 c | 4.97 ± 0.01 c | 5.28 ± 0.04 c | 5.36 ± 0.06 c | 4.75 ± 0.02 c |
| Al | 36.12 ± 0.20 a | 11.20 ± 0.23 e | 17.14 ± 0.21 c | 16.23 ± 0.04 c | 13.93 ± 0.05 d | 13.98 ± 0.15 d | 19.05 ± 0.07 b | 13.63 ± 0.11 d |
| Others | As 2.21 Pb 0.44 | As 1.23 Cd 0.11 Ni 0.45 | As 1.76 Ni 0.26 Pb 0.74 | Pb 0.67 | ND | ND | Cd 0.06 Ni 0.23 | Pb 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beranová, K.; Bharati, R.; Žiarovská, J.; Bilčíková, J.; Hamouzová, K.; Klíma, M.; Fernández-Cusimamani, E. Morphological, Cytological, and Molecular Comparison between Diploid and Induced Autotetraploids of Callisia fragrans (Lindl.) Woodson. Agronomy 2022, 12, 2520. https://doi.org/10.3390/agronomy12102520
Beranová K, Bharati R, Žiarovská J, Bilčíková J, Hamouzová K, Klíma M, Fernández-Cusimamani E. Morphological, Cytological, and Molecular Comparison between Diploid and Induced Autotetraploids of Callisia fragrans (Lindl.) Woodson. Agronomy. 2022; 12(10):2520. https://doi.org/10.3390/agronomy12102520
Chicago/Turabian StyleBeranová, Kateřina, Rohit Bharati, Jana Žiarovská, Jana Bilčíková, Kateřina Hamouzová, Miroslav Klíma, and Eloy Fernández-Cusimamani. 2022. "Morphological, Cytological, and Molecular Comparison between Diploid and Induced Autotetraploids of Callisia fragrans (Lindl.) Woodson" Agronomy 12, no. 10: 2520. https://doi.org/10.3390/agronomy12102520
APA StyleBeranová, K., Bharati, R., Žiarovská, J., Bilčíková, J., Hamouzová, K., Klíma, M., & Fernández-Cusimamani, E. (2022). Morphological, Cytological, and Molecular Comparison between Diploid and Induced Autotetraploids of Callisia fragrans (Lindl.) Woodson. Agronomy, 12(10), 2520. https://doi.org/10.3390/agronomy12102520





