Uniconazole and Adaptability of Transplantations by Enhancing the Competition Tolerance in a High Sowing Density of Rapeseed Blanket Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Design
2.2. Seeding and Pre-Seeding Processes
2.3. Pot Experiment
2.4. Sample Collection and Measurement
2.5. Data analysis
3. Results
3.1. Sowing Density Effect on Seedling Performance
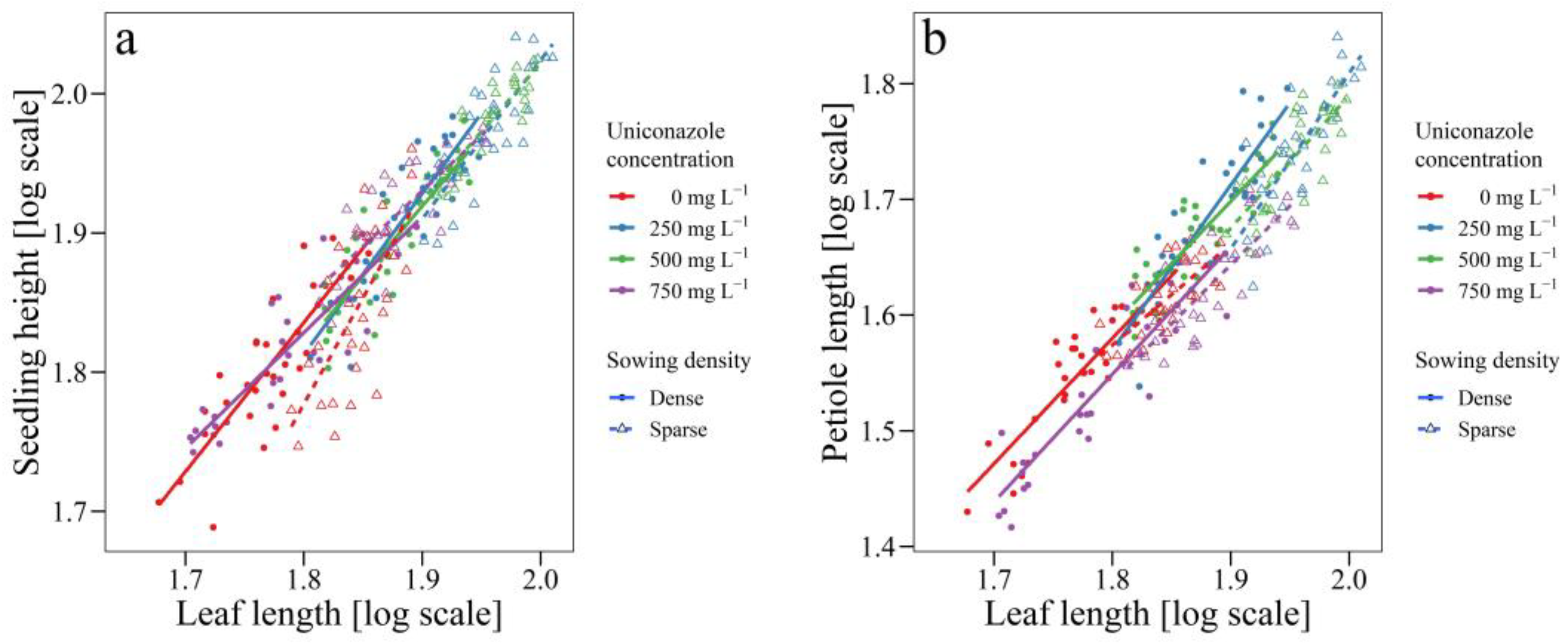
3.2. Uniconazole Effect on Seedling Architecture
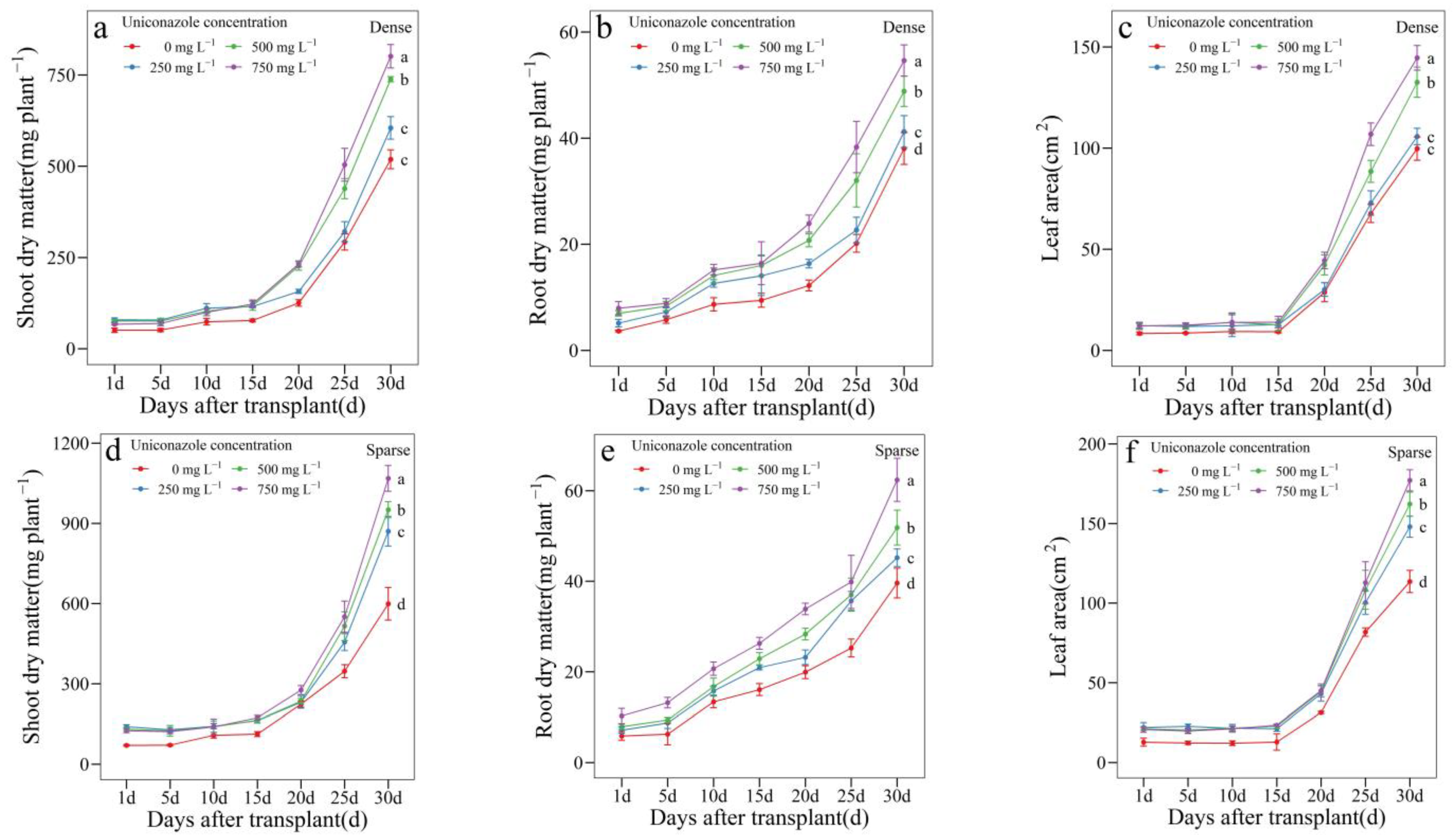
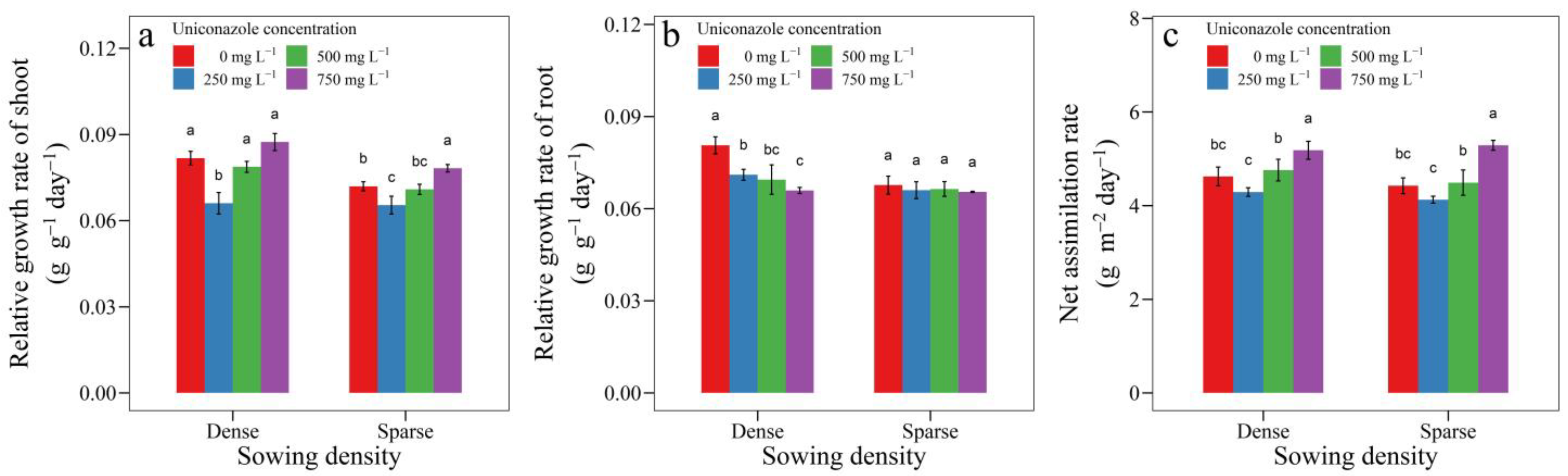
3.3. Uniconazole Effect on Seedling Growth and Allocation
3.4. Principal Coordinate Analysis
3.5. Integrated Evaluation of Seedling Performance during Transplant Shock and Mature Stages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, Z.; Ji, Y.; Sun, L.; Xu, X.; Fan, D.; Zhong, H.; Liang, Z.; Ficsher, G. Changes in production potentials of rapeseed in the Yangtze River Basin of China under climate change: A multi-model ensemble approach. J. Geogr. Sci. 2018, 28, 1700–1714. [Google Scholar] [CrossRef] [Green Version]
- Ji, C.; Zhai, Y.; Zhang, T.; Shen, X.; Bai, Y.; Hong, J. Carbon, energy and water footprints analysis of rapeseed oil production: A case study in China. J. Environ. Manag. 2021, 287, 112359. [Google Scholar] [CrossRef]
- Tao, J.; Wu, W.; Liu, W.; Xu, M. Exploring the spatio-temporal dynamics of winter rape on the middle reaches of Yangtze River Valley using time-series MODIS Data. Sustainability 2020, 12, 466. [Google Scholar] [CrossRef] [Green Version]
- Ma, P.; Lan, Y.; Lyu, T.; Zhang, Y.; Lin, D.; Li, F.; Li, Y.; Yang, Z.; Sun, Y.; Ma, J. Improving rice yields and nitrogen use efficiency by optimizing nitrogen management and applications to rapeseed in rapeseed-rice rotation system. Agronomy 2020, 10, 1060. [Google Scholar] [CrossRef]
- Anwar, S.; Lei, H.; Kuai, J.; Khan, S.; Fahad, S.; Zhou, G. Soaking seeds of winter rapeseed with Quizalofop-P-Ethyl alters plant growth and improves yield in a rice-rapeseed cropping system. Field Crops Res. 2017, 208, 11–17. [Google Scholar] [CrossRef]
- Tian, Z.; Ji, Y.; Xu, H.; Qiu, H.; Sun, L.; Zhong, H.; Liu, J. The potential contribution of growing rapeseed in winter fallow fields across Yangtze River Basin to energy and food security in China. Resour. Conserv. Recycl. 2021, 164, 105159. [Google Scholar] [CrossRef]
- Ren, Y.; Zhu, J.; Zhang, H.; Lin, B.; Hao, P.; Hua, S. Leaf carbohydrate metabolism variation caused by late planting in rapeseed (Brassica napus L.) at reproductive stage. Plants 2022, 11, 1696. [Google Scholar] [CrossRef]
- Wang, S.; Wang, E.; Wang, F.; Tang, L. Phenological development and grain yield of canola as affected by sowing date and climate variation in the Yangtze River Basin of China. Crop Pasture Sci. 2012, 63, 478–488. [Google Scholar] [CrossRef]
- Cong, R.; Wang, Y.; Li, X.; Ren, T.; Lu, J. Differential responses of seed yield and yield components to nutrient deficiency between direct sown and transplanted winter oilseed rape. Int. J. Plant Prod. 2020, 14, 77–92. [Google Scholar] [CrossRef]
- Ren, T.; Liu, B.; Lu, J.; Deng, Z.; Li, X.; Cong, R. Optimal plant density and N fertilization to achieve higher seed yield and lower N surplus for winter oilseed rape (Brassica napus L.). Field Crops Res. 2017, 204, 199–207. [Google Scholar] [CrossRef]
- Fischer, G.; Winiwarter, W.; Cao, G.Y.; Ermolieva, T.; Hizsnyik, E.; Klimont, Z.; Wiberg, D.; Zheng, X.Y. Implications of population growth and urbanization on agricultural risks in China. Popul. Environ. 2012, 33, 243–258. [Google Scholar] [CrossRef]
- Jiang, L.; Wu, S.; Liu, Y. Change analysis on the Spatio-Temporal patterns of main crop planting in the Middle Yangtze Plain. Remote Sens. 2022, 14, 1141. [Google Scholar] [CrossRef]
- Hu, Q.; Hua, W.; Yin, Y.; Zhang, X.; Liu, L.; Shi, J.; Zhao, Y.; Qin, L.; Chen, C.; Wang, H. Rapeseed research and production in China. Crop J. 2017, 5, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Wu, C.; Tang, Q.; Zhang, M.; Wang, G.; Wu, J. Critical equation of seedling block falling off in transplanting process and the optimization experiment of rape blanket seedling transplanter. Int. J. Agric. Biol. Eng. 2019, 12, 87–96. [Google Scholar] [CrossRef]
- Novoplansky, A. Picking battles wisely: Plant behaviour under competition. Plant Cell Environ. 2009, 32, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Q.; Zheng, J.; You, J.; Wang, L.; Yang, G.; Leng, S. Effects of nitrogen rate on growth and quality of rapeseed blanket seedling for mechanized transplanting. J. Plant Nutr. 2022, 45, 2515–2522. [Google Scholar] [CrossRef]
- Chang, S.; Li, C.; Yao, X.; Chen, S.; Jiao, X.; Liu, X.; Xu, Z.; Guan, R. Morphological, photosynthetic, and physiological responses of rapeseed leaf to different combinations of red and blue lights at the rosette stage. Front. Plant Sci. 2016, 7, 1144. [Google Scholar] [CrossRef] [Green Version]
- Ballare, C.L.; Pierik, R. The shade-avoidance syndrome: Multiple signals and ecological consequences. Plant Cell Environ. 2017, 40, 2530–2543. [Google Scholar] [CrossRef]
- Wang, X.; Gao, X.; Liu, Y.; Fan, S.; Ma, Q. Progress of research on the regulatory pathway of the plant shade-avoidance syndrome. Front. Plant Sci. 2020, 11, 439. [Google Scholar] [CrossRef] [Green Version]
- Kurepin, L.V.; Pharis, R.P. Light signaling and the phytohormonal regulation of shoot growth. Plant Sci. 2014, 229, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhu, J.; Hussain, N.; Ma, S.; Ye, G.; Zhang, D.; Hua, S. Seedling age and quality upon transplanting affect seed yield of canola (Brassica napus L.). Can. J. Plant Sci. 2014, 94, 1461–1469. [Google Scholar] [CrossRef] [Green Version]
- Izumi, K.; Kamiya, Y.; Sakurai, A.; Oshio, H.; Takahashi, N. Studies of sites of action of a new plant growth retardant (E)-1-(4-Chlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)-1-penten-3-ol (S-3307) and comparative effects of its stereoisomers in a cell-free system from cucurbita maxima. Plant Cell Physiol. 1985, 26, 821–827. [Google Scholar]
- Saito, S.; Okamoto, M.; Shinoda, S.; Kushiro, T.; Koshiba, T.; Kamiya, Y.; Hirai, N.; Todoroki, Y.; Sakata, K.; Nambara, E.; et al. A plant growth retardant, uniconazole, is a potent inhibitor of ABA catabolism in arabidopsis. Biosci. Biotechnol. Biochem. 2006, 70, 1731–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, E.; Ogura, T.; Takei, K.; Kojima, M.; Kitahata, N.; Sakakibara, H.; Asami, T.; Shimada, Y. Uniconazole, a cytochrome P450 inhibitor, inhibits trans-zeatin biosynthesis in arabidopsis. Phytochemistry 2013, 87, 30–38. [Google Scholar] [CrossRef]
- Luo, K.; Xie, C.; Wang, J.; Du, Q.; Cheng, P.; Wang, T.; Wu, Y.; Yang, W.; Yong, T. Uniconazole, 6-benzyladenine, and diethyl aminoethyl hexanoate increase the yield of soybean by improving the photosynthetic efficiency and increasing grain filling in maize-soybean relay strip intercropping system. J. Plant Growth Regul. 2021, 40, 1869–1880. [Google Scholar] [CrossRef]
- Cha-um, S.; Puthea, O.; Kirdmanee, C. An effective in-vitro acclimatization using uniconazole treatments and ex-vitro adaptation of Phalaenopsis orchid. Sci. Hortic. 2009, 121, 468–473. [Google Scholar] [CrossRef]
- Zhou, W.J.; Leul, M. Uniconazole-induced tolerance of rape plants to heat stress in relation to changes in hormonal levels, enzyme activities and lipid peroxidation. Plant Growth Regul. 1999, 27, 99–104. [Google Scholar] [CrossRef]
- Schluttenhofer, C.M.; Massa, G.D.; Mitchell, C.A. Use of uniconazole to control plant height for an industrial/pharmaceutical maize platform. Ind. Crops Prod. 2011, 33, 720–726. [Google Scholar] [CrossRef]
- Agehara, S.; Leskovar, D.I. Growth suppression by exogenous abscisic acid and uniconazole for prolonged marketability of tomato transplants in commercial conditions. Hortscience 2017, 52, 606–611. [Google Scholar] [CrossRef]
- Zhang, W.J.; Yao, X.; Duan, X.J.; Liu, Q.M.; Tang, Y.Q.; Li, J.Y.; Li, G.H.; Ding, Y.F.; Liu, Z.H. Foliar application uniconazole enhanced lodging resistance of hybrid indica rice by altering basal stem quality under poor light stress. Agron. J. 2022, 114, 524–544. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmad, S.; Kamran, M.; Yang, X.N.; Hou, F.J.; Yang, B.P.; Ding, R.X.; Liu, T.; Han, Q.F. Uniconazole and nitrogen fertilization trigger photosynthesis and chlorophyll fluorescence, and delay leaf senescence in maize at a high population density. Photosynthetica 2021, 59, 192–202. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmad, S.; Yang, X.N.; Meng, X.P.; Yang, B.P.; Liu, T.; Han, Q.F. Effect of uniconazole and nitrogen level on lodging resistance and yield potential of maize under medium and high plant density. Plant Biol. 2021, 23, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Wang, R.M.; Yan, J.Z.; Hu, J. Seed film coating with uniconazole improves rape seedling growth in relation to physiological changes under waterlogging stress. Plant Growth Regul. 2005, 47, 75–81. [Google Scholar] [CrossRef]
- Warton, D.I.; Duursma, R.A.; Falster, D.S.; Taskinen, S. smatr 3-an R package for estimation and inference about allometric lines. Methods Ecol. Evol. 2012, 3, 257–259. [Google Scholar] [CrossRef]
- Simier, M.; Thioulouse, J.; Olivier, J.-M. ADE-4 software: A tool for multivariate analysis and graphical display. Oceanis 1998, 24, 393–416. [Google Scholar]
- Valladares, F.; Niinemets, U. Shade tolerance, a key plant feature of complex nature and consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Shen, N.; Yu, D.; Liu, C. Effects of temporal heterogeneity of water supply and spatial heterogeneity of soil nutrients on the growth and intraspecific competition of bolboschoenus yagara depend on plant density. Front. Plant Sci. 2019, 9, 1987. [Google Scholar] [CrossRef] [Green Version]
- Clerget, B.; Bueno, C.; Domingo, A.J.; Layaoen, H.L.; Vial, L. Leaf emergence, tillering, plant growth, and yield in response to plant density in a high-yielding aerobic rice crop. Field Crops Res. 2016, 199, 52–64. [Google Scholar] [CrossRef]
- Sandhu, R.K.; Boyd, N.S.; Zotarelli, L.; Agehara, S.; Peres, N. Effect of planting density on the yield and growth of intercropped tomatoes and peppers in florida. Hortscience 2021, 56, 286–290. [Google Scholar] [CrossRef]
- Williams, M., II; Hausman, N.; Dhaliwal, D.; Grift, T.; Bohn, M. Economic optimum plant density of sweet corn does not increase root lodging incidence. Crop Sci. 2021, 61, 3637–3646. [Google Scholar] [CrossRef]
- Zuo, Q.; You, J.; Wang, L.; Zheng, J.; Li, J.; Qian, C.; Lin, G.; Yang, G.; Leng, S. A balanced sowing density improves quality of rapeseed blanket seedling. Agronomy 2022, 12, 1539. [Google Scholar] [CrossRef]
- Zhang, M.; Duan, L.; Tian, X.; He, Z.; Li, J.; Wang, B.; Li, Z. Uniconazole-induced tolerance of soybean to water deficit stress in relation to changes in photosynthesis, hormones and antioxidant system. J. Plant Physiol. 2007, 164, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Shi, Y. Preparation and application of a novel environmentally friendly organic seed coating for rice. J. Sci. Food Agric. 2009, 89, 2181–2185. [Google Scholar] [CrossRef]
- Pilar, M.C.; Ortega, N.; Perez-Mateos, M.; Busto, M.D. Alkaline Phosphatase-polyresorcinol complex: Characterization and application to seed coating. J. Agric. Food Chem. 2009, 57, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.O.; Omena-Garcia, R.P.; Oliveira, F.S.; Silva, W.A.; Hajirezaei, M.-R.; Vallarino, J.G.; Ribeiro, D.M.; Fernie, A.R.; Nunes-Nesi, A.; Araujo, W.L. Differential root and shoot responses in the metabolism of tomato plants exhibiting reduced levels of gibberellin. Environ. Exp. Bot. 2019, 157, 331–343. [Google Scholar] [CrossRef]
- Gao, X.-H.; Xiao, S.-L.; Yao, Q.-F.; Wang, Y.-J.; Fu, X.-D. An updated GA signaling ‘relief of repression’ regulatory model. Mol. Plant 2011, 4, 601–606. [Google Scholar] [CrossRef]
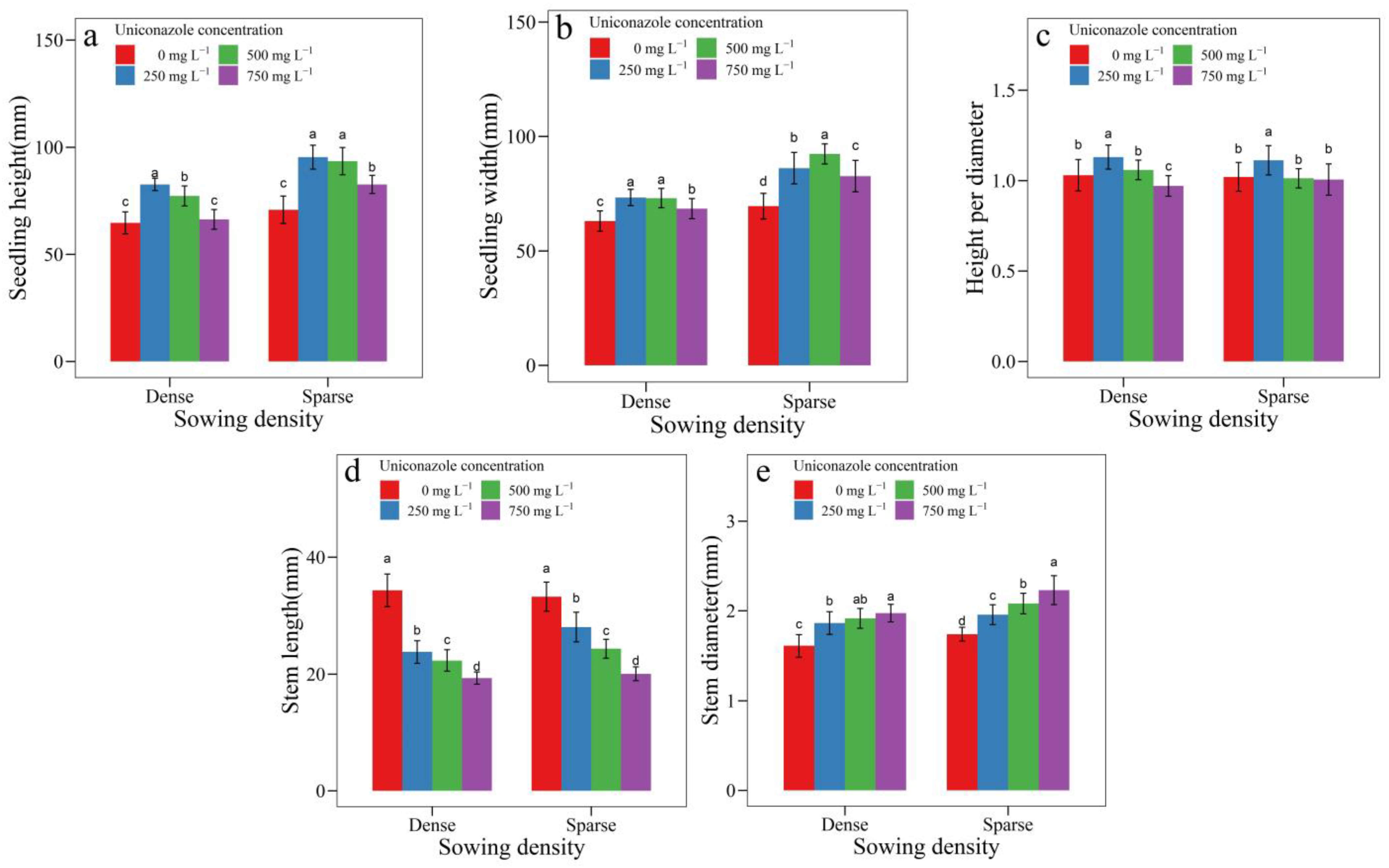
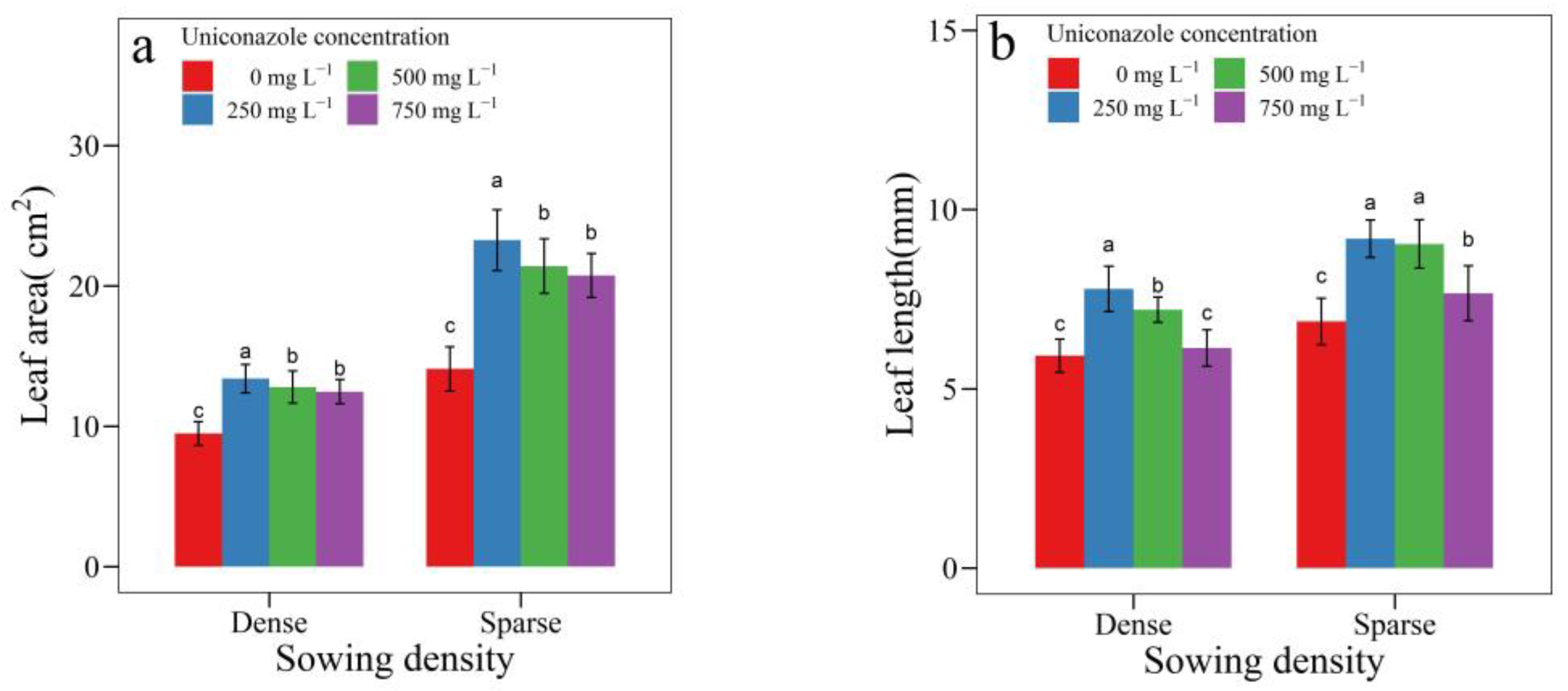
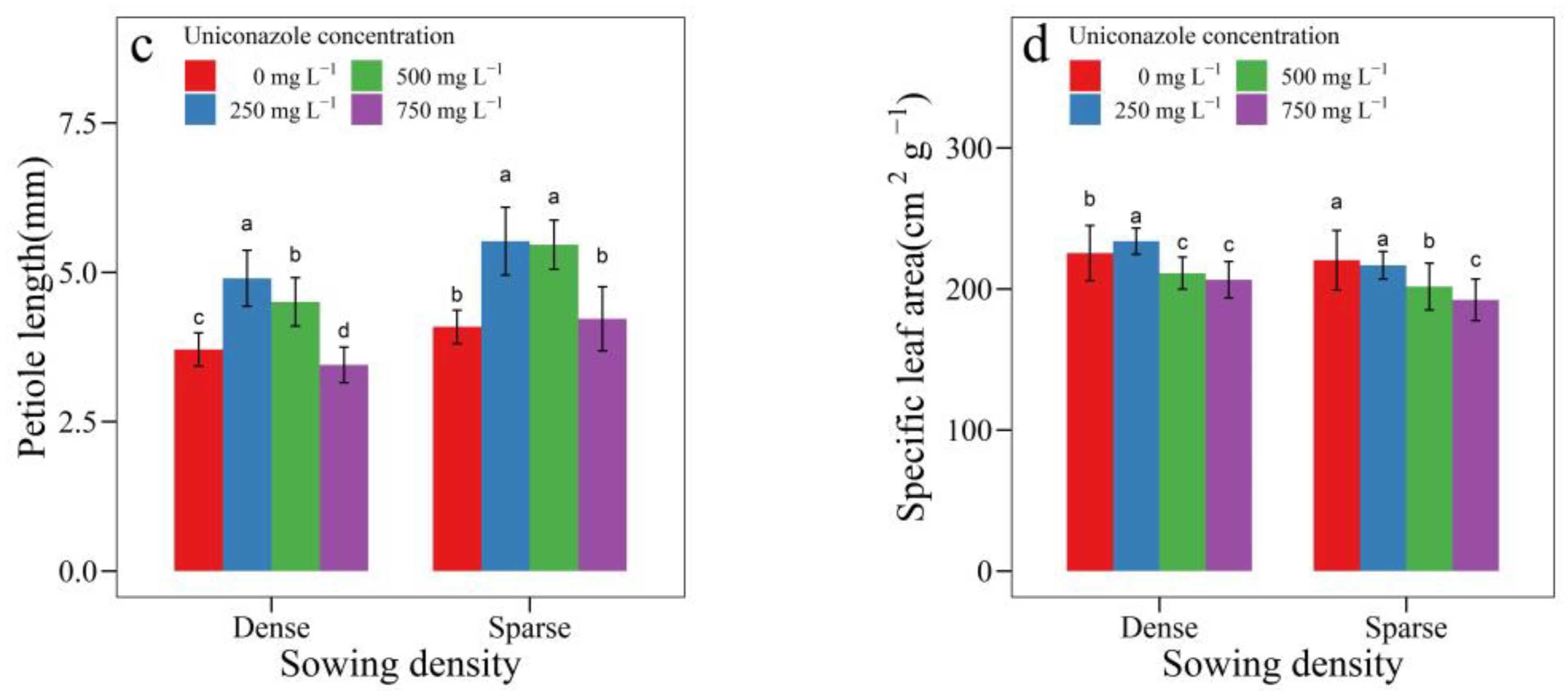
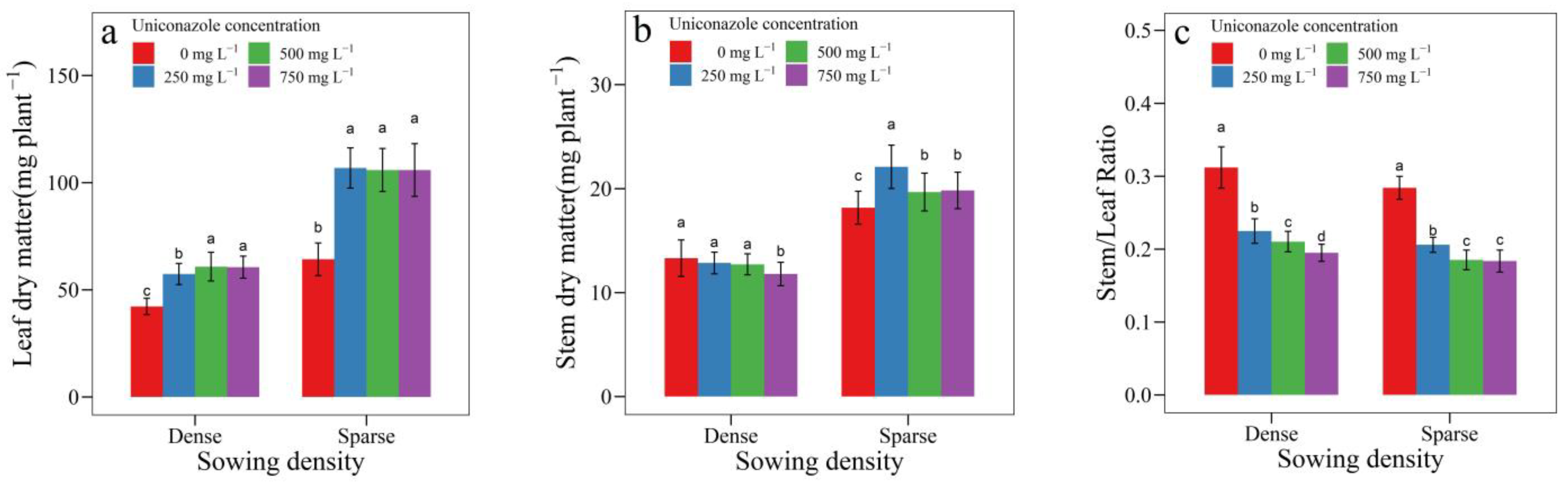


| SowingDensity | Seedling Height (mm) | Seedling Width (mm) | Height per Diameter | Stem Length (mm) | Stem Diameter (mm) | Leaf Area (cm2) | Leaf Length (cm) | Petiole Length (cm) |
|---|---|---|---|---|---|---|---|---|
| 200 | 80.29 a | 83.50 a | 0.97 c | 31.84 c | 2.11 a | 25.96 a | 3.67 a | 4.37 a |
| 400 | 71.09 b | 69.86 b | 1.03 abc | 33.20 bc | 1.73 b | 15.08 b | 2.83 b | 4.16 ab |
| 600 | 70.60 b | 63.66 c | 1.11 ab | 34.40 b | 1.71 b | 12.43 c | 2.75 b | 4.03 b |
| 800 | 63.89 c | 62.63 c | 1.01 bc | 34.50 b | 1.59 c | 9.59 d | 2.39 c | 3.55 c |
| 1000 | 62.71 c | 57.01 d | 1.12 a | 42.24 a | 1.37 d | 7.61 e | 2.27 c | 3.15 d |
| Sowing Density | Leaf Dry Matter (mg plant−1) | Stem Dry Matter (mg plant−1) | Stem/Leaf Ratio | Shoot Biomass (g/m2) | Root Biomass (g/m2) | Root/Shoot Ratio |
|---|---|---|---|---|---|---|
| 200 | 109.34 a | 24.32 a | 0.23 d | 133.13 c | 12.01 d | 0.09 c |
| 400 | 68.90 b | 18.91 b | 0.28 c | 132.33 c | 21.32 c | 0.16 b |
| 600 | 60.43 b | 18.79 b | 0.33 b | 182.74 a | 30.36 b | 0.17 b |
| 800 | 41.46 c | 13.30 c | 0.33 b | 165.63 ab | 41.07 a | 0.25 a |
| 1000 | 30.35 d | 11.69 d | 0.39 a | 157.43 b | 37.52 ab | 0.24 a |
| Sowing Density | Concentration | Intercept (logα) | Slope (β) | Significance of Slope | 95% Confidence Intervals of Slope |
|---|---|---|---|---|---|
| Dense | Seedling height vs. Leaf length | ||||
| 0 mg L−1 | −0.4401 | 1.2674 | a | 1.0323–1.5560 | |
| 250 mg L−1 | −0.5447 | 1.3029 | a | 1.0898–1.5577 | |
| 500 mg L−1 | −0.3226 | 1.1831 | ab | 0.9651–1.4503 | |
| 750 mg L−1 | 0.1528 | 0.9314 | b | 0.7838–1.1068 | |
| Petiole height vs. Leaf length | |||||
| 0 mg L−1 | −0.6297 | 1.2298 | b | 1.0257–1.4745 | |
| 250 mg L−1 | −1.4075 | 1.644 | a | 1.3561–1.9930 | |
| 500 mg L−1 | −0.6597 | 1.244 | b | 1.0253–1.5094 | |
| 750 mg L−1 | 0.6366 | 1.2148 | b | 1.0399–1.4273 | |
| Sparse | Seedling height vs. Leaf length | ||||
| 0 mg L−1 | −2.1771 | 2.1804 | a | 1.6606–2.8629 | |
| 250 mg L−1 | −0.7763 | 1.4059 | b | 1.1234–1.7595 | |
| 500 mg L−1 | −0.1709 | 1.0991 | bc | 0.9257–1.3049 | |
| 750 mg L−1 | 0.9749 | 0.975 | c | 0.7817–1.2160 | |
| Petiole height vs. Leaf length | |||||
| 0 mg L−1 | −0.6118 | 1.206 | b | 0.9264–1.5700 | |
| 250 mg L−1 | −1.8916 | 1.8577 | a | 1.4888–2.3179 | |
| 500 mg L−1 | −1.096 | 1.4499 | ab | 1.1470–1.8329 | |
| 750 mg L−1 | −0.6749 | 1.2219 | b | 0.9888–1.5101 | |
| Density | Concentration | Effective Branches | Main Effective Pods | Branch Effective Pods | Seeds per Silique | 1000-Grain Weight | Grain Yield |
|---|---|---|---|---|---|---|---|
| Dense | 0 mg L−1 | 6.11 c | 74.25 b | 199.13 f | 20.64 d | 4.13 c | 18.08 f |
| 250 mg L−1 | 7.33 b | 83.00 a | 249.67 e | 21.28 cd | 4.14 bc | 21.83 e | |
| 500 mg L−1 | 7.43 b | 83.33 a | 320.17 cd | 23.53 ab | 4.24 abc | 24.98 d | |
| 750 mg L−1 | 7.38 b | 85.29 a | 366.83 b | 23.61 ab | 4.37 a | 28.08 c | |
| Spare | 0 mg L−1 | 6.67 bc | 85.80 a | 309.20 d | 22.44 bc | 4.27 abc | 26.62 c |
| 250 mg L−1 | 7.00 bc | 85.14 a | 342.00 c | 23.35 ab | 4.34 a | 28.00 c | |
| 500 mg L−1 | 7.00 b | 85.83 a | 372.00 b | 23.83 a | 4.38 a | 30.64 b | |
| 750 mg L−1 | 8.50 a | 86.17 a | 413.86 a | 24.22 a | 4.32 ab | 32.52 a | |
| F-value | |||||||
| density | 1.863 ns | 16.820 ** | 245.631 ** | 16.395 ** | 8.8561 ** | 254.999 ** | |
| concentration | 7.548 ** | 6.761 ** | 116.771 ** | 13.297 ** | 3.326 * | 79.321 ** | |
| density concentration | 2.482 ns | 4.606 ** | 7.621 ** | 1.961 ns | 1.651 ns | 4.895 ** | |
| Stage | Indicator | Absolute Size (PCoA1) | Competition Tolerance (PCoa2) | ||
|---|---|---|---|---|---|
| Correlation | p-Value | Correlation | p-Value | ||
| Transplant shock stage | RGR of shoot | −0.3148 | 0.4916 | 0.6777 | 0.0943 |
| RGR of root | −0.3685 | 0.4160 | −0.5784 | 0.1737 | |
| NAR | 0.1698 | 0.7159 | 0.9171 | 0.0036 | |
| Growth of dry matter | 0.6835 | 0.0905 | 0.8398 | 0.0181 | |
| Extension of leaf area | 0.5933 | 0.1602 | 0.8662 | 0.0117 | |
| Yield components | Effective branches | 0.5427 | 0.2082 | 0.7470 | 0.0537 |
| main effective pots | 0.4543 | 0.3058 | 0.5761 | 0.1758 | |
| branches effective pots | 0.5359 | 0.2150 | 0.9095 | 0.0045 | |
| seed per silique | 0.1804 | 1.5561 | 0.8115 | 0.0267 | |
| 1000-grain-weight | 0.4275 | 0.3387 | 0.7026 | 0.0783 | |
| yield | 0.5486 | 0.2022 | 0.9108 | 0.0044 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Zhang, Y.; Leng, S.; Wang, Z.; Gong, C.; Zuo, Q.; Yang, G. Uniconazole and Adaptability of Transplantations by Enhancing the Competition Tolerance in a High Sowing Density of Rapeseed Blanket Seedlings. Agronomy 2022, 12, 2637. https://doi.org/10.3390/agronomy12112637
Zhou X, Zhang Y, Leng S, Wang Z, Gong C, Zuo Q, Yang G. Uniconazole and Adaptability of Transplantations by Enhancing the Competition Tolerance in a High Sowing Density of Rapeseed Blanket Seedlings. Agronomy. 2022; 12(11):2637. https://doi.org/10.3390/agronomy12112637
Chicago/Turabian StyleZhou, Xiangyu, Yu Zhang, Suohu Leng, Zeyu Wang, Chenhu Gong, Qingsong Zuo, and Guang Yang. 2022. "Uniconazole and Adaptability of Transplantations by Enhancing the Competition Tolerance in a High Sowing Density of Rapeseed Blanket Seedlings" Agronomy 12, no. 11: 2637. https://doi.org/10.3390/agronomy12112637





