Volatiles and Transcriptome Profiling Revealed the Formation of ‘Taro-like’ Aroma in the Leaf of Pumpkin (Cucurbita moschata)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Standards
2.3. Extraction of Volatile Compounds
2.4. GC-MS Analysis
2.5. Data Preprocessing and Statistical Analyses
2.6. GC-Olfactometry Analysis
2.7. cDNA Library Construction and RNA-Sequencing
2.8. Mapping of Reads to Reference Genome and Analysis of the Genes
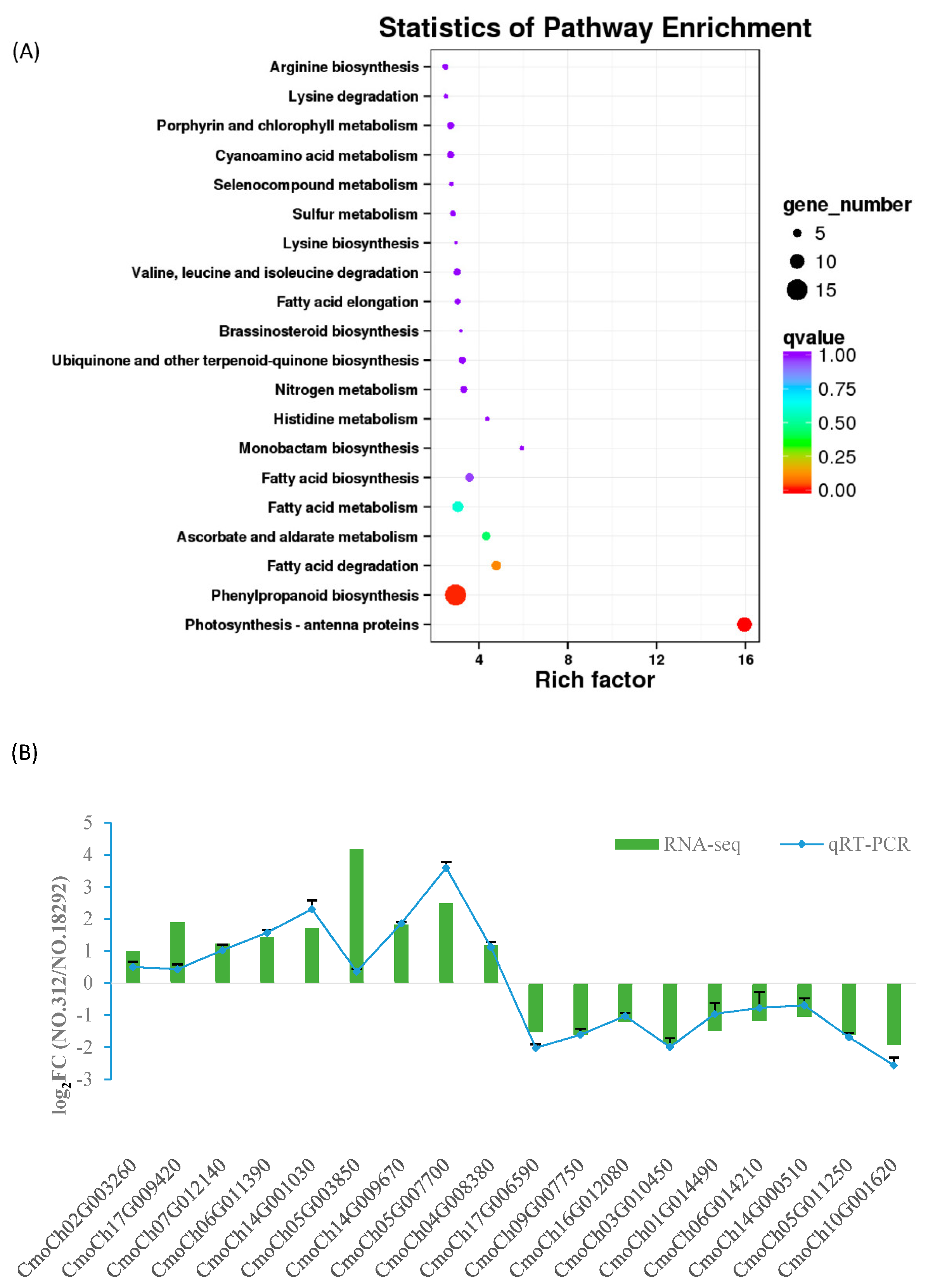
2.9. Validation of DEGs by Quantitative Real-Time PCR Analysis
3. Results
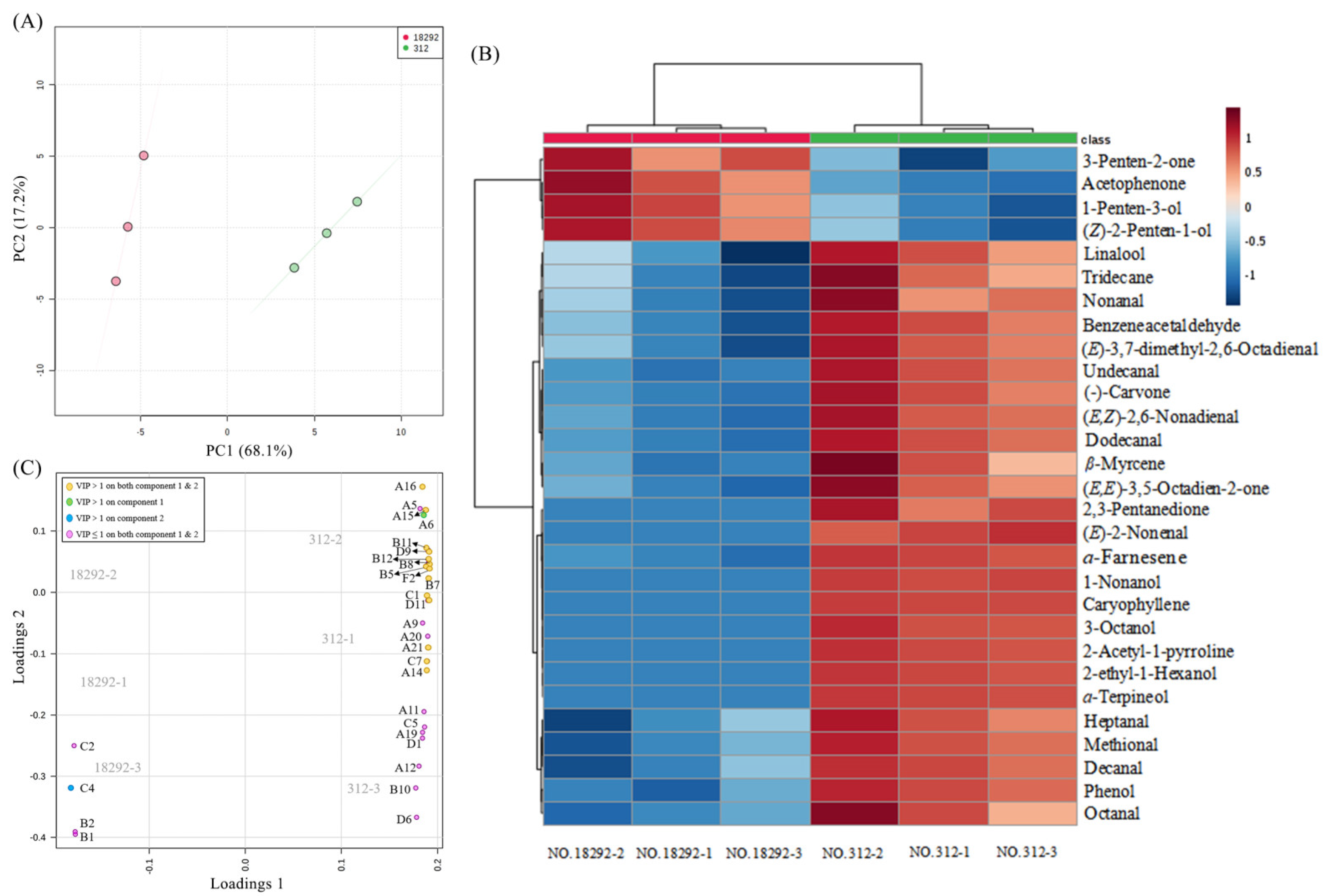
3.1. Identification of Volatile Compounds and Chemometric Analysis
3.2. Identification and Analysis of Differential Volatile Compounds
3.3. The Analysis of Key Volatile Compound Related to ‘Taro-like’ Aroma Using GC-O
3.4. Identification and Analysis of DEGs
4. Discussion
4.1. Analyses of Key Volatile Compounds Associated with the ‘Taro-like’ Aroma in Pumpkin
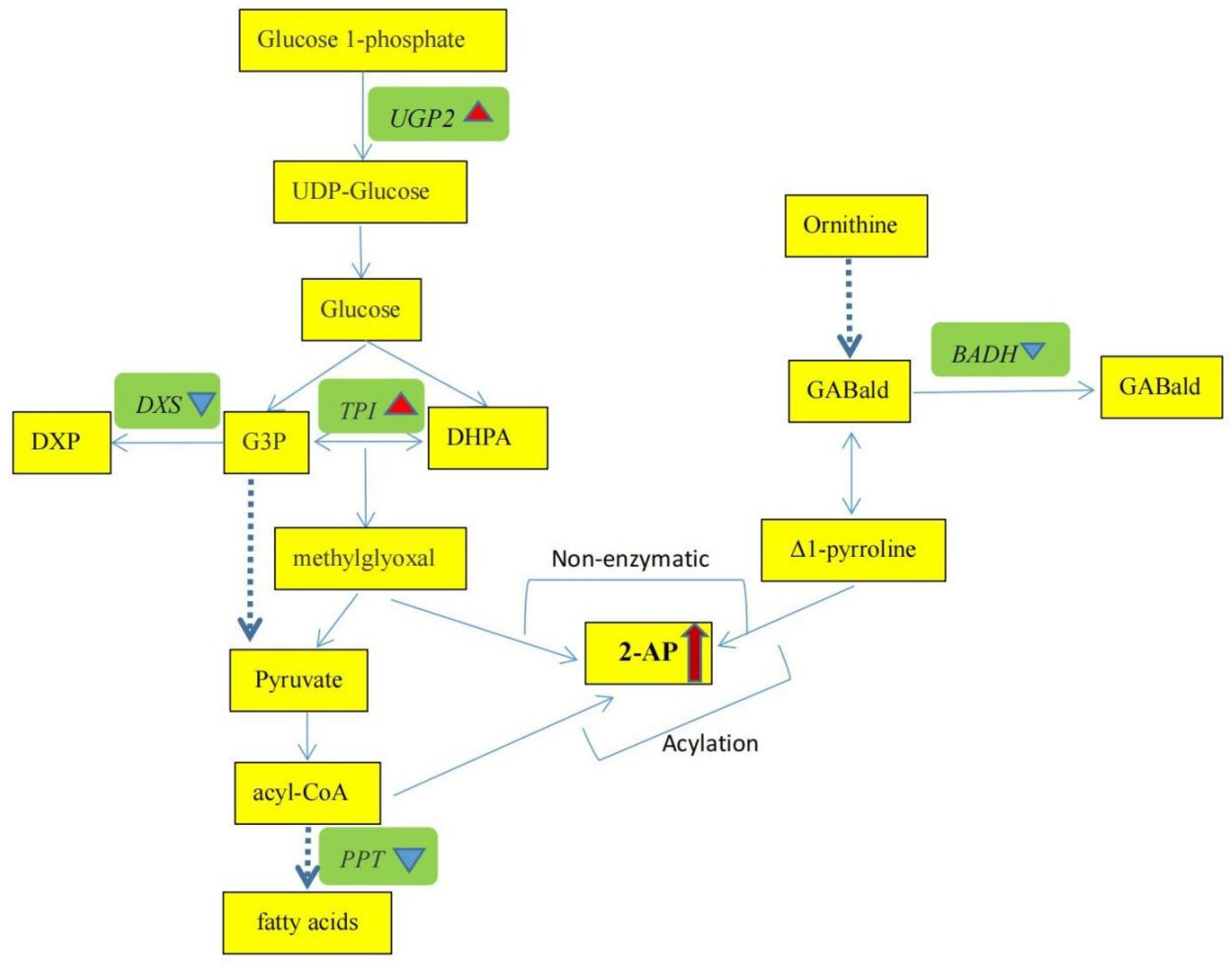
4.2. Genes’ Expression Patterns and Correlations among Volatile Compounds and Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shao, G.N.; Tang, A.; Tang, S.Q.; Luo, J.; Jiao, G.A.; Wu, J.L.; Hu, P.S. A New Deletion Mutation of Fragrant Gene and the Development of Three Molecular Markers for Fragrance in Rice. Plant Breed. 2011, 130, 172–176. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, P.; Chen, X.; Sun, Y.; Yue, C.; Ye, N. Transcriptome and Metabolite Profiling Reveal Novel Insights into Volatile Heterosis in the Tea Plant (Camellia sinensis). Molecules 2019, 24, 3380. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Chen, S.; Zhou, Z.; Li, X.; Chen, S.; Hu, J.; Lai, Z.; Sun, Y. Transcriptome Analysis Reveals the Effect of Short-Term Sunlight on Aroma Metabolism in Postharvest Leaves of Oolong Tea (Camellia sinensis). Food Res. Int. 2020, 137, 109347. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Chen, J.; Tang, N.; Gao, Y.; Hong, M.; Wei, W.; Cao, H. Transcriptome Analysis of Aroma Volatile Metabolism Change in Tomato (Solanum lycopersicum) Fruit under Di Ff Erent Storage Temperatures and 1- MCP Treatment. Postharvest Biol. Technol. 2018, 135, 57–67. [Google Scholar] [CrossRef]
- Li, J.X.; Zhong, Y.; Luo, J.N.; He, X.; Gong, H.; Yan, S.; Huang, H. Detection of Volatile Flavor Compounds in Leaf of Xiangyu Pumpkin Using Headspace Solid-Phase Microextraction-Gas Chromatography-Mass Spectrometry. J. Zhejiang Univ. 2019, 45, 175–180. [Google Scholar]
- LI, J.; Miao, A.; Zhao, G.; Liu, X.; Wu, H.; Luo, J.; Gong, H.; Zheng, X.; Deng, L.; Ma, C. Assessment of the ‘Taro-like’ Aroma of Pumpkin Fruit (Cucurbita moschata D.) via E-Nose, GC–MS and GC-O Analysis. Food Chem. X 2022, 15, 100435. [Google Scholar]
- Ho, C.T.; Zheng, X.; Li, S. Tea Aroma Formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Verzera, A.; Dima, G.; Tripodi, G.; Ziino, M. Fast Quantitative Determination of Aroma Volatile Constituents in Melon Fruits by Headspace—Solid-Phase Microextraction and Gas Chromatography—Mass Spectrometry. Food Anal. Methods 2011, 4, 141–149. [Google Scholar] [CrossRef]
- Leffingwell, J.C.; Alford, E.D.; Leffingwell, D.; Consulting, A. Identification of the Volatile Constituents of Raw Pumpkin (Cucurbita pepo L.) by Dynamic Headspace Analyses. Leffingwell Rep. 2015, 7, 293–301. [Google Scholar]
- Poehlmann, S.; Schieberle, P. Characterization of the Aroma Signature of Styrian Pumpkin Seed Oil (Cucurbita Pepo Subsp. Pepo Var. Styriaca) by Molecular Sensory Science. J. Agric. Food Chem. 2013, 61, 2933–2942. [Google Scholar] [CrossRef] [PubMed]
- Bandeira Reidel, R.V.; Cioni, P.L.; Pistelli, L. Volatiles from Different Plant Parts of Punica Granatum Grown in Tuscany (Italy). Sci. Hortic. 2018, 231, 49–55. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, C.; Xu, C.; Sun, J.; Grierson, D.; Zhang, B.; Chen, K. Integration of Metabolite Profiling and Transcriptome Analysis Reveals Genes Related to Volatile Terpenoid Metabolism in Finger Citron (C. medica Var. Sarcodactylis). Molecules 2019, 24, 2564. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, L.E.; Strickler, S.R.; Mueller, L.A.; Mazourek, M. An Acorn Squash (Cucurbita pepo ssp. Ovifera) Fruit and Seed Transcriptome as a Resource for the Study of Fruit Traits in Cucurbita. Hortic. Res. 2015, 2, 14070. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Luo, S.; Wang, R. The First Illumina-Based de Novo Transcriptome Sequencing and Analysis of Pumpkin (Cucurbita moschata Duch.) and SSR Marker Development. Mol. Breed. 2014, 34, 1437–1447. [Google Scholar] [CrossRef]
- Guo, W.; Chen, B.; Chen, X.; Guo, Y.; Yang, H.; Li, X.; Wang, G. Transcriptome Profiling of Pumpkin (Cucurbita moschata Duch.) Leaves Infected with Powdery Mildew. PLoS ONE 2018, 13, e0190175. [Google Scholar] [CrossRef]
- Luo, W.; Li, Y.; Sun, Y.; Lu, L.; Zhao, Z.; Zhou, J.; Li, X. Comparative RNA-Seq Analysis Reveals Candidate Genes Associated with Fruit Set in Pumpkin. Sci. Hortic. 2021, 288, 110255. [Google Scholar] [CrossRef]
- Kaur, S.; Panghal, A.; Garg, M.K.; Mann, S.; Khatkar, S.K.; Sharma, P.; Chhikara, N. Functional and Nutraceutical Properties of Pumpkin—A Review. Nutr. Food Sci. 2020, 50, 384–401. [Google Scholar] [CrossRef]
- Davoudi, M.; Chen, J.; Lou, Q. Genome-Wide Identification and Expression Analysis of Heat Shock Protein 70 (HSP70) Gene Family in Pumpkin (Cucurbita moschata) Rootstock under Drought Stress Suggested the Potential Role of These Chaperones in Stress Tolerance. Int. J. Mol. Sci. 2022, 23, 1918. [Google Scholar] [CrossRef]
- Errard, A.; Ulrichs, C.; Kühne, S.; Mewis, I.; Drungowski, M.; Schreiner, M.; Baldermann, S. Single- versus Multiple-Pest Infestation Affects Differently the Biochemistry of Tomato (Solanum lycopersicum ’Ailsa Craig’). J. Agric. Food Chem. 2015, 63, 10103–10111. [Google Scholar] [CrossRef]
- Chen, W.; Qi, D.; Wang, W.; Miao, A.; Ma, C. GC-MS Analysis Combined with Sensory Analysis Revealed the Various Aroma Characteristics of Black Tea Resulted from Different Grafting Rootstocks. J. Food Sci. 2021, 86, 813–823. [Google Scholar] [CrossRef]
- Qi, D.; Miao, A.; Cao, J.; Wang, W.; Chen, W.; Pang, S.; He, X.; Ma, C. Study on the Effects of Rapid Aging Technology on the Aroma Quality of White Tea Using GC–MS Combined with Chemometrics: In Comparison with Natural Aged and Fresh White Tea. Food Chem. 2018, 265, 189–199. [Google Scholar] [CrossRef]
- Hinge, V.R.; Patil, H.B.; Nadaf, A.B. Aroma Volatile Analyses and 2AP Characterization at Various Developmental Stages in Basmati and Non-Basmati Scented Rice (Oryza sativa L.) Cultivars. Rice 2016, 9, 38. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of Plant-Derived Flavor Compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- García-Parra, J.; González-Cebrino, F.; Ramírez, R. Volatile Compounds of a Pumpkin (Cucurbita moschata) Purée Processed by High Pressure Thermal Processing. J. Sci. Food Agric. 2020, 100, 4449–4456. [Google Scholar] [CrossRef] [PubMed]
- Steingass, C.B.; Jutzi, M.; Müller, J.; Carle, R.; Schmarr, H.G. Ripening-Dependent Metabolic Changes in the Volatiles of Pineapple (Ananas comosus (L.) Merr.) Fruit: II. Multivariate Statistical Profiling of Pineapple Aroma Compounds Based on Comprehensive Two-Dimensional Gas Chromatography-Mass Spectrometry. Anal. Bioanal. Chem. 2015, 407, 2609–2624. [Google Scholar] [CrossRef] [PubMed]
- Dumhai, R.; Wanchana, S.; Saensuk, C.; Choowongkomon, K.; Mahatheeranont, S.; Kraithong, T.; Toojinda, T.; Vanavichit, A. Scientia Horticulturae Discovery of a Novel CnAMADH2 Allele Associated with Higher Levels of 2- Acetyl-1-Pyrroline (2AP) in Yellow Dwarf Coconut (Cocos nucifera L.). Sci. Hortic. 2019, 243, 490–497. [Google Scholar] [CrossRef]
- Attar, U.; Hinge, V.; Zanan, R.; Adhav, R.; Nadaf, A. Identification of Aroma Volatiles and Understanding 2-Acetyl-1-Pyrroline Biosynthetic Mechanism in Aromatic Mung Bean (Vigna radiata (L.) Wilczek). Physiol. Mol. Biol. Plants 2017, 23, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Guo, X.; Qin, Z.; Yao, Y.; Hu, X.; Wu, J. Identification of Aroma-Active Compounds in Jiashi Muskmelon Juice by GC-O-MS and OAV Calculation. J. Agric. Food Chem. 2012, 60, 4179–4185. [Google Scholar] [CrossRef]
- Lin, D.; Chou, S.; Wang, A.Z.; Wang, Y.; Kuo, S.; Lai, C.; Chen, L.; Wang, C. A Proteomic Study of Rice Cultivar TNG67 and Its High Aroma Mutant. Plant Sci. 2014, 214, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Wakte, K.; Kad, T.; Zanan, R.; Nadaf, A. Mechanism of 2-Acetyl-1-Pyrroline Biosynthesis in Bassia Latifolia Roxb. Flowers. Physiol. Mol. Biol. Plants 2011, 17, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Wakte, K.; Zanan, R.; Hinge, V.; Khandagale, K.; Nadaf, A.; Henry, R. Thirty-Three Years of 2-Acetyl-1-Pyrroline, a Principal Basmati Aroma Compound in Scented Rice (Oryza sativa L.): A Status Review. J. Sci. Food Agric. 2017, 97, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Qi, Y.; Lei, Y.; Wang, S.; Hu, H.; Wei, A. Transcriptome and Metabolome Dynamics Explain Aroma Differences between Green and Red Prickly Ash Fruit. Foods 2021, 10, 391. [Google Scholar] [CrossRef]
- Costello, P.; Henschke, P. Mousy Off-Flavor of Wine: Precursors and Biosynthesis of the 2-Acetyltetrahydropyridine, and 2-Acetyl-1-Pyrroline by Lactobacillus Hilgardii DSM 20176. J. Agric. Food Chem. 2002, 50, 7070–7087. [Google Scholar] [CrossRef] [PubMed]
- Okpala, N.E.; Mo, Z.; Duan, M.; Tang, X. The Genetics and Biosynthesis of 2-Acetyl-1-Pyrroline in Fragrant Rice. Plant Physiol. Biochem. 2019, 15, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, L.M.T.; Fitzgerald, T.L.; Henry, R.J.; Jin, Q.; Waters, D.L.E. The Gene for Fragrance in Rice. Plant Physiol. Biochem. 2005, 3, 363–370. [Google Scholar] [CrossRef]
| No. | Volatiles | Retention Index (RI) | No. 18292:No. 312 | ||
|---|---|---|---|---|---|
| RI-Practical | RI-NIST 17 Library a | RI-STD b | Ratio of Peak Area | ||
| Aldehydes | |||||
| A1 | (E)-2-pentenal | 745 | 754 | - | 1.00:0.98 |
| A2 | hexanal | 795 | 800 | 797 | 1.00:0.95 |
| A3 | 2-hexenal | 850 | 851 | 852 | 1.00:0.96 |
| A4 | (E)-4-heptenal | 894 | 900 | - | 1.00:1.13 |
| A5 | heptanal | 897 | 901 | - | 1.00:2.11 |
| A6 | methional | 903 | 907 | - | 1.00:2.67 |
| A7 | (E,E)-2,4-hexadienal | 907 | 911 | - | 1.00:0.81 |
| A8 | benzaldehyde | 961 | 962 | 966 | 1.00:1.00 |
| A9 | octanal | 999 | 1003 | - | 1.00:1.88 |
| A10 | (E,E)-2,4-heptadienal | 1008 | 1012 | 1014 | 1.00:1.44 |
| A11 | benzeneacetaldehyde | 1043 | 1045 | 1048 | 1.00:2.27 |
| A12 | nonanal | 1100 | 1104 | 1100 | 1.00:1.60 |
| A13 | (E,E)-2,4-octadienal | 1107 | 1115 | - | 1.00:1.26 |
| A14 | (E,Z)-2,6-nonadienal | 1150 | 1155 | - | 1.00:2.61 |
| A15 | (E)-2-nonenal | 1157 | 1162 | 1162 | 0:1.00 |
| A16 | decanal | 1202 | 1206 | 1204 | 1.00:2.96 |
| A17 | 2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde | 1221 | 1220 | - | 1.00:1.17 |
| A18 | 2,2,6-trimethyl-1-cyclohexene-1-acetaldehyde | 1258 | 1254 | - | 1.00:1.39 |
| A19 | (E)-3,7-dimethyl-2,6-octadienal | 1264 | 1270 | - | 1.00:1.85 |
| A20 | undecanal | 1303 | 1307 | - | 1.00:3.43 |
| A21 | dodecanal | 1405 | 1409 | - | 1.00:4.77 |
| Alcohols | |||||
| B1 | 1-penten-3-ol | - | 684 | - | 1.00:0.54 |
| B2 | (Z)-2-penten-1-ol | 758 | 767 | - | 1.00:0.55 |
| B3 | 1-hexanol | 861 | 868 | 864 | 1.00:0.76 |
| B4 | cyclohexanol | 884 | 880 | - | 1.00:0.99 |
| B5 | phenol | 970 | 980 | - | 1.00:2.24 |
| B6 | 1-octen-3-ol | 975 | 980 | - | 1.00:0.96 |
| B7 | 3-octanol | 992 | 994 | - | 0:1.00 |
| B8 | 2-ethyl-1-hexanol | 1023 | 1030 | - | 0:1.00 |
| B9 | benzyl alcohol | 1032 | 1036 | 1041 | 1.00:0.76 |
| B10 | linalool | 1096 | 1099 | 1103 | 1.00:1.83 |
| B11 | 1-nonanol | 1166 | 1173 | - | 0:1.00 |
| B12 | α-terpineol | 1196 | 1189 | - | 0:1.00 |
| B13 | 1-decanol | 1266 | 1273 | - | 1.00:1.35 |
| Ketones | |||||
| C1 | 2,3-pentanedione | - | 698 | - | 0:1.00 |
| C2 | 3-penten-2-one | 728 | 733 | - | 1.00:0.54 |
| C3 | 2,2,6-trimethyl-cyclohexanone | 1035 | 1036 | - | 1.00:1.30 |
| C4 | acetophenone | 1065 | 1065 | - | 1.00:0.43 |
| C5 | (E,E)-3,5-octadien-2-one | 1065 | 1073 | - | 1.00:1.77 |
| C6 | 2,6,6-trimethyl-2-cyclohexene-1,4-dione | 1143 | 1144 | - | 1.00:0.72 |
| C7 | (-)-carvone | 1245 | 1242 | - | 1.00:3.87 |
| C8 | α-ionone | 1422 | 1426 | 1428 | 1.00:1.10 |
| C9 | trans-β-ionone | 1479 | 1486 | 1486 | 1.00:1.10 |
| Hydrocarbons | |||||
| D1 | β-myrcene | 985 | 991 | 985 | 1.00:1.77 |
| D2 | p-cymene | 1024 | 1022 | - | 1.00:0.79 |
| D3 | limonene | 1029 | 1030 | 1032 | 1.00:1.04 |
| D4 | γ-terpinene | 1057 | 1060 | - | 1.00:0.99 |
| D5 | naphthalene | 1190 | 1182 | - | 1.00:0.97 |
| D6 | tridecane | 1295 | 1300 | - | 1.00:1.54 |
| D7 | 1-tetradecene | 1387 | 1392 | - | 1.00:1.24 |
| D8 | tetradecane | 1395 | 1400 | - | 1.00:1.40 |
| D9 | caryophyllene | 1428 | 1419 | - | 0:1.00 |
| D10 | pentadecane | 1495 | 1500 | - | 1.00:1.21 |
| D11 | α-farnesene | 1499 | 1508 | - | 1.00:6.55 |
| Esters | |||||
| E1 | methyl salicylate | 1193 | 1192 | 1194 | 1.00:1.25 |
| E2 | dimethyl phthalate | 1445 | 1455 | - | 1.00:1.30 |
| Heterocyclic compounds | |||||
| F1 | 2-ethyl-furan | - | 703 | - | 1.00:0.98 |
| F2 | 2-acetyl-1-pyrroline | 917 | 922 | 920 | 0:1.00 |
| F3 | (R)-5,6,7,7a-tetrahydro-4,4,7a-trimethyl-2(4H)-benzofuranone | 1537 | 1532 | - | 1.00:1.34 |
| No. A | Volatile Compound | Aroma Description [8,22] B | p Value C | Derivative Way [23] |
|---|---|---|---|---|
| Aldehydes | ||||
| A5 | heptanal | grass, fresh, citrus, fat | 0.003 | fatty acid |
| A6 | methional | cooked potato | 0.001 | fatty acid |
| A9 | octanal | citrus, fat, green, lemon | 0.008 | fatty acid |
| A11 | benzeneacetaldehyde | green, sweet, flower | 0.002 | phenylpropanoid |
| A12 | nonanal | grass, citrus, floral | 0.008 | fatty acid |
| A14 | (E,Z)-2,6-nonadienal | green, metal | 0.001 | fatty acid |
| A15 | (E)-2-nonenal | cucumber, fat, green | 0 | fatty acid |
| A16 | decanal | fat, citrus, flower | 0.001 | fatty acid |
| A19 | (E)-3,7-dimethyl-2,6-octadienal | lemon | 0.003 | fatty acid |
| A20 | undecanal | oil, sweet | 0.001 | fatty acid |
| A21 | dodecanal | fresh, citrus | 0.001 | fatty acid |
| Alcohols | ||||
| B1 | 1-penten-3-ol | earth | 0.003 | fatty acid |
| B2 | (Z)-2-penten-1-ol | green, rubber | 0.003 | fatty acid |
| B5 | phenol | must | 0 | phenylpropanoid |
| B7 | 3-octanol | fat, metal | 0 | fatty acid |
| B8 | 2-ethyl-1-hexanol | heavy, earth | 0 | fatty acid |
| B10 | linalool | sweet, floral | 0.008 | terpenoid |
| B11 | 1-nonanol | cucumber, fat | 0 | fatty acid |
| B12 | α-terpineol | mint, oil, anise | 0 | terpenoid |
| ketones | ||||
| C1 | 2,3-pentanedione | caramel, fruit | 0 | fatty acid |
| C2 | 3-penten-2-one | sweet | 0.004 | fatty acid |
| C4 | acetophenone | almond, sweet, flower | 0.004 | phenylpropanoid |
| C5 | (E,E)-3,5-octadien-2-one | fruit, mushroom, fat | 0.004 | fatty acid |
| C7 | (-)-carvone | mint | 0.003 | fatty acid |
| Hydrocarbons | ||||
| D1 | β-myrcene | spice | 0.01 | terpenoid |
| D6 | ttridecane | alkane | 0.013 | fatty acid |
| D9 | caryophyllene | wood, earth | 0 | terpenoid |
| D11 | α-farnesene | wood, sweet | 0 | carotenoid |
| Heterocyclic compounds | ||||
| F2 | 2-acetyl-1-pyrroline | nut, roast, popcorn-like, sweet | 0 | amino acid |
| Retention Time | Odor Quality | Odor Intensity | Volatile Compounds | Identification |
|---|---|---|---|---|
| 12.61–12.91 | taro | 3 | 2-acetyl-1-pyrroline | RT, MS |
| 15.11–15.31 | rust | 2 | 3-octanol | RT, MS |
| 21.92–22.05 | floral | 1 | 1-nonanol | RT, MS |
| 25.96–26.15 | mint | 2 | (E)-3,7-dimethyl-2,6-octadienal | RT, MS |
| Gene Name | Gene ID | Expression Level-FPKM in No. 18292 | Expression Level-FPKM in No. 312 | Regulated (No. 312/No. 18292) |
|---|---|---|---|---|
| UGP2 | CmoCh14G008490 | 5.65 | 11.88 | up |
| TPI | CmoCh02G003260 | 9.20 | 21.08 | up |
| DXS | CmoCh16G008020 | 11.43 | 2.96 | down |
| PPT | CmoCh06G008220 | 49.06 | 15.29 | down |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Qiu, Y.; Luo, J.; Wang, W.; Wu, H.; Liu, X.; Zhao, G.; Gong, H.; Zheng, X.; Zhong, Y.; et al. Volatiles and Transcriptome Profiling Revealed the Formation of ‘Taro-like’ Aroma in the Leaf of Pumpkin (Cucurbita moschata). Agronomy 2022, 12, 2641. https://doi.org/10.3390/agronomy12112641
Zhao S, Qiu Y, Luo J, Wang W, Wu H, Liu X, Zhao G, Gong H, Zheng X, Zhong Y, et al. Volatiles and Transcriptome Profiling Revealed the Formation of ‘Taro-like’ Aroma in the Leaf of Pumpkin (Cucurbita moschata). Agronomy. 2022; 12(11):2641. https://doi.org/10.3390/agronomy12112641
Chicago/Turabian StyleZhao, Siying, Yuehan Qiu, Jianning Luo, Wenwen Wang, Haibin Wu, Xiaoxi Liu, Gangjun Zhao, Hao Gong, Xiaoming Zheng, Yujuan Zhong, and et al. 2022. "Volatiles and Transcriptome Profiling Revealed the Formation of ‘Taro-like’ Aroma in the Leaf of Pumpkin (Cucurbita moschata)" Agronomy 12, no. 11: 2641. https://doi.org/10.3390/agronomy12112641
APA StyleZhao, S., Qiu, Y., Luo, J., Wang, W., Wu, H., Liu, X., Zhao, G., Gong, H., Zheng, X., Zhong, Y., Yang, X., & Li, J. (2022). Volatiles and Transcriptome Profiling Revealed the Formation of ‘Taro-like’ Aroma in the Leaf of Pumpkin (Cucurbita moschata). Agronomy, 12(11), 2641. https://doi.org/10.3390/agronomy12112641






