Changes in Biochemical and Microbiological Quality of Silage Produced with the Use of Innovative Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material Source and Bale Preparation
2.2. Tested Films
2.3. Microbiological Analyses
2.4. Analyses of Silage Biochemical Properties
2.5. Statistical Analyses
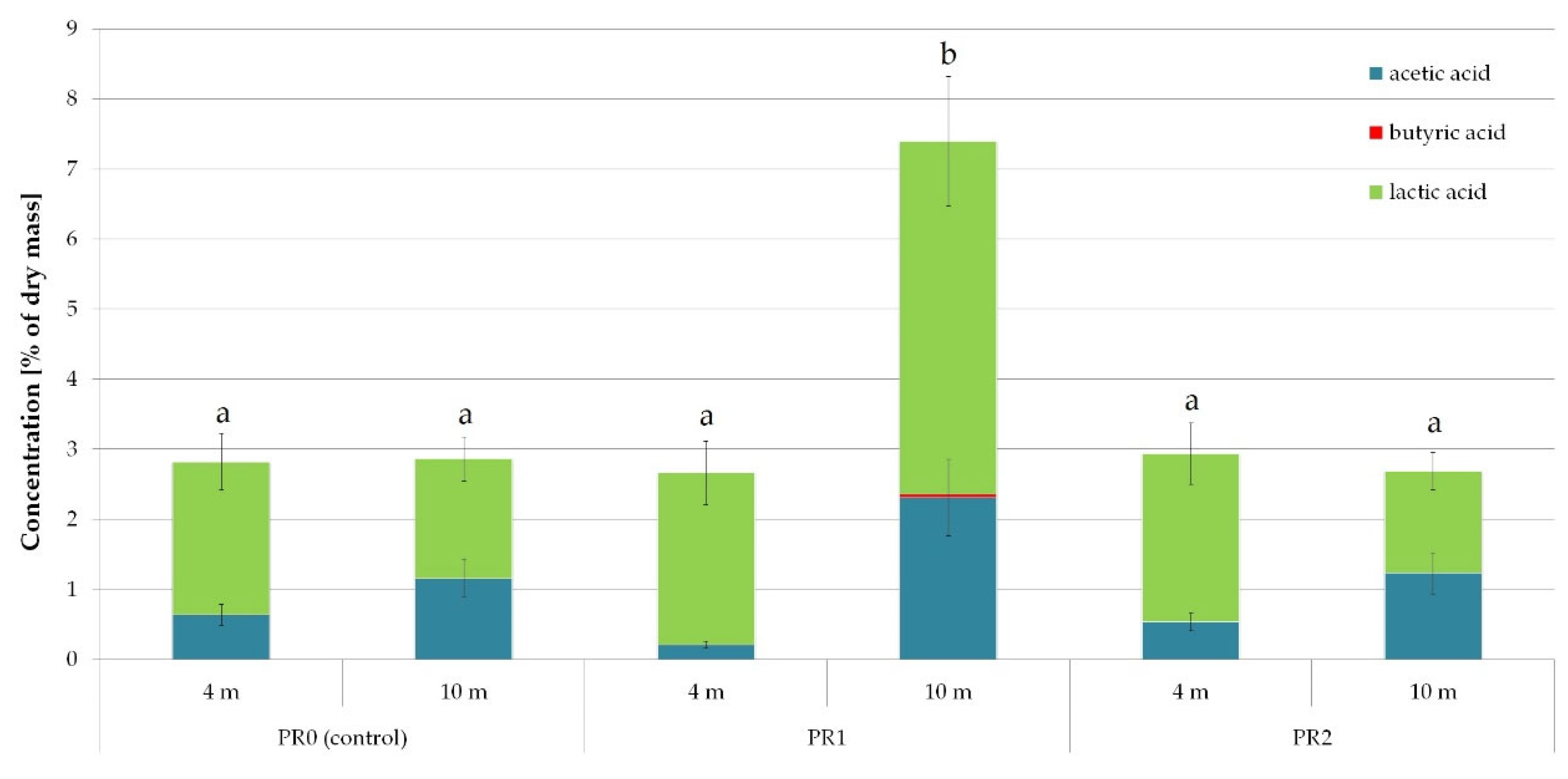
3. Results and Discussion
3.1. Nanosilver and Microcellulose Addition to the Wrapping Film
3.2. Preparation of Films with Recycled PE
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Supel, P.; Kaszycki, P.; Kasperczyk, M.; Kacorzyk, P. Impact of innovative films used for the production of silage on biochemical and microbial product qualities. Biol. Life Sci. Forum 2021, 3, 17. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Rinne, M. Highlights of progress in silage conservation and future perspectives. Grass Forage Sci. 2018, 73, 40–52. [Google Scholar] [CrossRef]
- Szterk, P.; Mikołajczak, J. Wykorzystanie folii biodegradowalnej przy produkcji kiszonek. Wiadomości Zootech. 2007, 45, 39–47. (In Polish) [Google Scholar]
- Korpysz, K.; Gach, S. Properties of the stretch film used for green fodder balles wrapping vs. quality of the silage. J. Res. Appl. Agric. Eng. 2011, 56, 76–81. (In Polish) [Google Scholar]
- Avila, C.L.S.; Carvalho, B.F. Silage fermentation-updates focusing on the performance of micro-organisms. J. Appl. Microbiol. 2019, 128, 966–984. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Benno, Y.; Ogawa, M.; Ohmomo, S.; Kumai, S.; Nakase, T. Influence of Lactobacillus spp. from an inoculant and of Weissella and Leuconostoc spp. from forage crops on silage fermentation. Appl. Env. Microbiol. 1998, 64, 2982–2987. [Google Scholar] [CrossRef] [Green Version]
- Grabowicz, M.; Kaszkowiak, J.; Dorszewski, P.; Szterk, P. Przydatność folii oksybiodegradowalnej do przechowywania kiszonek. Logistyka 2015, 4, 1869–1876. (In Polish) [Google Scholar]
- Savoie, P.; Jofriet, J.C. Silage storage. In Silage Science and Technology; Buxton, D.R., Muck, R.E., Harrison, J.H., Eds.; American Society of Agronomy: Madison, WI, USA, 2003; Volume 42, pp. 405–467. [Google Scholar]
- Borreani, G.; Piano, S.; Tabacco, E. Aerobic stability of maize silage stored under plastic films with different oxygen permeability. J. Sci. Food Agric. 2014, 94, 2684–2690. [Google Scholar] [CrossRef]
- Coblentz, W.K.; Akins, M.S. Silage review: Recent advances and future technologies for baled silages. J. Dairy Sci. 2018, 101, 4075–4092. [Google Scholar] [CrossRef]
- Gach, S.; Korpysz, K.; Skonieczny, I. Wybrane aspekty sporządzania kiszonek w belach osłanianych folią. In Współczesne Zagadnienia Rozwoju Sektora Energetycznego i Rolniczego, Wyd; SGGW: Warsaw, Poland, 2010. (In Polish) [Google Scholar]
- Borreani, G.; Tabacco, E. Low permeability to oxygen of a new barrier film prevents butyric acid bacteria spore formation in farm corn silage. J. Dairy Sci. 2008, 91, 4272–4281. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Fenlon, J.S. A meta-analysis comparing standard polyethylene and oxygen barrier film in terms of losses during storage and aerobic stability of silage. Grass Forage Sci. 2014, 69, 385–392. [Google Scholar] [CrossRef]
- Kacorzyk, P.; Kasperczyk, M.; Szewczyk, W.; Lepiarczyk, A. Porównanie niektórych parametrów folii stretch z polietylenu z dwoma rodzajami folii biodegradowalnej w produkcji kiszonki. Fragm. Agron. 2017, 34, 28–33. (In Polish) [Google Scholar]
- Briassoulis, D.; Hiskakis, M.; Babou, E. Technical specifications for mechanical recycling of agricultural plastic waste. Waste Manag. 2013, 33, 1516–1530. [Google Scholar] [CrossRef] [PubMed]
- San Miguel, G.; Serrano, D.P.; Aguado, J. Valorization of Waste Agricultural Polyethylene Film by Sequential Pyrolysis and Catalytic Reforming. Ind. Eng. Chem. Res. 2009, 48, 8697–8703. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and Chemical Recycling of Solid Plastic Waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Schyns, Z.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, 2000415. [Google Scholar] [CrossRef]
- Lawrence, M. Disposal of Plastics. In A Guide to the Manufacture, Performance, and Potential of Plastics in Agriculture; Orzolek, M., Ed.; Elsevier: Oxford, UK, 2017; pp. 187–196. [Google Scholar]
- Korol, J.; Hejna, A.; Wypiór, K.; Mijalski, K.; Chmielnicka, E. Wastes from Agricultural Silage Film Recycling Line as a Potential Polymer Materials. Polymers 2021, 13, 1383. [Google Scholar] [CrossRef]
- ERDE Recycling: Recovery and Recycling Systems for Agricultural Plastics. Available online: https://mag.k-online.com/en/Menue/Hot_Topics/Archive_Hot_Topics/ERDE_Recycling_recovery_and_recycling_systems_for_agricultural_plastics (accessed on 21 September 2022).
- Horodytska, O.; Valdés, F.J.; Fullana, A. Plastic Flexible Films Waste Management—A State of Art Review. Waste Manag. 2018, 77, 413–425. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Zhang, X.; Wu, H.; Shen, J.; Chen, R.; Xiong, Y.; Li, J.; Guo, S. Progress on the Layer-by-Layer Assembly of Multilayered Polymer Composites: Strategy, Structural Control and Applications. Prog. Polym. Sci. 2019, 89, 76–107. [Google Scholar] [CrossRef]
- Iyer, K.A.; Zhang, L.; Torkelson, J.M. Direct Use of Natural Antioxidant-rich Agro-wastes as Thermal Stabilizer for Polymer: Processing and Recycling. ACS Sustain. Chem. Eng. 2016, 4, 881–889. [Google Scholar] [CrossRef]
- Muck, R.E. Recent advances in silage microbiology. Agric. Food Sci. 2013, 22, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Driehuis, F. Silage and the safety and quality of dairy foods: A review. Agric. Food Sci. 2013, 22, 16–34. [Google Scholar] [CrossRef]
- Radkowski, A.; Radkowska, I. Ocena jakości i wartości pokarmowej kiszonek z runi łąkowej wybranych gospodarstw Polski południowo-wschodniej. Wiadomości Zootech. 2014, 1, 32–37. (In Polish) [Google Scholar]
- Micek, P. Analizy chemiczne obnażają błędy podczas kiszenia traw. Top Agrar Polska. Top Bydło 2007, 4, 10–13. (In Polish) [Google Scholar]
- Kung, L.J.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Supel, P.; Kaszycki, P.; Kasperczyk, M.; Kacorzyk, P. Dynamics of microbiota changes of silage environment resulting from the use of new generation films. Przem. Chem. 2018, 97, 1333–1339. (In Polish) [Google Scholar] [CrossRef]
- Bathelt, P. Jakość Sianokiszonki w Zależności od Użytej Folii. Master’s Thesis, University of Agriculture in Krakow, Krakow, Poland, 2017. (In Polish). [Google Scholar]
- Queiroz, O.C.M.; Arriola, K.G.; Daniel, J.L.P.; Adesogan, A.T. Effects of 8 chemical and bacterial additives on the quality of corn silage. J. Dairy Sci. 2013, 96, 5836–5843. [Google Scholar] [CrossRef]
- Gajewska, J.; Błaszczyk, M. Probiotyczne bakterie fermentacji mlekowej (LAB). Post. Mikrobiol. 2012, 51, 55–65. (In Polish) [Google Scholar]
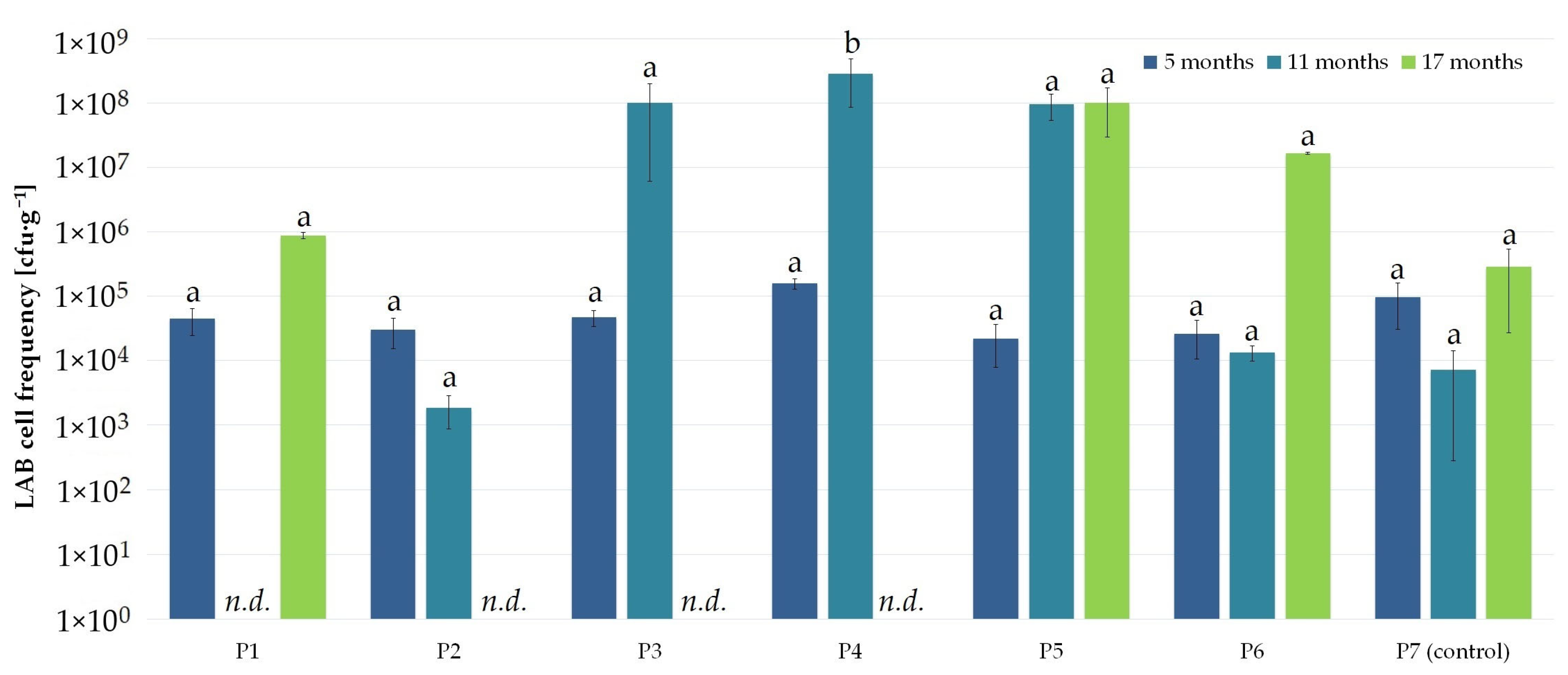
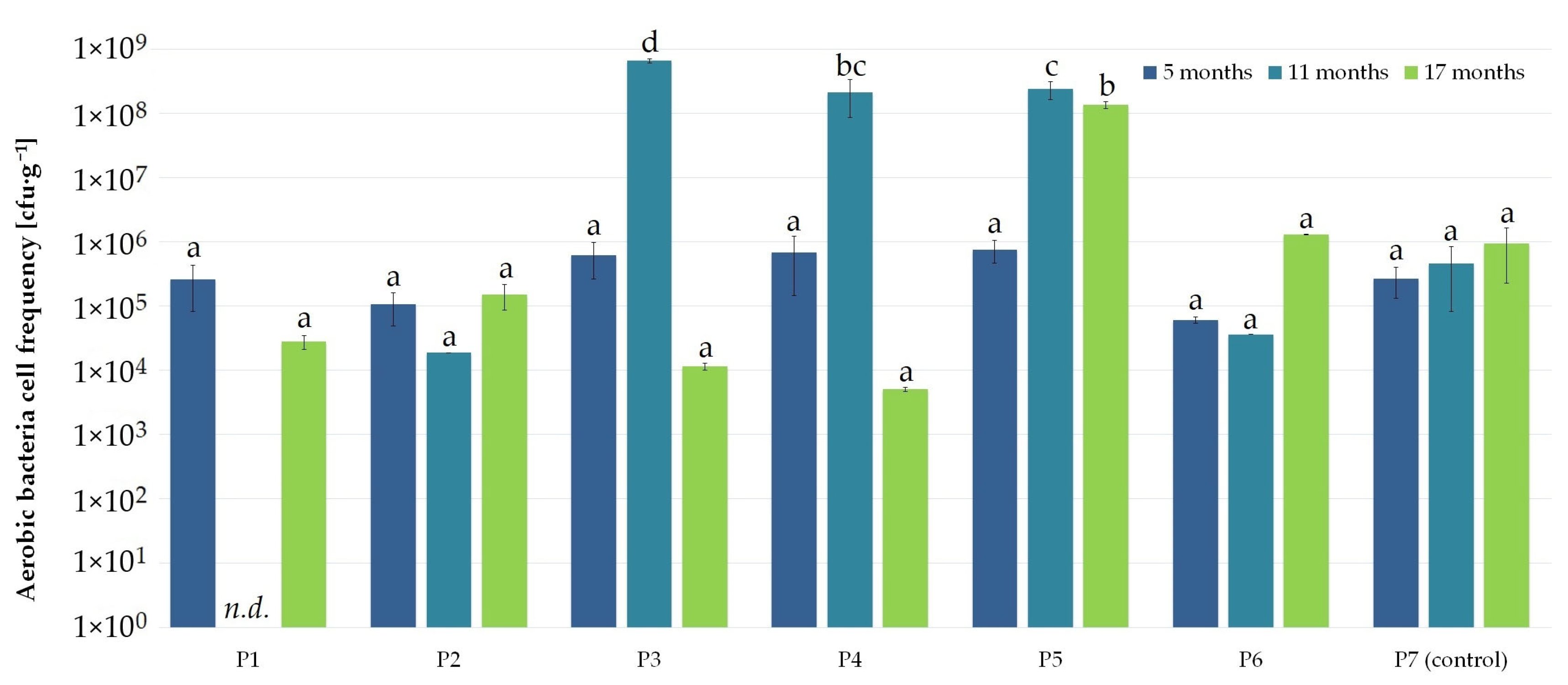

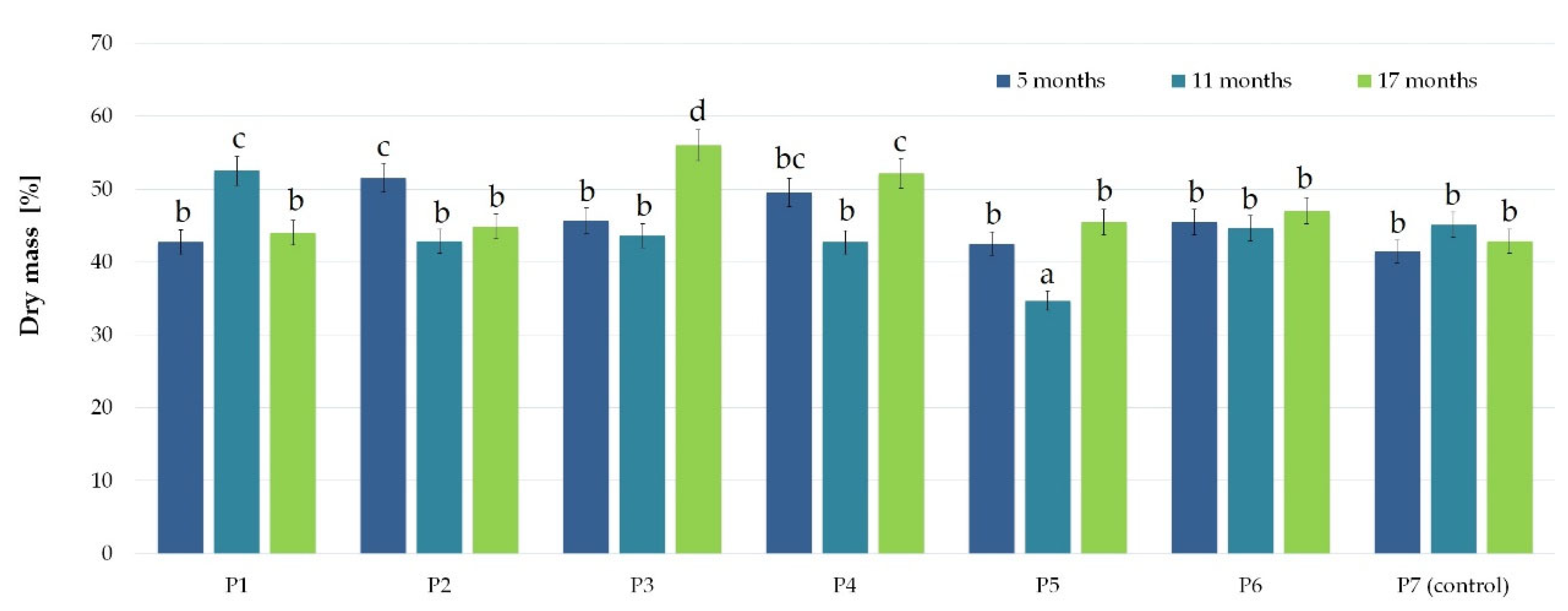



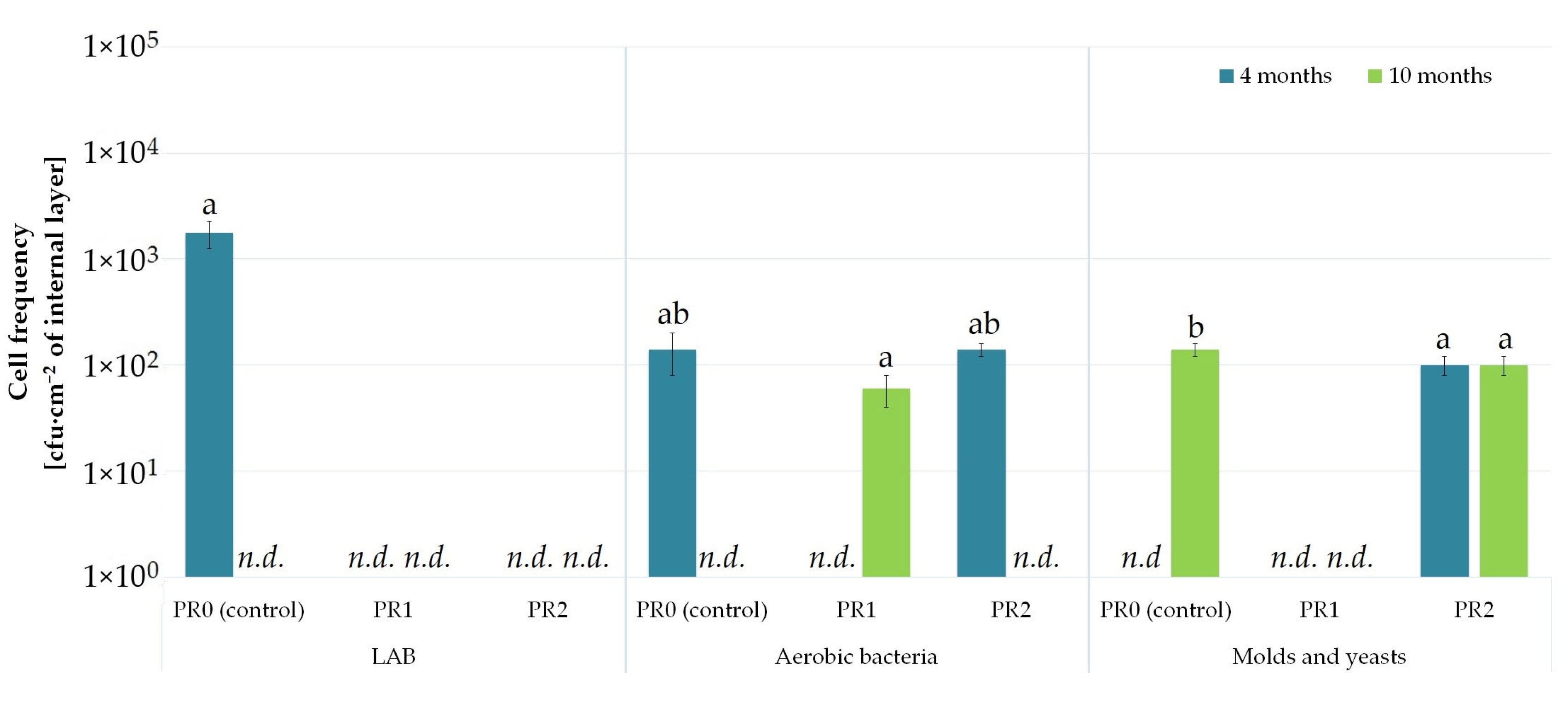


| Microbial Colonization of the External (*) and Internal (+) Film Layer | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| [Months] | Lactic Acid Bacteria | Aerobic Bacteria | Molds and Yeasts | |||||||
| Sample Variant | 5 | 11 | 17 | 5 | 11 | 17 | 5 | 11 | 17 | |
| P1 | ***/++ | */- | */- | ***/++ | */+ | **/+ | */++ | */- | */- | |
| P2 | **/++ | **/+ | ***/+++ | */+ | **/++ | ***/+++ | **/+ | **/+ | ***/+++ | |
| P3 | ***/++ | ***/+++ | ***/+++ | **/++ | ***/+++ | ***/+++ | **/+ | **/+++ | ***/+++ | |
| P4 | ***/++ | */+ | ***/++ | **/+ | **/++ | ***/+++ | **/++ | */+ | **/++ | |
| P5 | */+ | **/+ | ***/+++ | */++ | **/++ | ***/+++ | **/++ | **/++ | ***/++ | |
| P6 | ***/+ | -/- | ***/+++ | **/++ | **/++ | ***/+++ | **/+ | -/+ | ***/++ | |
| P7 (control) | **/+++ | -/- | */+ | **/++ | */+ | ***/+++ | **/+ | -/- | **/+ | |
| Total Sugar | Total Protein | Crude Fiber | |
|---|---|---|---|
| P1 | 6.4 ± 0.6 a | 14.0 ± 0.6 a | 29.3 ± 1.0 a |
| P2 | 8.0 ± 0.7 ab | 15.0 ± 0.6 a | 28.1 ± 0.9 a |
| P3 | 11.5 ± 1.1 b | 13.1 ± 0.5 a | 28.6 ± 0.9 a |
| P4 | 9.9 ± 0.9 ab | 13.0 ± 0.5 a | 29.3 ± 1.0 a |
| P5 | 6.6 ± 0.6 a | 13.7 ± 0.6 a | 29.5 ± 1.0 a |
| P6 | 7.8 ± 0.7 ab | 14.2 ± 0.6 a | 30.0 ± 1.0 a |
| P7 (control) | 6.5 ± 0.6 a | 13.4 ± 0.6 a | 29.4 ± 1.0 a |
| Dry Mass [%] | pH | ||||
|---|---|---|---|---|---|
| [Months] | 4 | 10 | 4 | 10 | |
| Sample | |||||
| PR0 (control) | 39.14 ± 1.50 b | 42.79 ± 1.64 bc | 4.69 ± 0.06 b | 4.85 ± 0.06 bc | |
| PR1 | 46.59 ± 1.78 c | 32.32 ± 1.24 a | 4.82 ± 0.06 b | 4.32 ± 0.06 a | |
| PR2 | 38.98 ± 1.49 b | 41.14 ± 1.57 b | 4.83 ± 0.06 b | 5.01 ± 0.06 c | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Supel, P.; Kaszycki, P.; Kasperczyk, M.; Kacorzyk, P. Changes in Biochemical and Microbiological Quality of Silage Produced with the Use of Innovative Films. Agronomy 2022, 12, 2642. https://doi.org/10.3390/agronomy12112642
Supel P, Kaszycki P, Kasperczyk M, Kacorzyk P. Changes in Biochemical and Microbiological Quality of Silage Produced with the Use of Innovative Films. Agronomy. 2022; 12(11):2642. https://doi.org/10.3390/agronomy12112642
Chicago/Turabian StyleSupel, Paulina, Paweł Kaszycki, Mirosław Kasperczyk, and Piotr Kacorzyk. 2022. "Changes in Biochemical and Microbiological Quality of Silage Produced with the Use of Innovative Films" Agronomy 12, no. 11: 2642. https://doi.org/10.3390/agronomy12112642





