Investigation of Indigenous Entomopathogenic Nematodes in Guangxi and Its Biological Control of Spodoptera frugiperda
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sample Collection
2.2. Isolation of EPNs with Galleria Mellonella
2.3. The Test Insects
2.4. Molecular Identification of EPN Species
2.5. Virulence of EPNs against S. frugiperda
2.5.1. Screening of EPNs in Guangxi
2.5.2. Virulence of Highly Effective EPNs to the Third-Instar Larvae of S. frugiperda
2.5.3. Virulence of Highly Effective EPNs to the Sixth-Instar Larvae of S. frugiperda
2.5.4. Virulence of Highly Effective EPNs to S. frugiperda Pupae
2.5.5. Pot Experiment
2.6. Statistical Analysis
3. Results
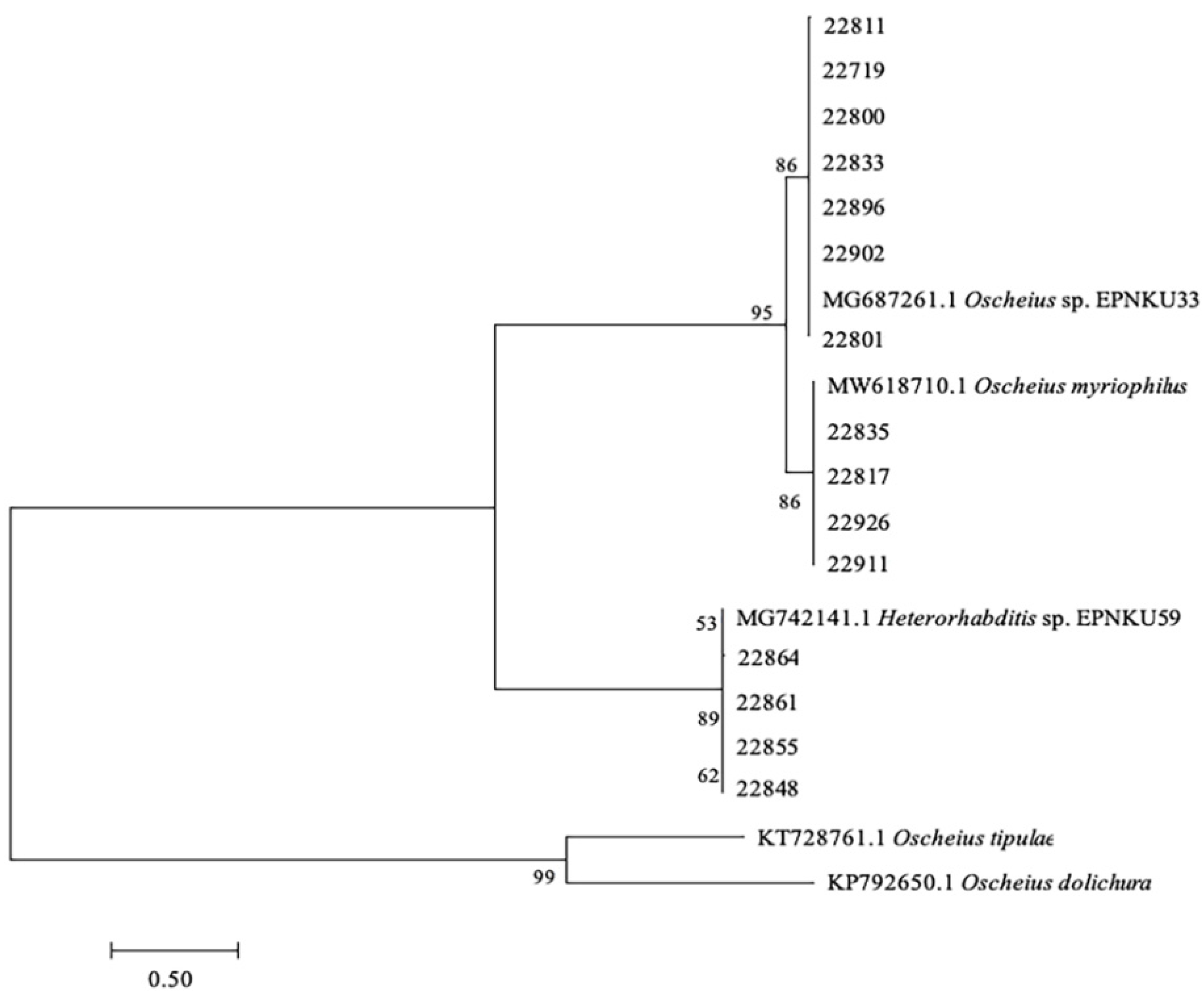
3.1. Identification of EPNs from Soil Samples from Guangxi
3.2. Screening of Highly Virulent EPNs in Guangxi
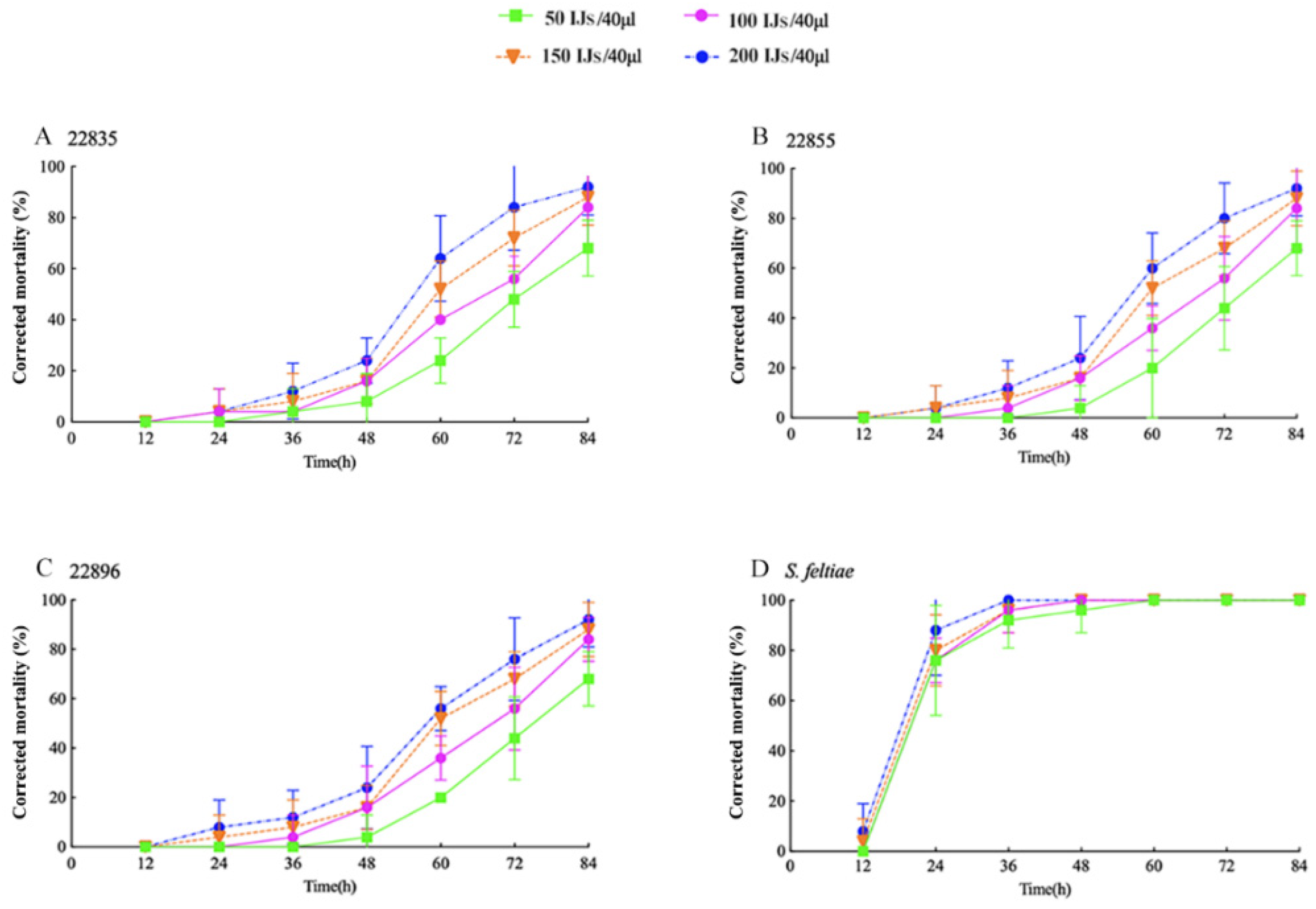
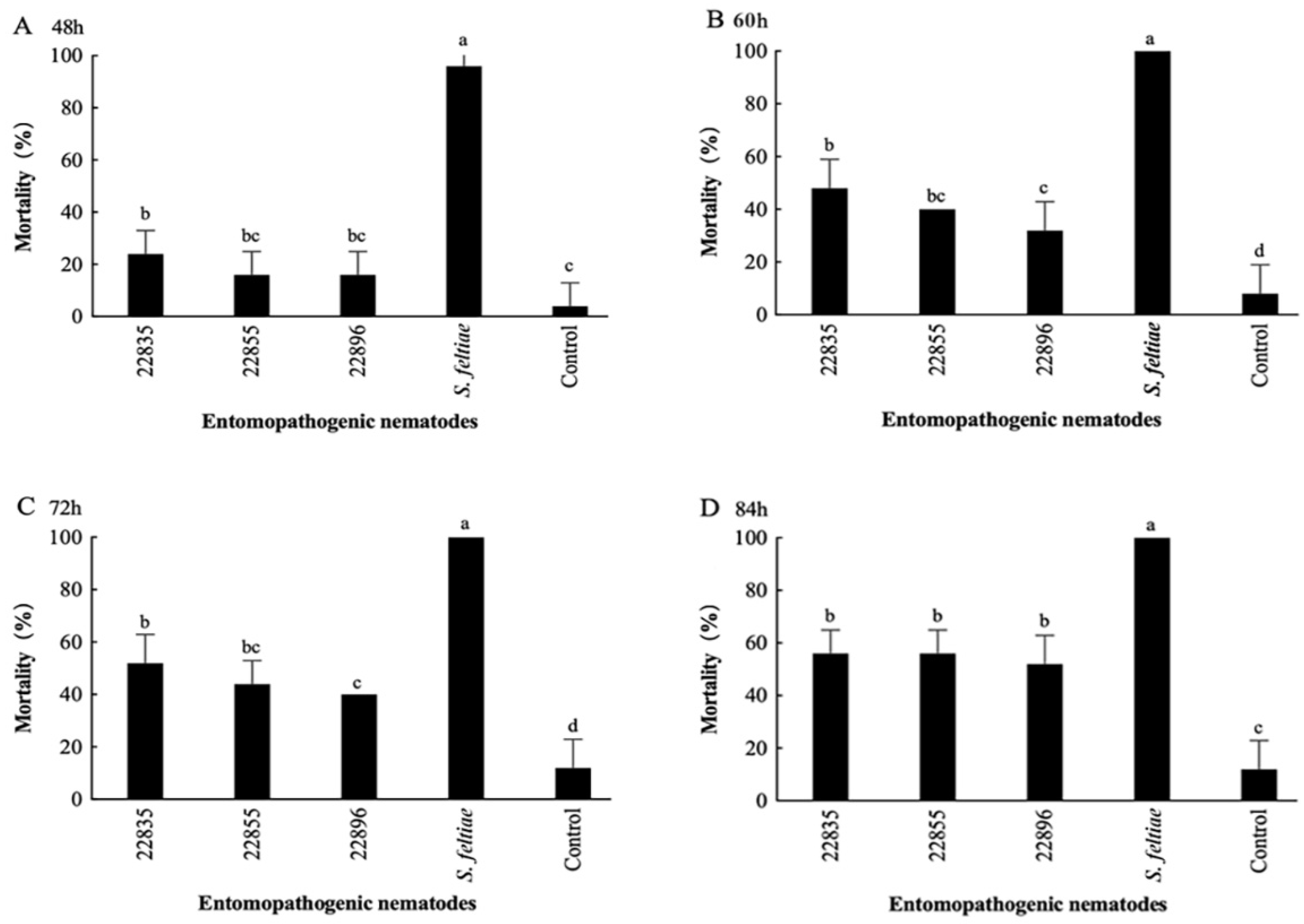
3.3. Virulence of EPNs at Different Concentrations to the Third-Instar Larvae of S. frugiperda
3.4. Virulence of Highly Effective EPNs to the Sixth-Instar Larvae of S. frugiperda
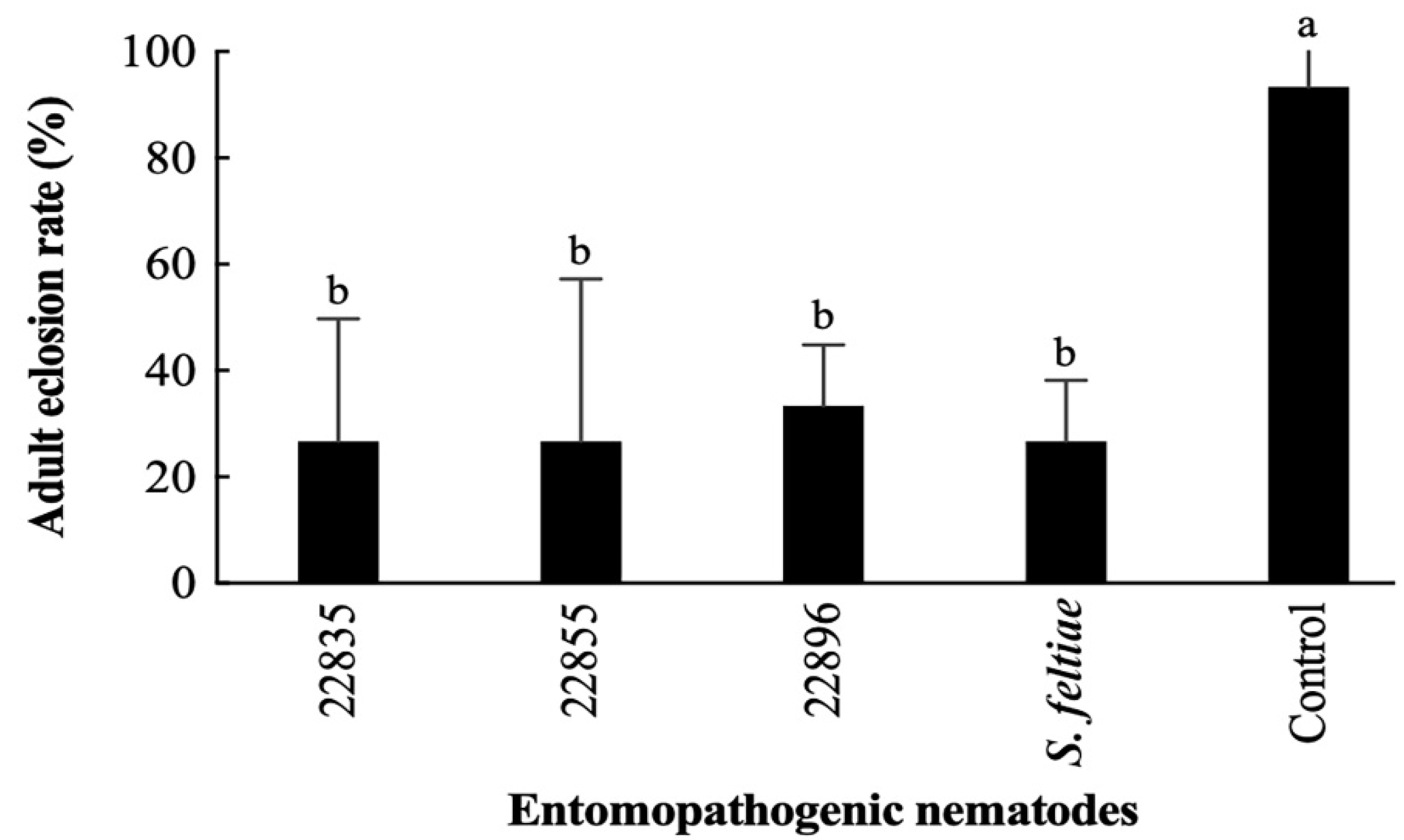
3.5. Virulence of Highly Effective EPNs to S. frugiperda Pupae
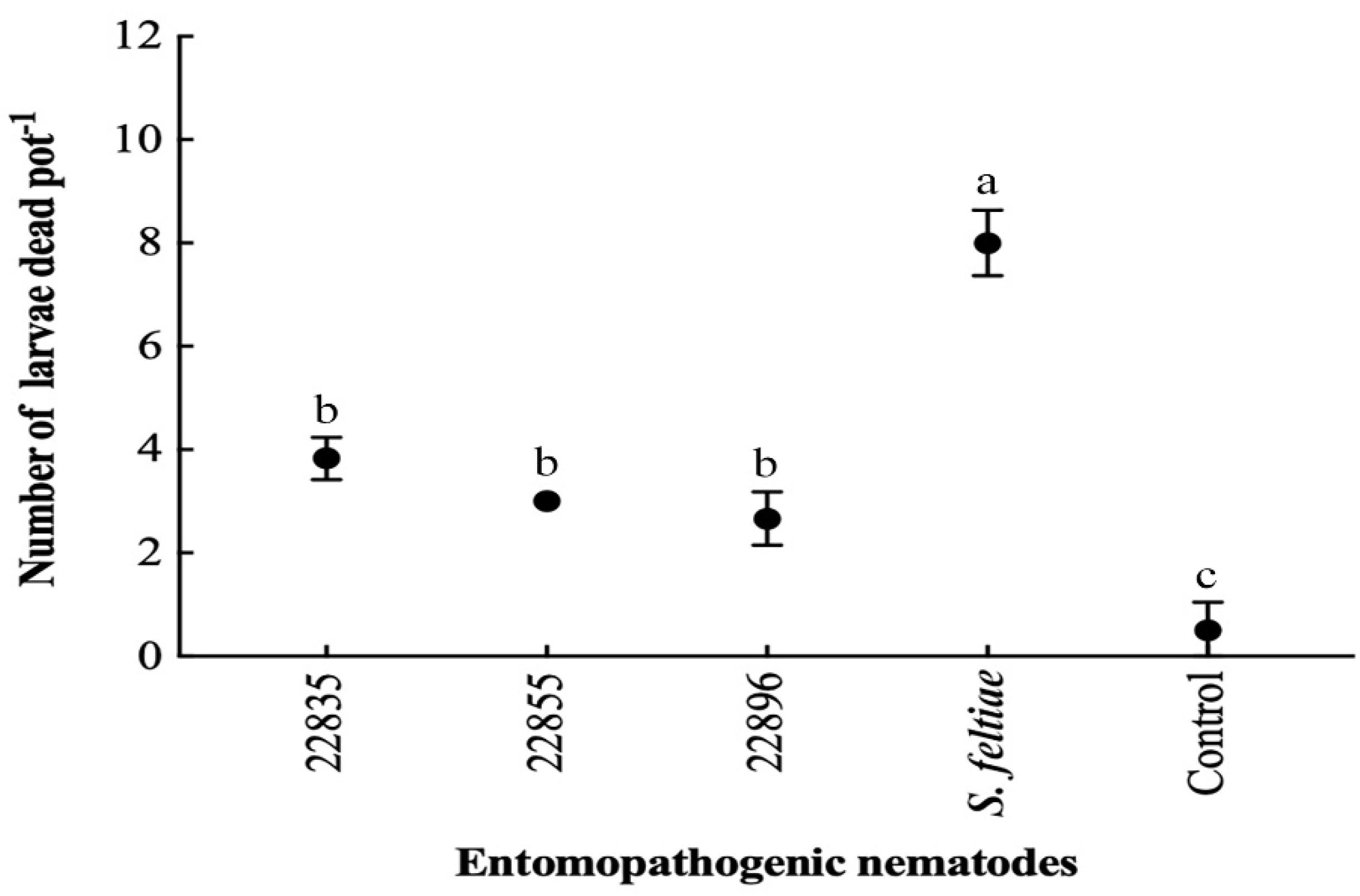
3.6. Pot Experiment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.X.; Gao, H.; Yu, R.N.; Zhang, Y.L.; Feng, F.; Tang, J.; Li, B. Identification and characterization of G protein-coupled receptors in Spodoptera frugiperda (Insecta: Lepidoptera). Gen. Comp. Endocrinol. 2022, 317, 113976. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Y.; Liu, J.; Wu, Q.L.; Ci, R.Z.G.; Zeng, J. Investigation on winter breding and overwintering areas of Spodoptera frugiperda in China. Plant Prot. 2021, 47, 212–217. [Google Scholar]
- Wu, Q.L.; Jiang, Y.Y.; Wu, K.M. Analysis of migration routes of the fall armyworm Spodoptera frugiperda (J.E. Smith) from Myanmar to China. Plant Prot. 2019, 45, 1–6. [Google Scholar]
- Zhang, L.; Jin, M.H.; Zhang, D.D.; Jiang, Y.Y.; Liu, J.; Wu, K.M.; Xiao, Y.T. Molecular identification of invasive fall armyworm Spodoptera frugiperda in Yunnan Province. Plant Prot. 2019, 45, 19–24. [Google Scholar]
- Liang, C.P.; Liang, J.R.; He, J. Biological characteristics of Metarhizium anisopliae and its infection to Spodoptera frugiperda. Acta Agric. Univ. Jiangxiensis 2022, 44, 386–392. [Google Scholar]
- Carvalho, R.A.; Omoto, C.; Field, L.M.; Williamson, M.S.; Bass, C. Investigating the molecular mechanisms of organophosphate and pyrethroid resistance in the fall armyworm Spodoptera frugiperda. PLoS ONE 2013, 8, e62268. [Google Scholar] [CrossRef] [Green Version]
- do Nascimento, A.R.B.; Farias, J.R.; Bernardi, D.; Horikoshi, R.J.; Omoto, C. Genetic basis of Spodoptera frugiperda (Lepidoptera: Noctuidae) resistance to the chitin synthesis inhibitor lufenuron. Pest Manag. Sci. 2016, 72, 810–815. [Google Scholar] [CrossRef]
- Acharya, R.; Hwang, H.-S.; Mostafiz, M.M.; Mostafiz, Y.-S.; Lee, K.-Y. Susceptibility of various developmental stages of the fall armyworm, Spodoptera frugiperda, to entomopathogenic nematodes. Insects 2020, 11, 868. [Google Scholar] [CrossRef]
- Malhat, F.M.; Haggag, M.N.; Loutfy, N.M.; Osman, M.A.M.; Ahmed, M.T. Residues of organochlorine and synthetic pyrethroid pesticides in honey, an indicator of ambient environment, a pilot study. Chemosphere 2015, 120, 457–461. [Google Scholar] [CrossRef]
- Mostafalou, S.; Abdollahi, M. Pesticides and human chronic diseases: Evidences, mechanisms, and perspectives. Toxicol. Appl. Pharmacol. 2013, 268, 157–177. [Google Scholar] [CrossRef]
- Blanco, C.A.; Portilla, M.; Jurat-Fuentes, J.L.; Sánchez, J.F.; Viteri, D.; Vega-Aquino, P.; Terán-Vargas, A.P.; Azuara-Domínguez, A.; López, J.D.; Arias, R. Susceptibility of isofamilies of Spodoptera frugiperda (Lepidoptera: Noctuidae) to Cry1Ac and Cry1Fa proteins of Bacillus thuringiensis. Southwest. Entomol. 2010, 35, 409–415. [Google Scholar] [CrossRef]
- Huang, F.; Qureshi, J.A.; Meagher, R.L.; Reisig, D.D.; Head, G.P.; Andow, D.A.; Ni, X.; Kerns, D.; Buntin, G.D.; Niu, Y. Cry1F resistance in fall armyworm Spodoptera frugiperda: Single gene versus pyramided Bt maize. PLoS ONE 2014, 9, e112958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinelli, S.; de Carvalho, R.A.; Dourado, P.M.; Head, G.P. Resistance of Spodoptera frugiperda to Bacillus thuringiensis proteins in the western Hemisphere. In Bacillus thuringiensis and Lysinibacillus sphaericus; Springer: Cham, Switzerland, 2017; pp. 273–288. [Google Scholar]
- Chandrasena, D.I.; Signorini, A.M.; Abratti, G.N.; Storer, P.M.; Olaciregui, L.A.; Alves, P.; Pilcher, C.D. Characterization of field-evolved resistance to Bacillus thuringiensis-derived Cry1F-endotoxin in Spodoptera frugiperda populations from Argentina. Pest Manag. Sci. 2018, 74, 746–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, X.; Gu, X.H.; Han, R.C. Research advances in using entomopathogenic nematodes to control the fall armyworm, Spodoptera frugiperda. Environ. Entomol. 2019, 41, 695–700. [Google Scholar]
- Brixey, J. The potential for biological control to reduce Hylobius abietis damage. For. Comm. Res. Inf. Note 1997, 273, 7. [Google Scholar]
- Sisay, B.; Simiyu, J.; Mendesil, E.; Likhayo, P.; Ayalew, G.; Mohamed, S.; Subramanian, S.; Tefera, T. Fall armyworm, Spodoptera frugiperda infestations in East Africa: Assessment of damage and parasitism. Insects 2019, 10, 195. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, X.; Song, L.; Zheng, L.; Wei, X.; Gu, X.; Cui, Y.; Hu, B.; Yoshiga, T.; Abd-Elgawad, M.M.; et al. Evaluation of indigenous entomopathogenic nematodes in Southwest China as potential biocontrol agents against Spodoptera litura (Lepidoptera: Noctuidae). J. Nematol. 2021, 53, 1–17. [Google Scholar] [CrossRef]
- Yang, L.K.; Zhu, X.F.; Qian, X.J.; Liu, W.H.; Jiang, H.X.; Liu, C.Z. Pathogenicity of five entomopathogenic nematodes from Gansu Province on Spodoptera frugiperda. Pratac. Sci. 2021, 38, 2069–2076. [Google Scholar]
- Kaya, H.K.; Gaugler, R. Entomopathogenic nematodes. Annu. Rev. Entomol. 1993, 38, 181–206. [Google Scholar] [CrossRef]
- Stock, S.P.; Kusakabe, A.; Orozco, R.A. Secondary metabolites produced by Heterorhabditis symbionts and their application in agriculture: What we know and what to do next. J. Nemato. 2017, 49, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Ji, G.H.; Qian, X.J.; Xing, Y.F.; Liu, C.Z. Changes of the activities of protective enzymes in Pieris rapae infected by Steinernema feltiae. Plant Prot. 2009, 35, 66–69. [Google Scholar]
- Zhang, S.J.; Qian, X.J.; Li, C.J.; Pan, F.J.; Xu, Y.L. Pathogenicity of entomopathogenic nematode on Xestia c-nigrum in Soybean Field. Soybean Sci. 2013, 32, 63–67. [Google Scholar]
- Andalo, V.; Santos, V.; Moreira, G.F.; Moreira, C.C.; Junior, A.M. Evaluation of entomopathogenic nematodes under laboratory and greenhouses conditions for the control of Spodoptera frugiperda. Cienc. Rural. 2010, 40, 1860–1866. [Google Scholar] [CrossRef] [Green Version]
- Caccia, M.G.; Del Valle, E.; Doucet, M.E.; Lax, P. Susceptibility of Spodoptera frugiperda and Helicoverpa gelotopoeon (Lepidoptera: Noctuidae) to the entomopathogenic nematode Steinernema diaprepesi (Rhabditida: Steinernematidae) under laboratory conditions. Chil. J. Agric. Res. 2014, 74, 123–126. [Google Scholar] [CrossRef]
- Garcia, L.C.; Raetano, C.G.; Leite, L.G. Application technology for the entomopathogenic nematodes Heterorhabditis indica and Steinernema sp. (Rhabditida: Heterorhabditidae and Steinernematidae) to control Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) in corn. Neotrop. Entomol. 2008, 37, 305–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viteri, D.M.; Linares, A.M.; Flores, L. Use of the entomopathogenic nematode Steinernema carpocapsae in combination with low-toxicity insecticides to control fall armyworm (Lepidoptera: Noctuidae) Larvae. Fla. Entomol. 2018, 101, 327–329. [Google Scholar] [CrossRef] [Green Version]
- Leyva-Hernandez, H.A.; Garcia-Gutierrez, C.; Ruiz-Vega, J.; Calderon-Vazquez, C.L.; Luna-Gonzalez, A.; Garcia-Salas, S. Evaluation of the Virulence of Steinernema riobrave and Rhabditis blumi against third instar larvae of Spodoptera frugiperda. Southwest. Entomol. 2018, 43, 189–197. [Google Scholar] [CrossRef]
- Li, J.J.; Feng, W.Y. Spatial and temporal changes of vegetation net primary productivity and its relation with climatic factors in Guangxi, China. In Proceedings of the International Conference on Intelligent Earth Observing and Applications, Guilin, China, 23–24 October 2015; SPIE: Bellingham, WA, USA, 2015; Volume 9808. [Google Scholar]
- Noujeim, E.; Sakr, J.; Fanelli, E.; Troccoli, A.; Pages, S.; Tarasco, E.; De Luca, F. Phylogenetic relationships of entomopathogenic nematodes and their bacterial symbionts from coastal areas in Lebanon. Redia 2016, 99, 127–137. [Google Scholar]
- Bedding, R.; Akhurst, R. A simple technique for the detection of insect paristic rhabditid nematodes in soil. Nematologica 1975, 21, 109–110. [Google Scholar] [CrossRef]
- White, G. A method for obtaining invective nematode larvae from culture. Science 1927, 66, 302–303. [Google Scholar] [CrossRef]
- Holterman, M.; Van der Wurff, A.; Van den Elsen, S. Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown clades. Mol. Biol. Evol. 2006, 23, 1792–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrain, T.C.; Wakarchuk, D.A.; Levesque, A.C.; Hamilton, R.I. Intraspecific rDNA restriction fragment length polymorphism in the Xiphinema americanum group. Fundam. Appl. Nematol. 1992, 15, 563–573. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Hall, T. BioEdit version 7.1.7. 2012. Available online: http://www.mbio.ncsu.edu/bioedit/bioedit.html (accessed on 15 April 2022).
- Castro-Ortega, I.D.; Caspeta-Mandujano, J.M.; Suarez-Rodriguez, R.; Pena-Chora, G.; Ramirez-Trujillo, J.A.; Cruz-Perez, K.; Sosa, I.A.; Hernandez-Velazquez, V.M. Oscheius myriophila (Nematoda: Rhabditida) isolated in sugar cane soils in Mexico with potential to be used as entomopathogenic nematode. J. Nematol. 2020, 52, 1–8. [Google Scholar] [CrossRef]
- Goda, N.; Mirzaei, M.; Brunetti, M. Potentially entomopathogenic nematode isolated from Popillia japonica: Bioassay, molecular characterization and the associated microbiota. Bull. Insectology 2020, 73, 295–301. [Google Scholar]
- Ye, W.M.; Foye, S.; MacGuidwin, A.E.; Steffan, S. Incidence of Oscheius onirici (Nematoda: Rhabditidae), a potentially entomopathogenic nematode from the marshlands of Wisconsin. J. Nematol. 2018, 50, 9–26. [Google Scholar] [CrossRef] [Green Version]
- Fallet, P.; Gianni, L.D.; Machado, R.A.R.; Bruno, P.; Bernal, J.S.; Karangwa, P.; Kajuga, J.; Waweru, B.; Bazagwira, D.; Degen, T.; et al. Comparative screening of Mexican, Rwandan and commercial entomopathogenic nematodes to be used against invasive fall armyworm, Spodoptera frugiperda. Insects 2022, 13, 205. [Google Scholar] [CrossRef]
- Patil, J.; Linga, V.; Vijayakumar, R.; Subaharan, L.; Navik, O.; Bakthavatsalam, N.; Mhatrec, P.H.; Sekhard, J. Biocontrol potential of entomopathogenic nematodes for the sustainable management of Spodoptera frugiperda (Lepidoptera: Noctuidae) in maize. Pest Manag. Sci. 2022, 78, 2883–2895. [Google Scholar] [CrossRef]
- Kapranas, A.; Sbaiti, I.; Degen, T.; Turlings, T.C.J. Biological control of cabbage fly Delia radicum with entomopathogenic nematodes: Selecting the most effective nematode species and testing a novel application method. Biol. Control 2020, 144, 104212. [Google Scholar] [CrossRef]
- Öğretmen, A.; Yüksel, E.; Canhilal, R. Susceptibility of larvae of wireworms (Agriotes spp.) (Coleoptera: Elateridae) to some Turkish isolates of entomopathogenic nematodes under laboratory and field conditions. Biol. Control 2020, 149, 104320. [Google Scholar] [CrossRef]
- Patil, J.; Vijayakumar, R.; Linga, V.; Sivakumar, G. Susceptibility of Oriental armyworm, Mythimna separata (Lepidoptera: Noctuidae) larvae and pupae to native entomopathogenic nematodes. J. Appl. Entomol. 2020, 144, 647–654. [Google Scholar] [CrossRef]
- Yan, X.; Shahid Arain, M.; Lin, Y.; Gu, X.; Zhang, L.; Li, J.; Han, R. Efficacy of entomopathogenic nematodes against the tobacco cutworm, Spodoptera litura (Lepidoptera: Noctuidae). J. Econ. Entomol. 2020, 113, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.R.; Li, Z.Y.; Dai, Q.X.; Lu, Y.Y.; Chen, K.W.; Wang, L. Virulence of four entomopathogenic nematodes on fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Bios. 2020, 29, 82–89. [Google Scholar]
- Kaya, H.K.; Hara, A.H. Susceptibility of various species of lepidopterous pupae to the entomogenous nematode Neoaplectana carpocapsae. J. Nematol. 1981, 13, 291–294. [Google Scholar] [PubMed]
- Kondo, E.; Ishibashi, N. Infection efficacy of Steinernema feltiae (DD-136) to the common cutworm, Spodoptera litura (Lepidoptera: Noctuidae), on the soil. Appl. Entomol. Zool. 1986, 21, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, K.; Gopalakrishnan, C. Effect of entomogenous nemamode, Steinernema feltiae (Rhabditida: Steinernematldae) to the pre-pupa, pupa and adult of Spodoptera litura (Noctuidae: Lepidoptera). Indian J. Nematol. 1987, 17, 273–276. [Google Scholar]
- Fuxa, J.R.; Richter, A.R.; Acudelo-Silva, F. Effect of host age and nematode strain on susceptibility of Spodoptera frugiperda to Steinernema feltiae. J. Nematol. 1988, 20, 91–95. [Google Scholar]
- Lacey, L.A.; Georgis, R. Entomopathogenic nematodes for control of insect pests above and below ground with comments on commercial production. J. Nematol. 2012, 44, 218–225. [Google Scholar]
- Acar, I.; Sipes, B. Enhancing the biological control potential of Steinernema feltiae with protection from desiccation and UV radiation. Biol. Control 2022, 169, 104874. [Google Scholar] [CrossRef]
- Odendaal, D.; Addison, M.F.; Malan, A.P. Entomopathogenic nematodes for the control of the codling moth (Cydia pomonella L.) in field and laboratory trials. J. Helminthol. 2016, 90, 615–623. [Google Scholar] [CrossRef]


| EPNs | LT50 (h) | 95% FL | χ2 | p |
|---|---|---|---|---|
| 22719 | 66.312 | 61.764–71.545 | 1.459 | 0.918 |
| 22800 | 67.231 | 62.613–72.706 | 0.371 | 0.996 |
| 22801 | 68.593 | 63.828–74.495 | 3.365 | 0.644 |
| 22811 | 67.054 | 62.089–73.206 | 1.498 | 0.913 |
| 22817 | 66.258 | 61.564–71.800 | 0.577 | 0.989 |
| 22833 | 66.846 | 62.075–72.621 | 0.86 | 0.973 |
| 22835 | 58.634 | 48.805–71.115 | 10.303 | 0.067 |
| 22848 | 65.116 | 60.165–71.121 | 1.504 | 0.913 |
| 22855 | 60.135 | 55.672–64.891 | 0.909 | 0.97 |
| 22861 | 66.217 | 61.769–71.291 | 1.83 | 0.872 |
| 22864 | 66.281 | 61.205–72.678 | 1.847 | 0.87 |
| 22896 | 61.388 | 57.068–65.917 | 2.311 | 0.802 |
| 22902 | 67.36 | 62.593–73.202 | 0.543 | 0.99 |
| 22911 | 66.717 | 62.290–71.815 | 1.141 | 0.95 |
| 22926 | 65.681 | 61.096–70.983 | 1.557 | 0.906 |
| Source | DF | F Value | p |
|---|---|---|---|
| EPN strains (S) | 3 | 10.446 | 0.003 |
| Concentration (C) | 3 | 9 | 0.005 |
| S × C | 9 | 1.287 | 0.262 |
| Error | 64 | ||
| Corrected total | 79 |
| EPNs | LT50 (h) | 95% FL | χ2 | p |
|---|---|---|---|---|
| 22835 | 73.821 | 61.145–100.628 | 3.291 | 0.655 |
| 22855 | 82.603 | 67.903–117.839 | 6.825 | 0.234 |
| 22896 | 80.415 | 69.855–104.185 | 0.554 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.; Fang, M.; Sun, J.; Wei, X.; Ruan, W. Investigation of Indigenous Entomopathogenic Nematodes in Guangxi and Its Biological Control of Spodoptera frugiperda. Agronomy 2022, 12, 2536. https://doi.org/10.3390/agronomy12102536
Wang A, Fang M, Sun J, Wei X, Ruan W. Investigation of Indigenous Entomopathogenic Nematodes in Guangxi and Its Biological Control of Spodoptera frugiperda. Agronomy. 2022; 12(10):2536. https://doi.org/10.3390/agronomy12102536
Chicago/Turabian StyleWang, Ailing, Ming Fang, Jie Sun, Xianqin Wei, and Weibin Ruan. 2022. "Investigation of Indigenous Entomopathogenic Nematodes in Guangxi and Its Biological Control of Spodoptera frugiperda" Agronomy 12, no. 10: 2536. https://doi.org/10.3390/agronomy12102536
APA StyleWang, A., Fang, M., Sun, J., Wei, X., & Ruan, W. (2022). Investigation of Indigenous Entomopathogenic Nematodes in Guangxi and Its Biological Control of Spodoptera frugiperda. Agronomy, 12(10), 2536. https://doi.org/10.3390/agronomy12102536






